Impact of Prescription Opioid Detoxification on Quality of Life and Pain Levels
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Quality of Life Assessment
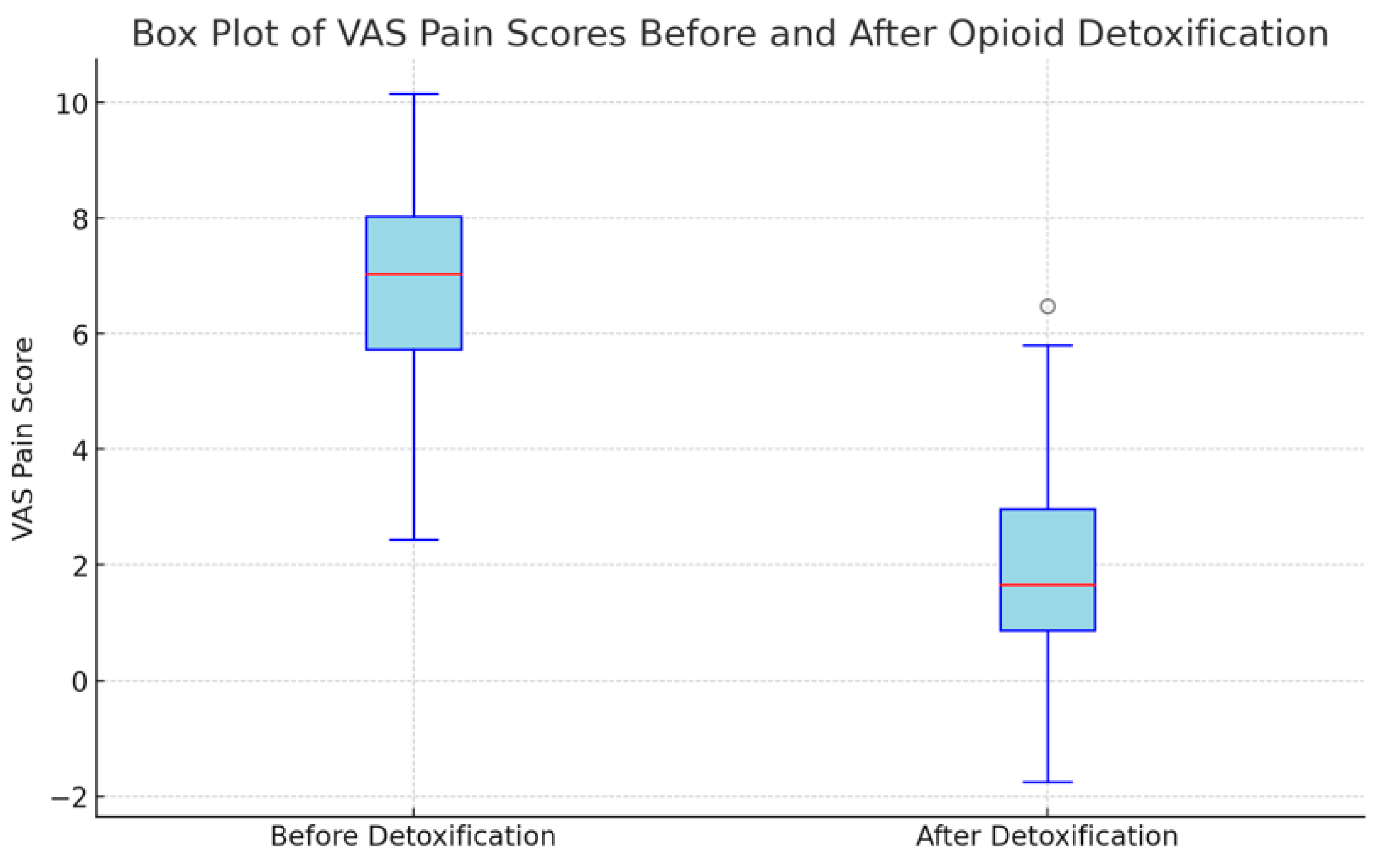
3.2. Pain Scores
3.3. Opioid Usage Cessation
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baldini, A.; Von Korff, M.; Lin EH, B. A review of potential adverse effects of long-term opioid therapy: A practitioner’s guide. Prim. Care Companion CNS Disord. 2012, 14, 27252. [Google Scholar] [CrossRef] [PubMed]
- Veiga, D.; Monteiro-Soares, M.; Mendonça, L.; Sampaio, R.; Castro-Lopes, J.; Azevedo, L. Effectiveness of opioids for chronic noncancer pain: A two-year multicenter, prospective cohort study with propensity score matching. J. Pain Off. J. Am. Pain Soc. 2018, 20, 706–715. [Google Scholar] [CrossRef]
- Bell, J.; Strang, J. Medication Treatment of Opioid Use Disorder. Biol. Psychiatry 2020, 87, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Fishbain, D.A.; Cole, B.; Lewis, J.; Rosomoff, H.L.; Rosomoff, R.S. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008, 9, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Vowles, K.E.; McEntee, M.L.; Julnes, P.S.; Frohe, T.; Ney, J.P.; van der Goes, D.N. Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain 2015, 156, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Conrardy, M.; Lank, P.; Cameron, K.A.; McConnell, R.; Chevrier, A.; Sears, J.; Ahlstrom, E.; Wolf, M.S.; Courtney, D.M.; McCarthy, D.M. Emergency department patient perspectives on the risk of addiction to prescription opioids. Pain Med. 2016, 17, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kosten, T.R.; George, T.P. The neurobiology of opioid dependence: Implications for treatment. Sci. Pract. Perspect. 2002, 1, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Cragg, A.; Hau, J.P.; Woo, S.A.; Liu, C.; Doyle-Waters, M.M.; Hohl, C.M. Risk factors for addiction among patients receiving prescribed opioids: A systematic review protocol. Syst. Rev. 2017, 6, 265. [Google Scholar] [CrossRef] [PubMed]
- Stoicea, N.; Russell, D.; Weidner, G.; Durda, M.; Joseph, N.C.; Yu, J.; Bergese, S.D. Opioid-induced hyperalgesia in chronic pain patients and the mitigating effects of gabapentin. Front. Pharmacol. 2015, 6, 104. [Google Scholar] [CrossRef][Green Version]
- Lee, M.; Silverman, S.M.; Hansen, H.; Patel, V.B.; Manchikanti, L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011, 14, 145–161. [Google Scholar] [CrossRef]
- Koponen, M.E.; Forget, P. Pharmacological interventions for opioid-induced hyperalgesia: A scoping review of preclinical trials. J. Clin. Med. 2022, 11, 7060. [Google Scholar] [CrossRef]
- Nadeau, S.E.; Wu, J.K.; Lawhern, R.A. Opioids and chronic pain: An analytic review of the clinical evidence. Front. Pain Res. 2021, 2, 721357. [Google Scholar] [CrossRef] [PubMed]
- O’Brien MD, C.; Wand AP, F. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing 2020, 49, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Uhl, G.R.; Drgon, T. Genetic Contributions to Individual Differences in Vulnerability to Addiction and Abilities to Quit. In Drug Abuse and Addiction in Medical Illness; Verster, J., Brady, K., Galanter, M., Conrod, P., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Mistry, C.J.; Bawor, M.; Desai, D.; Marsh, D.C.; Samaan, Z. Genetics of Opioid Dependence: A Review of the Genetic Contribution to Opioid Dependence. Curr. Psychiatry Rev. 2014, 10, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Chantarujikapong, S.I.; Scherrer, J.F.; Eisen, S.A.; Lyons, M.J.; Goldberg, J.; Tsuang, M.; True, W.R. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 2000, 61, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Berrettini, W. A brief review of the genetics and pharmacogenetics of opioid use disorders. Dialogues Clin. Neurosci. 2017, 19, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Laubner, G.; Zakarauskaitė, B.; Pociuvienė, G.; Tamošauskas, R.; Badaras, R. Change of quality of life in patients with chronic pain with prescription opioid usage after opioid detoxification. J. Opioid Manag. 2022, 18, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Bennett, H.; Fitzmaurice, G.; Hill, K.; Provost, S.; Weiss, R. Health-related quality of life among prescription opioid-dependent patients: Results from a multi-site study. Am. J. Addict. 2015, 24, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Badaras, R.; Jovaisa, T.; Lapinskiene, I.; Ivaskevicius, J. Dose escalation of naltrexone to reduce stress responses associated with opioid antagonist induction: A double-blind randomized trial. J. Addict. Med. 2020, 14, 253–260. [Google Scholar] [CrossRef]
- Handelsman, L.; Cochrane, K.J.; Aronson, M.J.; Ness, R.; Rubinstein, K.J.; Kanof, P.D. Two new rating scales for opiate withdrawal. Am. J. Drug Alcohol Abus. 1987, 13, 293–308. [Google Scholar] [CrossRef]
- Rugiene, R.; Dadoniene, J.; Venalis, A.; Stropuviene, S. Reumatinemis ligomis serganciu ligoniu ir kontrolines grupes tiriamuju gyvenimo kokybes palyginimas [Comparison of health-related quality of life between patients with rheumatic diseases and a control group]. Medicina 2005, 41, 561–565. [Google Scholar] [PubMed]
- Norkienė, I.; Urbanavičiūtė, I.; Kezytė, G.; Vicka, V.; Jovaisa, T. Impact of pre-operative health-related quality of life on outcomes after heart surgery. ANZ J. Surg. 2018, 88, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Staniute, M. Su sveikata susijusios gyvenimo kokybes vertinimas naudojant SF-36 klausimyna. Biol. Psichiatr. Psichofarmakol. 2007, 9, 22–25. [Google Scholar]
- Volkow, N.D.; McLellan, A.T. Opioid Abuse in Chronic Pain-Misconceptions and Mitigation Strategies. N. Engl. J. Med. 2016, 374, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Krebs, E.E.; Gravely, A.; Nugent, S.; Jensen, A.C.; DeRonne, B.; Goldsmith, E.S.; Noorbaloochi, S. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: The SPACE randomized clinical trial. JAMA 2018, 319, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.D.; Howe, C.Q. Opioid therapy for chronic pain in the United States: Promises and perils. Pain 2013, 154 (Suppl. S1), S94–S100. [Google Scholar] [CrossRef] [PubMed]
- Compton, P.; Weaver, M.F. Responsible opioid use. J. Pain Palliat. Care Pharmacother. 2015, 29, 166–168. [Google Scholar] [CrossRef]
- Manhapra, A.; Becker, W.C. Pain and Addiction: An Integrative Therapeutic Approach. Med. Clin. N. Am. 2018, 102, 745–763. [Google Scholar] [CrossRef]

| VAS Score | Pain Description |
|---|---|
| 0 | No pain |
| 1–3 | Mild pain |
| 4–6 | Moderate pain |
| 7–9 | Severe pain |
| 10 | Worst pain imaginable |
| Average length of opioid usage (months ± SD) (n = 44) | 57.8 ± 66.9 | |
| Average detoxification duration (days ± SD) (n = 44) | 9.2 ± 3.2 | |
| Pain level (n = 44) | ||
| VAS before detoxification (points ± SD) | 6.68 ± 2 | |
| VAS after detoxification (points ± SD) | 2.17 ± 1.93 | |
| Reason for opioid use; pain-causing disease (n = 44) | ||
| Oncological disease | 14 (31.81%) | |
| Neurological disease | 14 (31.81%) | |
| Musculoskeletal disease | 10 (22.72%) | |
| Rheumathoidal disease | 2 (4.54%) | |
| Gastrointestinal | 2 (4.54%) | |
| Other disease | 2 (4.54%) | |
| VAS for each disease before and after detoxification (n = 44) (points ± SD) | ||
| Oncological disease (n = 14) | 5.9 ± 2.59 | 1.60 ± 1.88 |
| Neurological disease (n = 14) | 6.07 ± 1.55 | 1.78 ± 1.63 |
| Musculoskeletal disease (n = 10) | 7.7 ± 2.13 | 2.6 ± 2.46 |
| Rheumathoidal disease (n = 2) | 6.75 ± 1.06 | 2.5 ± 0 |
| Gastrointestinal (n = 2) | 8.5 ± 0.71 | 2.25 ± 2.48 |
| Other (n = 2) | 8.5 ± 0.71 | 4.75 ± 1.77 |
| Opioid analgesics (used alone and in combinations) (n = 44) | ||
| Tramadol | 12 (27.27%) | |
| Codeine | 9 (20.45%) | |
| Morphine | 9 (20.45%) | |
| Fentanyl | 4 (9.09%) | |
| Pethidine | 2 (4.54%) | |
| Methadone | 1 (2.27%) | |
| Oxycodone | 1 (2.27%) | |
| Combined Opioid Usage | ||
| Morphine and Fentanyl | 2 (4.54%) | |
| Fentanyl and Tramadol | 2 (4.54%) | |
| Morphine and Tramadol | 1 (2.27%) | |
| Codeine, Morphine, and Tramadol | 1 (2.27%) | |
| Average initial morphine equivalent dose before treatment (mg/d ± SD) (n = 44) | 142 ± 157 |
| Single opioid | |
| Tramadol (n = 12) | 74.58 ± 79.47 |
| Codeine (n = 9) | 20.54 ± 22.71 |
| Morphine (n = 9) | 200 ± 211.82 |
| Fentanyl (n = 4) | 323.12 ± 151.67 |
| Pethidine (n = 2) | 100 ± 0 |
| Methadone (n = 1) | 70.5 |
| Oxycodone (n = 1) | 450 |
| Combined opioids | |
| Morphine and Fentanyl (n = 2) | 315 ± 75 |
| Fentanyl and Tramadol (n = 2) | 213.75 ± 116.25 |
| Morphine and Tramadol (n = 1) | 90 |
| Codeine, Morphine, and Tramadol (n = 1) | 41.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laubner Sakalauskienė, G.; Stražnickaitė, I.; Miškinytė, S.; Zdanavičius, L.; Šipylaitė, J.; Badaras, R. Impact of Prescription Opioid Detoxification on Quality of Life and Pain Levels. Clin. Pract. 2024, 14, 1529-1537. https://doi.org/10.3390/clinpract14040123
Laubner Sakalauskienė G, Stražnickaitė I, Miškinytė S, Zdanavičius L, Šipylaitė J, Badaras R. Impact of Prescription Opioid Detoxification on Quality of Life and Pain Levels. Clinics and Practice. 2024; 14(4):1529-1537. https://doi.org/10.3390/clinpract14040123
Chicago/Turabian StyleLaubner Sakalauskienė, Gabija, Indrė Stražnickaitė, Sigutė Miškinytė, Linas Zdanavičius, Jūratė Šipylaitė, and Robertas Badaras. 2024. "Impact of Prescription Opioid Detoxification on Quality of Life and Pain Levels" Clinics and Practice 14, no. 4: 1529-1537. https://doi.org/10.3390/clinpract14040123
APA StyleLaubner Sakalauskienė, G., Stražnickaitė, I., Miškinytė, S., Zdanavičius, L., Šipylaitė, J., & Badaras, R. (2024). Impact of Prescription Opioid Detoxification on Quality of Life and Pain Levels. Clinics and Practice, 14(4), 1529-1537. https://doi.org/10.3390/clinpract14040123






