Differences in Efficacy between Antibacterial Lock Therapy and the Standard of Care for CVC-Related Infections: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Source and Search Strategy
2.2. Study Selection and Data Extraction
2.3. Data Analysis
2.4. Quality and Risk of Bias Assessment
3. Results
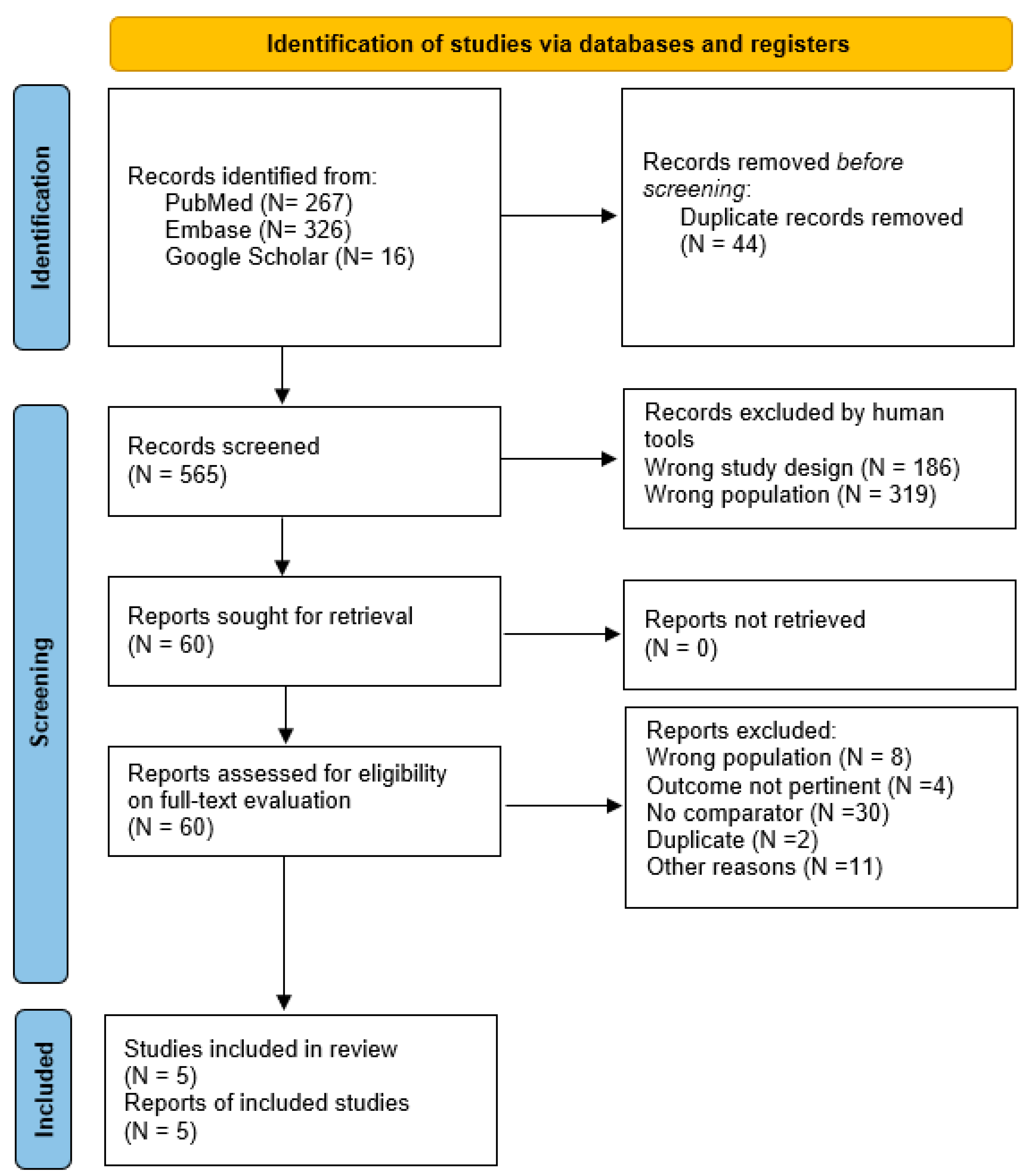
3.1. Search Results
3.2. Study Characteristics
3.3. Study Quality and Risk of Bias
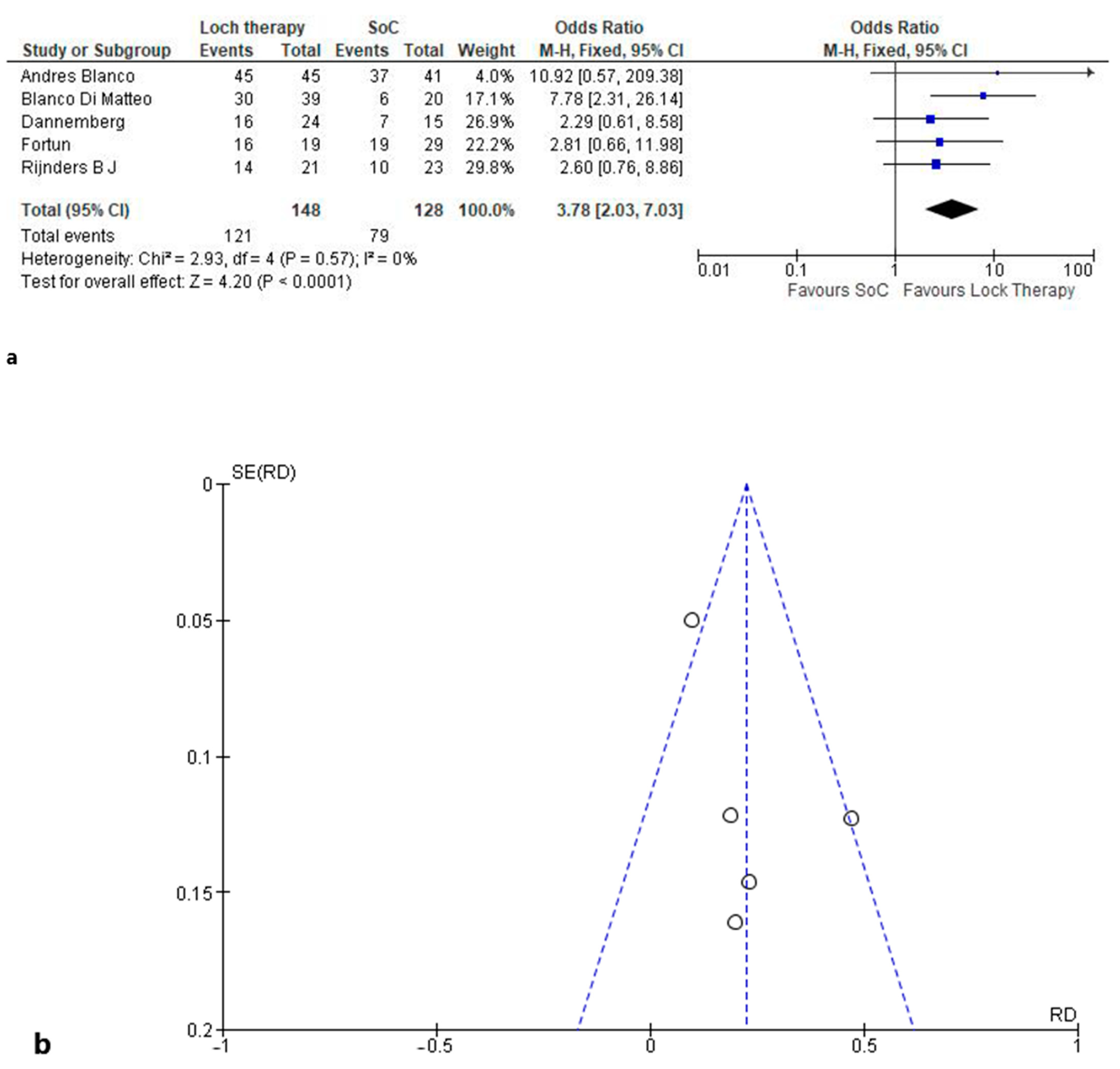
3.3.1. Microbiological Healing
3.3.2. Adverse Events Favors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- María, L.T.; Alejandro, G.S.; Jesús, P.-G.M. Central venous catheter insertion: Review of recent evidence. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 135–140. [Google Scholar] [CrossRef]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. National Kidney Foundation. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75 (Suppl. S2), S1–S164, Erratum in Am. J. Kidney Dis. 2021, 77, 551. [Google Scholar] [CrossRef]
- Rupp, M.E.; Karnatak, R. Intravascular Catheter-Related Bloodstream Infections. Infect. Dis. Clin. N. Am. 2018, 32, 765–787. [Google Scholar] [CrossRef]
- Kim, E.Y.; Saunders, P.; Yousefzadeh, N. Usefulness of anti-infective lock solutions for catheter-related bloodstream infections. Mt. Sinai J. Med. 2010, 77, 549–558. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Lock Therapy of CVC (Central Venous Catheter) in Patients with Bacterial Infection: A Network Systematic Review of Metanalyses to Compare Efficacy of Daptomycin. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=267985. (accessed on 13 August 2021).
- Hamilton: McMaster University (Developed by Evidence Prime, Inc.). Gradepro GDT. GRADEpro Guideline Development Tool. 2015. Available online: www.guidelinedevelopment.org (accessed on 5 November 2015).
- Blanco-Di Matteo, A.; Garcia-Fernandez, N.; Aguinaga Pérez, A.; Carmona-Torre, F.; Oteiza, A.C.; Leiva, J.; Del Pozo, J.L. In Vivo Effectiveness of Several Antimicrobial Locks to Eradicate Intravascular Catheter Coagulase-Negative Staphylococci Biofilms. Antimicrob. Agents Chemother. 2023, 67, e0126422. [Google Scholar] [CrossRef]
- Blanco-Di Matteo, A.; Garcia-Fernandez, N.; Aguinaga Pérez, A.; Carmona-Torre, F.; Oteiza, A.C.; Leiva, J.; Del Pozo, J.L. Pre-Emptive Antimicrobial Locks Decrease Long-Term Catheter-Related Bloodstream Infections in Hemodialysis Patients. Antibiotics 2022, 11, 1692. [Google Scholar] [CrossRef]
- Dannenberg, C.; Bierbach, U.; Rothe, A.; Beer, J.; Körholz, D. Ethanol-lock technique in the treatment of bloodstream infections in pediatric oncology patients with broviac catheter. J. Pediatr. Hematol. Oncol. 2003, 25, 616–621. [Google Scholar] [CrossRef]
- Fortún, J.; Grill, F.; Martín-Dávila, P.; Blázquez, J.; Tato, M.; Sánchez-Corral, J.; García-San Miguel, L.; Moreno, S. Treatment of long-term intravascular catheter-related bacteraemia with antibiotic-lock therapy. J. Antimicrob. Chemother. 2006, 58, 816–821. [Google Scholar] [CrossRef]
- Rijnders, B.J.; Van Wijngaerden, E.; Vandecasteele, S.J.; Stas, M.; Peetermans, W.E. Treatment of long-term intravascular catheter-related bacteraemia with antibiotic lock: Randomized, placebo-controlled trial. J. Antimicrob. Chemother. 2005, 55, 90–94. [Google Scholar] [CrossRef]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45, Erratum in Clin. Infect. Dis. 2010, 50, 1079; Erratum in Clin. Infect. Dis. 2010, 50, 457. [Google Scholar] [CrossRef]
- Maiefski, M.; Rupp, M.E.; Hermsen, E.D. Ethanol lock technique: Review of the literature. Infect. Control Hosp. Epidemiol. 2009, 30, 1096–1108, Erratum in Infect. Control Hosp. Epidemiol. 2010, 31, 202. [Google Scholar] [CrossRef]
- Jaffer, Y.; Selby, N.M.; Taal, M.W.; Fluck, R.J.; McIntyre, C.W. A meta-analysis of hemodialysis catheter locking solutions in the prevention of catheter-related infection. Am. J. Kidney Dis. 2008, 51, 233–241. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef]
- Carratalà, J.; Niubó, J.; Fernández-Sevilla, A.; Juvé, E.; Castellsagué, X.; Berlanga, J.; Liñares, J.; Gudiol, F. Randomized, double-blind trial of an antibiotic-lock technique for prevention of gram-positive central venous catheter-related infection in neutropenic patients with cancer. Antimicrob. Agents Chemother. 1999, 43, 2200–2204. [Google Scholar] [CrossRef]
- Kim, S.H.; Song, K.I.; Chang, J.W.; Kim, S.B.; Sung, S.A.; Jo, S.K.; Cho, W.Y.; Kim, H.K. Prevention of uncuffed hemodialysis catheter-related bacteremia using an antibiotic lock technique: A prospective, randomized clinical trial. Kidney Int. 2006, 69, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Liangos, O.; Gul, A.; Madias, N.E.; Jaber, B.L. Long-term management of the tunneled venous catheter. Semin. Dial. 2006, 19, 158–164. [Google Scholar] [CrossRef]
- Kammoun, M.; Jarraya, A.; Ammar, S. Improvement of Broviac catheter-related outcomes after the implementation of a quality management system: A before-and-after prospective observational study. J. Neonatal Surg. 2023, 12, 3. [Google Scholar] [CrossRef]
- Benvenuti, S.; Finetti, F.; Porteri, E.; Ceresoli, R.; Pintossi, C.; Zanatta, F.; Bartolini, G.; Facchini, F.; Annovazzi, C.; Alberti, D. Guidewire replacement of valved tunneled-cuffed silicone catheters with power injectable polyurethane tunneled-cuffed catheters or with ports. J. Vasc. Access. 2024, 11297298231218593. [Google Scholar] [CrossRef]
- MacRae, J.M.; Ahmed, A.; Johnson, N.; Levin, A.; Kiaii, M. Central vein stenosis: A common problem in patients on hemodialysis. ASAIO J. 2005, 51, 77–81. [Google Scholar] [CrossRef]
- Trebuian, C.I.; Marza, A.M.; Cindrea, A.C.; Petrica, A.; Onea, S.; Sutoi, D.; Barsac, C.; Crintea-Najette, I.; Popa, D.; Chioibas, R.; et al. Risk Assessment of Venous Thromboembolism among Septic Shock Patients: Single versus Concurrent Insertion of Central Venous Catheters. Medicina 2024, 60, 785. [Google Scholar] [CrossRef]
- Alonso, B.; Fernández-Cruz, A.; Díaz, M.; Sánchez-Carrillo, C.; Martín-Rabadán, P.; Bouza, E.; Muñoz, P.; Guembe, M. Can vancomycin lock therapy extend the retention time of infected long-term catheters? APMIS 2020, 128, 433–439. [Google Scholar] [CrossRef]
- Broom, J.; Woods, M.; Allworth, A.; McCarthy, J.; Faoagali, J.; Macdonald, S.; Pithie, A. Ethanol lock therapy to treat tunnelled central venous catheter-associated blood stream infections: Results from a prospective trial. Scand. J. Infect. Dis. 2008, 40, 399–406. [Google Scholar] [CrossRef]
- Bookstaver, P.B.; Gerrald, K.R.; Moran, R.R. Clinical outcomes of antimicrobial lock solutions used in a treatment modality: A retrospective case series analysis. Clin. Pharmacol. 2010, 2, 123–130. [Google Scholar] [CrossRef]
- Aslam, S.; Trautner, B.W.; Ramanathan, V.; Darouiche, R.O. Pilot trial of N-acetylcysteine and tigecycline as a catheter-lock solution for treatment of hemodialysis catheter-associated bacteremia. Infect. Control Hosp. Epidemiol. 2008, 29, 894–897. [Google Scholar] [CrossRef]
- Brescia, F.; Pittiruti, M.; Scoppettuolo, G.; Zanier, C.; Nadalini, E.; Bottos, P.; Moreal, C.; Da Ros, V.; Fabiani, F. Taurolidine lock in the treatment of colonization and infection of totally implanted venous access devices in cancer patients. J. Vasc. Access. 2023, 24, 87–91. [Google Scholar] [CrossRef]
- Devautour, C.; Poey, N.; Lagier, J.; Launay, E.; Cerdac, A.; Vergnaud, N.; Berneau, P.; Parize, P.; Ferroni, A.; Tzaroukian, L.; et al. Salvage strategy for long-term central venous catheter-associated Staphylococcus aureus infections in children: A multi-centre retrospective study in France. J. Hosp. Infect. 2024, 150, 125–133. [Google Scholar] [CrossRef]
- Arechabala, M.C.; Catoni, M.I.; Claro, J.C.; Rojas, N.P.; Rubio, M.E.; Calvo, M.A.; Letelier, L.M. Antimicrobial lock solutions for preventing catheter-related infections in haemodialysis. Cochrane Database Syst. Rev. 2018, 4, CD010597. [Google Scholar] [CrossRef]
- Padilla-Orozco, M.; Mendoza-Flores, L.; Herrera-Alonso, A.; Garza González, E.; Gutiérrez Ferman, J.L.; Rodríguez-López, J.M.; Bocanegra-Ibarias, P.; Camacho-Ortiz, A. Generalized and Prolonged Use of Gentamicin-Lock Therapy Reduces Hemodialysis Catheter-Related Infections Due to Gram Negatives. Nephron 2019, 143, 86–91. [Google Scholar] [CrossRef]
- Mandolfo, S.; Anesi, A.; Maggio, M.; Rognoni, V.; Galli, F.; Forneris, G. High success rate in salvage of catheter-related bloodstream infections due to Staphylococcus aureus, on behalf of project group of Italian society of nephrology. J. Vasc. Access. 2020, 21, 336–341. [Google Scholar] [CrossRef]
- Moran, J.; Sun, S.; Khababa, I.; Pedan, A.; Doss, S.; Schiller, B. A randomized trial comparing gentamicin/citrate and heparin locks for central venous catheters in maintenance hemodialysis patients. Am. J. Kidney Dis. 2012, 59, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm formation: A clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef]
| Study, Year (Ref) | Study Population | Population Characteristics | Antibacterial Lock Therapy | Standard of Care | Study Duration | Outcome(s) | Results | Notes |
|---|---|---|---|---|---|---|---|---|
| Andres Blanco 2022 [8] | Patients with CVC-related infections Hemodialysis patients | N = 86 Age = 68 years Men = 57% | Teicoplanin 10 ng/mL Daptomycin 5 ng/mL Vancomycin 10 ng/mL N = 45 | Heparin 500 IU N = 41 | 30 days | Microbiological healing | 45/45 vs. 37/41 | No adverse events were reported. |
| Blanco Di Matteo 2022 [9] | Pediatric patients with CVC-related infections Hemodialysis patients | N = 59 Age = 70 years Male = 33% | Teicoplanin 10 ngml Daptomycin 5 ng/mL Vancomycin 10 ng/mL N = 39 | Heparin 500 IU N = 20 | Microbiological healing | 30/39 vs. 6/20 | CVC replacement was needed in 4/39 vs. 9/20 patients. No other adverse events were reported. | |
| Dannemberg 2003 [10] | Patients with CVC-related infections Oncological children and adolescents | N = 39 Age = 12 years Men = 57% | Ethanol N=24 | Heparin N=15 | 28 days | Microbiological healing | 16/24 vs. 7/15 | Mild grade I elevation in the liver enzymes was noted in 8/24 vs. 3/15 patients. |
| Fortun J 2006 [11] | Patients with CVC-related infections Hospitalized patients with afferents by different wards. | N = 48 Age = 56 years Men = 40% | Vancomycin 2 ng/mL Gentamycin 2 ng/mL Ciprofloxacin 2 ng/mL N = 19 | Heparin 20 IU/mL N = 29 | 14 days | Microbiological healing | 16/19 vs. 19/29 | CVC replacement was needed in 1/19 vs. 7/29 patients. Mortality was reported in 3/19 and 7/29. Only 1/19 and 2/29 deaths were related to CVC infections. |
| Rijnders B.J. 2004 [12] | Patients with CVC-related infections Hospitalized patients with afferents by different wards. | N = 44 Age = 48 years Men = not reported | Ceftazidime 500 mg/L Vancomycin 500 mg/L N = 21 | Heparin 100 IU/mL N = 23 | 180 days | Microbiological healing | 14/21 vs. 10/23 | CVC replacement was needed in 3/21 vs. 9/23 patients. No other adverse events were reported. |
| Recommendation | Grade of Evidence | |
|---|---|---|
| 1 | KDOQI considers it reasonable to assess or check the vascular access and surrounding area by physical exam prior to every cannulation (if AV access) or connection (if CVC) for potential complications. | Expert Opinion |
| 2 | KDOQI recommends rope ladder cannulation as the preferred cannulation technique for AVFs. | Conditional Recommendation, Moderate Quality of Evidence |
| 3 | KDOQI considers it reasonable to limit AV access through buttonhole cannulation only in special circumstances given the associated increased risks of infection and related adverse consequences. | Expert Opinion |
| 4 | KDOQI considers it reasonable to avoid buttonhole cannulation in synthetic PTFE grafts due to potential serious consequences. | Expert Opinion |
| 5 | KDOQI suggests that when select buttonhole cannulation is performed, the use of buttonhole cannulation devices to facilitate cannulation should be at the discretion and expertise of the cannulator. | Conditional Recommendation, Low Quality of Evidence |
| 6 | KDOQI considers it reasonable to use skilled cannulators with established high rates of cannulation success to perform the initial AV access cannulation on patients to help avoid primary infiltration injury of the AV access. | Expert Opinion |
| 7 | KDOQI considers it reasonable to have structured training and supervision of dialysis technicians and nurses before and during their initial cannulation attempts and regular training updates to maintain cannulation competency. | Expert Opinion |
| 8 | KDOQI considers it reasonable to support and educate eligible patients on self-cannulation of their AV access (AVF or AVG). | Expert Opinion |
| 9 | KDOQI suggests the use of a catheter care protocol for the exit site and hub care to reduce catheter-related bloodstream infections and the treatment of catheter dysfunction. | Strong Recommendation, Moderate Quality of Evidence |
| 10 | KDOQI considers it reasonable, in addition to correct hand hygiene/washing, to use aseptic techniques and masks for patients and staff performing the catheter connection and disconnection procedures. | Expert Opinion |
| 11 | KDOQI considers it reasonable to cleanse the catheter hub when connecting and disconnecting the catheter with a chlorhexidine-based solution. If chlorhexidine is contraindicated (e.g., sensitivity or allergy), a povidone–iodine solution (preferably with alcohol) is a reasonable substitute and should be used. | Expert Opinion |
| 12 | KDOQI considers it reasonable at the time of the change of the catheter dressing to cleanse the skin surrounding the catheter exit site with a chlorhexidine-based solution. If chlorhexidine is contraindicated (e.g., sensitivity or allergy), a povidone–iodine solution (preferably with alcohol) is a reasonable substitute and should be used. | Expert Opinion |
| 13 | There is inadequate evidence for KDOQI to make a recommendation on the specific chlorhexidine formulation to use for infection prophylaxis, and this should be based on the clinician’s best clinical judgment and local practical considerations. | NA |
| 14 | There is inadequate evidence to demonstrate a difference in catheter-related infections with the use of a transparent film dressing compared with a nontransparent dressing; thus, the choice of catheter dressing material should be based on the clinician’s discretion that considers the patient’s circumstances and uses best clinical judgment. | NA |
| 15 | KDOQI considers it reasonable to use a topical antiseptic or antibiotic barrier at the catheter exit site in addition to cleansing until the exit site is healed to reduce the risk of a catheter-related infection. | Expert Opinion |
| 16 | There is inadequate evidence to demonstrate a difference in catheter-related infections between the use of various antiseptic or antibiotic topical exit site barriers; thus, the choice of topical exit site barrier should be based on the clinician’s discretion and best clinical judgment. | NA |
| 17 | KDOQI considers it reasonable to follow these catheter care practices. The frequency of the change of the catheter dressing should be based on the clinician’s discretion and best clinical judgment, with a minimum of once-weekly catheter dressings that should be protected against wet and dirty environments, particularly when the exit site is not yet fully healed (e.g., swimming and showering should be avoided). | Expert Opinion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, V.; Farina, A.; Maressa, V.; Cernaro, V.; Gembillo, G.; Messina, R.M.; Longhitano, E.; Ferio, C.; Venanzi Rullo, E.; Santoro, D. Differences in Efficacy between Antibacterial Lock Therapy and the Standard of Care for CVC-Related Infections: A Systematic Review and Meta-Analysis. Clin. Pract. 2024, 14, 1538-1549. https://doi.org/10.3390/clinpract14040124
Calabrese V, Farina A, Maressa V, Cernaro V, Gembillo G, Messina RM, Longhitano E, Ferio C, Venanzi Rullo E, Santoro D. Differences in Efficacy between Antibacterial Lock Therapy and the Standard of Care for CVC-Related Infections: A Systematic Review and Meta-Analysis. Clinics and Practice. 2024; 14(4):1538-1549. https://doi.org/10.3390/clinpract14040124
Chicago/Turabian StyleCalabrese, Vincenzo, Alessandra Farina, Veronica Maressa, Valeria Cernaro, Guido Gembillo, Roberta Maria Messina, Elisa Longhitano, Cinzia Ferio, Emanuele Venanzi Rullo, and Domenico Santoro. 2024. "Differences in Efficacy between Antibacterial Lock Therapy and the Standard of Care for CVC-Related Infections: A Systematic Review and Meta-Analysis" Clinics and Practice 14, no. 4: 1538-1549. https://doi.org/10.3390/clinpract14040124
APA StyleCalabrese, V., Farina, A., Maressa, V., Cernaro, V., Gembillo, G., Messina, R. M., Longhitano, E., Ferio, C., Venanzi Rullo, E., & Santoro, D. (2024). Differences in Efficacy between Antibacterial Lock Therapy and the Standard of Care for CVC-Related Infections: A Systematic Review and Meta-Analysis. Clinics and Practice, 14(4), 1538-1549. https://doi.org/10.3390/clinpract14040124








