1. Introduction
Retinopathy of Prematurity (ROP) remains a significant cause of childhood blindness worldwide, affecting the most vulnerable population of premature neonates [
1,
2]. The pathophysiology of ROP is complex, involving the interplay of genetic, metabolic, and environmental factors, with oxygen therapy and respiratory support being pivotal [
3,
4]. Recent advancements in neonatal care have improved survival rates of premature infants; however, this has concurrently increased the incidence of ROP, highlighting the necessity for accurate predictive markers for early identification and intervention. Insulin-like Growth Factor 1 (IGF1) and Tumor Necrosis Factor-alpha (TNF-alpha) have emerged as useful biomarkers due to their roles in angiogenesis and inflammatory pathways, respectively, which are crucial in the pathogenesis of ROP [
5,
6].
Extensive research has demonstrated the involvement of IGF1 in retinal vascular development and maturation, while TNF-alpha, a pro-inflammatory cytokine, has been implicated in the exacerbation of retinal neovascularization, suggesting its potential role as a biomarker for ROP risk [
7,
8]. The dynamic balance between these markers reflects not only the disease’s multifactorial nature but also the potential interventional points where therapeutic strategies could be targeted [
9,
10]. Therefore, understanding the correlation between these plasma levels and ROP development could offer a groundbreaking approach to managing this condition.
Moreover, other biological markers such as Lactate Dehydrogenase (LDH), Creatine Phosphokinase (CPK), and glucose levels have been studied in the context of ROP, indicating a broader metabolic disturbance in premature neonates at risk [
11,
12,
13]. These markers, involved in energy metabolism and cellular injury, may provide additional insights into the systemic condition of these infants, further refining risk assessment models.
Real-world data underscore the urgency and the potential impact of this research. Globally, an estimated 15 million infants are born preterm each year, with a significant proportion developing ROP [
14]. In regions with advanced neonatal care, the survival of extremely low-birth-weight infants (<1000 g) has dramatically increased, along with the prevalence of ROP [
15,
16]. This scenario presents a growing challenge to healthcare systems and calls for innovative approaches to predict, prevent, and manage this potentially blinding condition effectively.
Therefore, this study aims to establish a correlation between plasma levels of IGF1 and TNF-alpha in predicting the risk of developing ROP in premature neonates with respiratory distress syndrome treated with various modalities of respiratory support. It also explores the association between different ROP severity grades and plasma levels of glucose, LDH, CPK, and other biological markers. Through this approach, we seek to identify potential predictive biomarkers that can guide clinical interventions, improving the prognosis and management of ROP in this high-risk population.
3. Results
3.1. ROP vs. Non-ROP
In
Table 1, we examined the background characteristics of neonates categorized into two groups: those with any stage of ROP (
n = 47) and those without ROP (
n = 48). The distribution of gender across groups was not statistically significant, with 48.9% males in the Any ROP group compared to 72.9% in the No ROP group (
p = 0.134). A notable difference was observed in the gestational age of neonates, where the Any ROP group had a mean gestational age of 31.9 weeks (SD = 1.6), which was significantly lower than the 30.6 weeks (SD = 2.1) observed in the No ROP group, with a
p-value of 0.0010.
The birth weight of neonates did not show a statistically significant difference between the two groups, with the Any ROP group having a mean birth weight of 1527 g (SD = 275) compared to 1494 g (SD = 228) in the No ROP group (p = 0.5255). APGAR scores at 1 min post-birth indicated a statistically significant difference, with the Any ROP group having a lower mean score (6.2, SD = 2.0) compared to the No ROP group (7.1, SD = 1.4), with a p-value of 0.0126. However, the APGAR scores at 5 min, while still lower in the Any ROP group (6.9, SD = 1.8) compared to the No ROP group (7.5, SD = 1.6), did not reach statistical significance (p = 0.0891).
Monitored pregnancies were significantly less prevalent in the Any ROP group (48.9%) compared to the No ROP group (72.9%), with a p-value of 0.0165. This association may indicate a link between less monitored pregnancies and increased ROP risk. On the other hand, the mode of birth, as indicated by cesarean birth rates, did not significantly differ between groups (55.3% in Any ROP vs. 60.4% in No ROP; p = 0.6320).
Lactate dehydrogenase levels were measured at two different time intervals: day 1–3 and day 7–10 post-birth. Initially, LDH levels did not show a statistically significant difference between the groups (869 ± 552 U/L in the Any ROP group vs. 740 ± 614 U/L in the No ROP group; p = 0.2846). However, by day 7–10, LDH levels in the Any ROP group were significantly higher (599 ± 268 U/L) compared to those in the No ROP group (435 ± 253 U/L), with a p-value of 0.0028.
The study also investigated glucose levels, finding that neonates with ROP had lower mean glucose levels (3.1 ± 1.5 mmol/L) compared to those without ROP (3.8 ± 1.9 mmol/L), with the difference reaching statistical significance (p = 0.0495). Also, CPK levels, another marker investigated, did not significantly differ between the groups (282 ± 180 U/L in Any ROP vs. 259 ± 166 U/L in No ROP; p = 0.5188).
A noteworthy finding of the study was the significantly higher levels of TNF-alpha, a pro-inflammatory cytokine, in the Any ROP group both on the first day of life (24.9 ± 18.8 pg/mL vs. 14.2 ± 12.3 pg/mL in No ROP;
p = 0.0014) and at 2 weeks of life (38.2 ± 45.3 pg/mL vs. 16.9 ± 24.0 pg/mL in No ROP;
p = 0.0051). Lastly, the analysis of IGF1 levels revealed significantly lower IGF1 levels in the Any ROP group both at the first collection (61.4 ± 35.6 ng/mL) and at the second collection (57.9 ± 32.8 ng/mL) compared to the No ROP group (91.6 ± 44.6 ng/mL and 90.1 ± 48.2 ng/mL, respectively), with
p-values of 0.0004 and 0.0003 (
Table 2).
The utilization of High-Flow Nasal Cannula (HFNC) therapy showed no significant difference between the two groups, with 17.0% in the Any ROP group and 16.7% in the No ROP group (p-value = 0.9631). Similarly, the application of Nasal Continuous Positive Airway Pressure (nCPAP) was relatively balanced between the groups, with 46.8% in the Any ROP group compared to 52.1% in the No ROP group (p-value = 0.6071).
Synchronized Intermittent Mandatory Ventilation (SIMV) or Synchronized Intermittent Positive Pressure Ventilation (SIPPV) usage was observed in 36.2% of neonates with ROP versus 31.3% without ROP, with a p-value of 0.6119. The duration of oxygen therapy, a critical factor in ROP management, showed no significant difference between the groups, with neonates in the Any ROP group receiving an average of 7.8 days (SD = 4.1) of therapy compared to 7.4 days (SD = 3.6) in the No ROP group (p = 0.6143).
White matter injury, a severe complication, was observed in 38.3% of neonates with ROP and 33.3% of those without, resulting in a non-significant
p-value of 0.6137. The prevalence of Intraventricular Hemorrhage (IVH) was also analyzed, with 12.8% in the Any ROP group and 10.4% in the No ROP group, yielding a
p-value of 0.7204. Moreover, a significant finding of this study was the use of laser treatment, exclusively administered to neonates in the Any ROP group (17.0%), with none in the No ROP group undergoing this intervention, resulting in a
p-value of 0.0028 (
Table 3).
3.2. Analysis by ROP Grades
The distribution of gender among the groups revealed a statistically significant difference (p = 0.0156), with a higher percentage of males in the non-ROP group (72.9%) and ROP Aggressive Posterior group (80.0%), compared to the lower percentages observed in the ROP Grade I and II (48.5%) and ROP Grade III (33.3%) groups. Gestational age showed a significant variation across the groups (p = 0.0227), with the ROP Grade III group having the lowest mean gestational age (29.0 weeks, SD = 1.7), followed by the ROP Aggressive Posterior group (30.6 weeks, SD = 1.5) and the non-ROP group (30.6 weeks, SD = 1.4). The ROP Grade I and II group had a slightly higher mean gestational age (31.0 weeks, SD = 1.2).
Birth weight also varied significantly across the groups (p = 0.0089), with the lowest mean birth weight observed in the ROP Grade III group (1274 g, SD = 215). The ROP Aggressive Posterior and non-ROP groups had similar mean birth weights (1556 g, SD = 204, and 1494 g, SD = 231, respectively).
The APGAR scores at 1 min (
p = 0.0294) and 5 min (
p = 0.0367) post-birth showed statistical significance across the groups. The ROP Grade III and ROP Grade I and II groups had lower APGAR scores compared to the non-ROP group. Monitored pregnancies were less frequent in the groups with ROP, with a statistically significant difference (
p = 0.0471). The rate of Cesarean births, although not statistically significant (
p = 0.0553), varied across the groups, with the highest percentage observed in the ROP Grade I and II group (66.7%), as described in
Table 4.
Lactate dehydrogenase levels were evaluated at two intervals: day 1–3 and day 7–10. An exceptional elevation was noted in the LDH levels during days 1–3 for the ROP Grade III group (1659.6 ± 394 U/L), significantly higher than the other groups, with a p-value of 0.0065. This pronounced increase suggests that severe ROP might be associated with higher levels of cellular distress or injury early post-birth. Conversely, LDH levels during days 7–10, while still elevated in the ROP Grade III group, showed a more widespread significance across the groups (p = 0.0320), indicating ongoing cellular stress beyond the immediate postnatal period. Glucose levels across the groups revealed a statistically significant difference (p = 0.0462), with the lowest levels observed in the ROP Grade III group (3.0 ± 1.4 mmol/L).
Creatine phosphokinase levels demonstrated a marked increase in the ROP Grade III group (450.4 ± 229 U/L), significantly differing from the other groups (
p = 0.0184). The study also investigated levels of TNF-alpha. Notably, TNF-alpha levels on the first day of life were highest in the ROP Aggressive Posterior group (69.7 ± 22.5 pg/mL), suggesting an early inflammatory response in neonates who develop severe forms of ROP (
p = 0.0012). Insulin-like Growth Factor 1 (IGF1) levels were assessed at two points, revealing significantly lower levels in neonates with ROP compared to those without, both initially and subsequently (
p-values of 0.0138 and 0.0044, respectively), as presented in
Table 5.
The utilization of HFNC and nCPAP did not exhibit a statistically significant difference across the groups, with p-values of 0.7584 and 0.2104, respectively. However, the application of SIMV/SIPPV showed a significant disparity (p = 0.0017), particularly highlighting an elevated usage in neonates diagnosed with ROP Aggressive Posterior (80.0%) and ROP Grade III (66.7%) compared to those with ROP Grade I and II (21.2%) and without ROP (31.3%). The duration of oxygen therapy revealed a significant difference across the groups (p = 0.0088), with neonates in the ROP Grade III group receiving the longest duration of therapy (12.1 ± 3.1 days), implying that prolonged exposure to oxygen therapy might be associated with higher severity levels of ROP.
Analysis of white matter injury and intraventricular hemorrhage showed no significant associations with ROP severity, with
p-values of 0.3146 and 0.8893, respectively. A striking result was observed in the administration of laser treatment, where a significant difference was noted (
p < 0.0001). All neonates with ROP Aggressive Posterior received laser treatment, and a substantial proportion of those with ROP Grade III were treated as well (33.3%), whereas none in the ROP Grade I and II and non-ROP groups received this intervention, as described in
Table 6.
3.3. Risk Factors
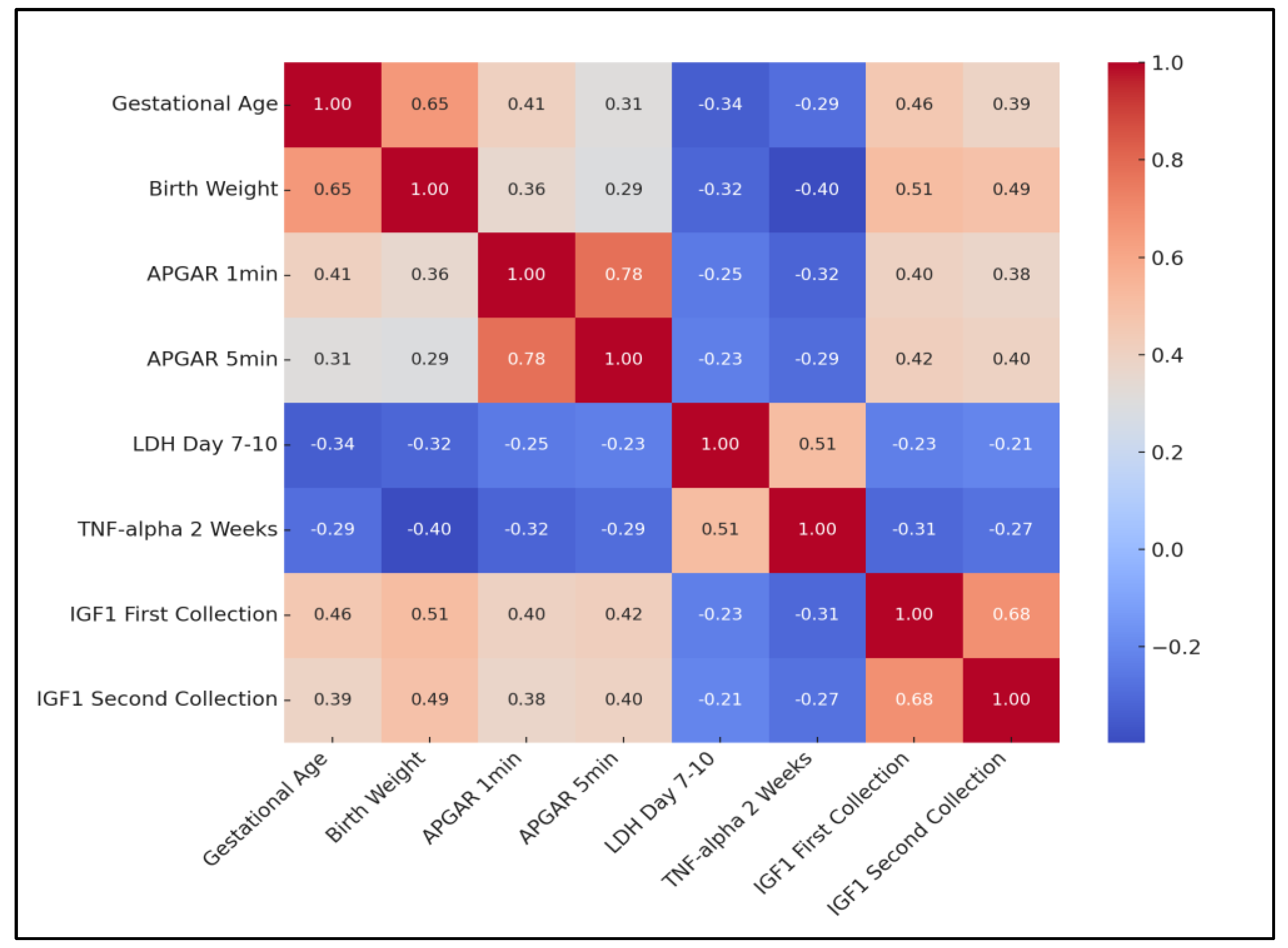
The analysis revealed a significant positive correlation between gestational age and birth weight (rho = 0.651, p = 0.0004), indicating that higher gestational ages are typically associated with higher birth weights. A strong positive correlation was also observed between APGAR scores at 1 min and 5 min post-birth (rho = 0.779, p < 0.0001), suggesting that neonates’ condition tends to remain consistent in the immediate post-birth period.
Conversely, a negative correlation was identified between gestational age and LDH levels on day 7–10 (rho = −0.341, p = 0.0123), as well as between gestational age and TNF-alpha levels at 2 weeks (rho = −0.288, p = 0.0214). Interestingly, a positive correlation emerged between birth weight and both IGF1 measurements (first collection: rho = 0.512, p = 0.0012; second collection: rho = 0.489, p = 0.0023), suggesting that higher birth weights are associated with higher levels of IGF1.
Moreover, TNF-alpha levels at 2 weeks showed a significant positive correlation with LDH day 7–10 (rho = 0.512,
p = 0.0004), indicating that higher inflammatory responses are associated with greater cellular stress or damage. Finally, the strong positive correlation between IGF1 measurements at two different time points (rho = 0.678,
p < 0.0001) indicates that IGF1 levels tend to remain consistent over time in neonates, as presented in
Table 7 and
Figure 1.
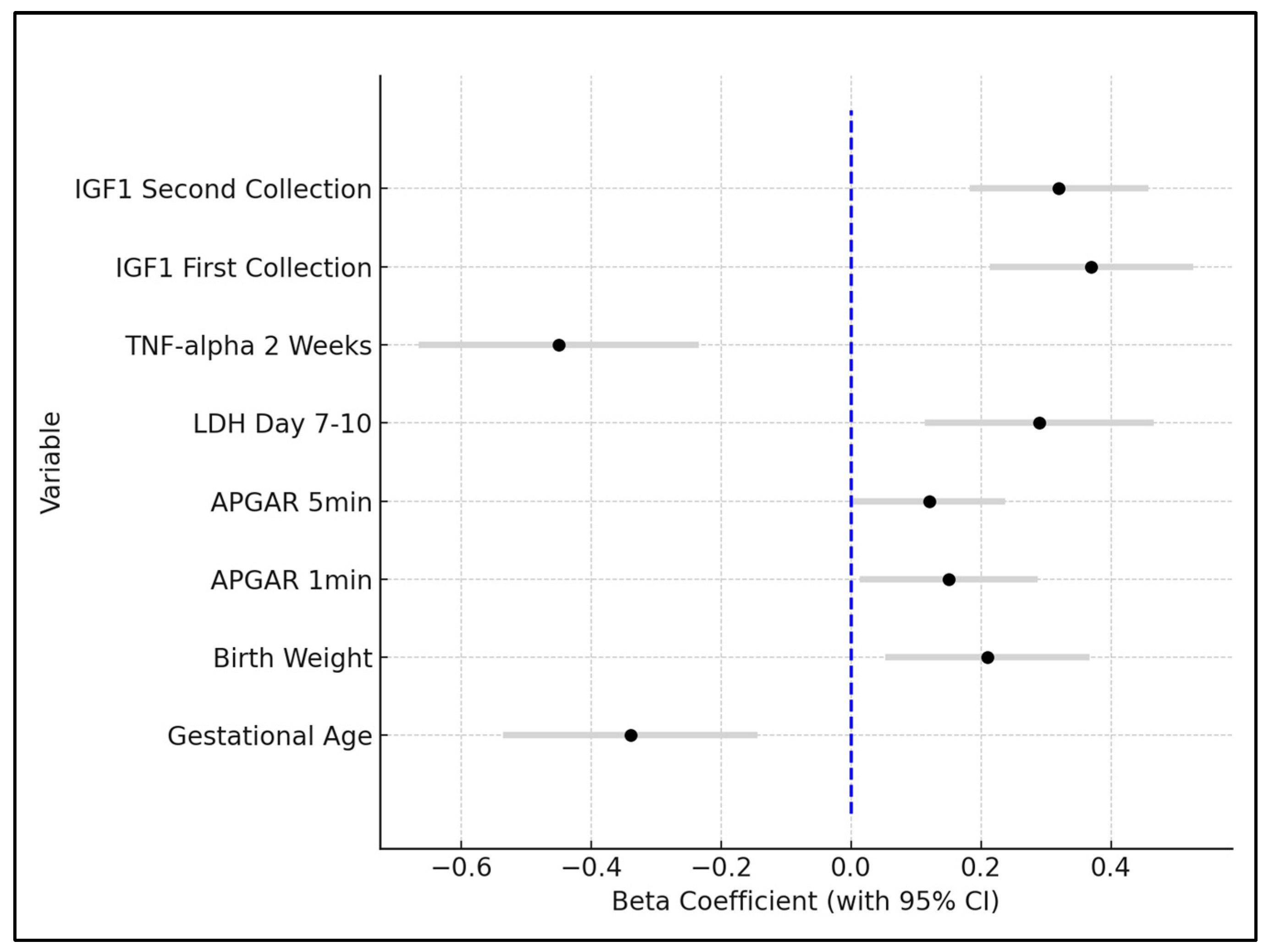
Gestational age emerged as a significant negative predictor for ROP, with a beta coefficient of −0.34 (p = 0.0123). This indicates that for each week increase in gestational age, the risk of ROP decreases, underlining the critical role of prematurity in ROP development. The confidence interval ranged from −0.536 to −0.144, reinforcing the robustness of this finding. Birth weight showed a positive association with decreased ROP risk, evidenced by a beta coefficient of 0.21 (p = 0.0427). This suggests that higher birth weights are protective against ROP, a finding supported by the confidence interval ranging from 0.0532 to 0.3668.
APGAR scores at 1 min and 5 min post-birth were also significant predictors, with beta coefficients of 0.15 (p = 0.0189) and 0.12 (p = 0.0345), respectively. These positive coefficients imply that higher APGAR scores, indicative of better neonatal condition at birth, are associated with a lower risk of ROP. Lactate dehydrogenase (LDH) levels on day 7–10 post-birth were positively correlated with an increased risk of ROP, demonstrated by a beta coefficient of 0.29 (p = 0.0214). This highlights the potential role of cellular injury or stress, as measured by LDH, in the pathogenesis of ROP.
TNF-alpha levels at 2 weeks showed a significant negative relationship with ROP development, with a beta coefficient of −0.45 (
p = 0.0014), suggesting that higher levels of this inflammatory marker are linked to an increased risk of ROP. Insulin-like Growth Factor 1 (IGF1) levels, both at the first and second collection, were found to be significant protective factors against ROP, with beta coefficients of 0.37 (
p = 0.0032) and 0.32 (
p = 0.0028), respectively. These findings indicate that higher levels of IGF1 are associated with a reduced risk of developing ROP, as seen in
Table 8 and
Figure 2.
The sensitivity and specificity analysis of TNF-alpha and IGF1 as predictive biomarkers for ROP in premature neonates revealed that both markers had an AUC of 0.616, indicating a good predictive ability. The optimal threshold for TNF-alpha was identified at 24.9 pg/mL and for IGF1 at 31.1 ng/mL. While both markers demonstrated a high sensitivity of 88.2%, suggesting they are effective in identifying neonates with ROP, their specificity was only 63.7%, indicating a considerably lower ability in correctly identifying neonates without ROP.
4. Discussion
The current study brought to light critical insights into the laboratory parameters associated with varying degrees of ROP severity. Through our analysis, it became evident that specific biochemical markers play a significant role in the progression and risk assessment of ROP. Notably, the study’s findings on lactate dehydrogenase levels, glucose concentrations, TNF-alpha, and IGF1 levels offer a nuanced understanding of the biological underpinnings that may influence ROP development. LDH levels, particularly on day 7–10 post-birth, showcased a notable increase in neonates with ROP compared to those without, highlighting a potential marker of cellular stress or damage in the context of ROP. This increase was most pronounced in the group with ROP Grade III, suggesting a correlation between elevated LDH levels and more severe forms of ROP. The role of LDH as an indicator of tissue breakdown or hypoxia aligns with the pathophysiology of ROP, where ischemic insult can precipitate retinal neovascularization [
21,
22].
Furthermore, the study’s findings on glucose levels reinforced the complexity of metabolic regulation in preterm infants and its potential impact on ROP development. Neonates with ROP exhibited lower glucose levels than their counterparts without ROP, indicating that hypoglycemia might be associated with an increased risk or severity of ROP. This relationship between glucose levels and ROP underscores the importance of glucose monitoring and management in preterm infants to potentially mitigate ROP risk.
The investigation into TNF-alpha levels provided compelling evidence of the role of inflammation in ROP. Significantly higher levels of TNF-alpha were observed in the ROP groups, especially on the first day of life and at 2 weeks, underscoring the inflammatory nature of ROP pathogenesis. This pro-inflammatory cytokine’s elevated levels in neonates with more severe ROP point to inflammation as a critical factor in disease progression, suggesting that anti-inflammatory strategies might hold therapeutic potential in managing ROP. Moreover, the analysis of IGF1 showed that lower levels of IGF1 were consistently found in neonates with ROP, highlighting its protective role against the disease, not only emphasizing the importance of IGF1 in vascular development and overall neonatal growth but also suggesting that increased IGF1 levels could be a potential strategy in preventing ROP or reducing its severity [
23].
The association between IGF1/TNF-α and ROP is hypothesized to stem from their roles in inflammation and vascular regulation. TNF-α, a pro-inflammatory cytokine, may contribute to the vascular abnormalities seen in ROP by promoting inflammation and neovascularization, critical factors in the pathogenesis of ROP. Conversely, IGF1, known for its role in normal vascular development, may be protective by supporting vascular stability and integrity. Lower levels of IGF1 and elevated levels of TNF-α could disrupt normal retinal vascular development, thus predisposing neonates to ROP. Both IGF1 and TNF-α have been implicated in various complications associated with prematurity, such as bronchopulmonary dysplasia and necrotizing enterocolitis, primarily through their roles in inflammation and growth regulation. Our study specifically focused on the correlation of these biomarkers with ROP development. However, while collecting data, we accounted for potential confounders, including other neonatal outcomes and clinical interventions, to isolate the specific impact of IGF1 and TNF-α levels on ROP risk, ensuring a more accurate analysis of their roles in this specific condition.
Cakir’s et al. exploration of the relationship between hyperglycemia, insulin insensitivity, low IGF1 levels, and the development and severity of ROP in extremely preterm infants offers compelling evidence that supports our findings on the predictive value of IGF1 levels [
23]. Their observation that the highest mean plasma glucose tertile correlated with increased ROP prevalence (34 of 39 neonates) and severity (71% with ROP stage 3 or higher) resonates with our study’s indication that lower IGF1 levels are associated with increased ROP risk. Moreover, their finding that recombinant human IGF1 (rh-IGF1) treatment reduced neovascularization and improved retinal revascularization underscores the therapeutic potential of IGF1, aligning with our conclusion that IGF1 levels play a crucial role in ROP development. Also, another study, focusing on a new method to identify newborns at risk of ROP based on IGF1 levels and the presence of sepsis, further validates our approach by demonstrating a 100% negative predictive value for ROP screening [
24]. Their protocol, which effectively reduced unnecessary screenings by 79.1% without missing any ROP cases, provides a practical framework that complements our study’s emphasis on IGF1 as a biomarker for ROP risk.
Similarly, another study [
25] illustrated a clear association between low postnatal serum IGF1 levels and severe ROP in a racially diverse U.S. cohort, adding an essential dimension to our study’s results, being among first in the Romanian region, by confirming the relevance of this association across varied populations. Their observation that mean IGF1 levels were lowest in infants developing stage 3 ROP (17.0 ng/mL) compared to those with no ROP (20.0 ng/mL) during PMA weeks 28–33, even after adjusting for birth weight and gestational age, resonates with our findings of significantly lower IGF1 levels being predictive of ROP development. Conversely, another study by Jafari et al. [
26] did not find a significant association between IGF-1 levels and ROP, despite noting differences in IGF1 levels based on birth weight categories. This discrepancy could hint at the complexity of ROP’s pathophysiology, suggesting that while IGF1 is a critical factor, its impact might be modulated by other variables.
The findings from our study, emphasizing the predictive role of IGF1 and TNF-alpha levels in ROP development, resonate with insights from other recent investigations. The study by Tan et al. [
27] underscored the complexity of ROP pathogenesis by categorizing potential biomarkers, including cytokines and growth factors, which aligns with our observation of lower IGF1 levels being significantly associated with ROP. Specifically, our analysis showing a substantial decrease in IGF1 levels in ROP cases (61.4 ng/mL for any ROP vs. 91.6 ng/mL for no ROP) mirrors the trends noted in the literature where low serum IGF1 was consistently linked to severe ROP outcomes. Concurrently, Hellgren’s et al. [
28] longitudinal analysis revealed a negative correlation between IL-6 levels and IGF1, especially notable between 5 and 8 weeks post-birth, complementing our findings on the role of TNF-alpha. This parallel between the elevated pro-inflammatory markers and lower IGF1 in ROP development not only corroborates our results but also suggests an intricate interplay between inflammation and growth regulation in ROP pathogenesis. Noteworthy is the observed increase in IL-6 and TNF-α levels 24 h post-birth in infants who later developed ROP, which, alongside our findings of a significant relationship between TNF-alpha levels and ROP severity, underscores the potential of integrating inflammatory markers with IGF1 profiling to enhance ROP risk assessment and management strategies.
The clinical relevance of this study’s findings lies in the potential for improved ROP risk stratification and early intervention strategies for preterm neonates by assessing the IGF1. Additionally, the significant correlation between TNF-alpha levels and ROP severity suggests that inflammation plays a key role in ROP pathogenesis, opening suggestions for therapeutic interventions aimed at reducing inflammation. Importantly, these findings advocate for the integration of biomarker monitoring into routine neonatal care for preterm infants, facilitating a more personalized approach to ROP management. This approach not only has the potential to improve patient outcomes but also to optimize resource allocation by focusing efforts on neonates at highest risk, ultimately enhancing the quality of care for this vulnerable population.
The identification of solid predictive biomarkers for ROP can significantly enhance early intervention strategies. By accurately predicting the risk of ROP before the onset of visible symptoms, healthcare providers can tailor preventative measures, adjust the intensity of monitoring, and potentially initiate early treatments such as optimizing oxygen therapy and other modifiable factors. This proactive approach could reduce the incidence and severity of ROP, improving visual outcomes and reducing long-term complications associated with this condition.
One notable limitation of this study lies in its single-center design, which may constrain the generalizability of the findings to broader neonatal populations with varying demographic and clinical characteristics. Additionally, the study’s relatively small sample size, especially when stratifying neonates by the severity of ROP, may limit the statistical power to detect subtle differences or interactions between biomarkers and ROP outcomes. While the study meticulously controlled for a range of biological markers, it did not encompass all potential confounding variables, such as maternal health factors, prenatal care quality, and postnatal interventions other than respiratory support, which might influence ROP development. The reliance on blood samples at only two time points also may not fully capture the dynamic nature of changes in IGF1 and TNF-alpha levels and their relationship with ROP progression over time. Further research, preferably multi-centered with larger cohorts and a broader scope of investigated variables, is required to validate these findings and explore additional predictive biomarkers for ROP. Moreover, future analyses will incorporate statistical methods appropriate for repeated measures to better account for the correlations between measurements taken at different time points on the same subjects.