Safety and Efficacy of Very Early Conversion to Belatacept in Pediatric Kidney Transplantation with Transplant-Associated Thrombotic Microangiopathy: Case Study and Review of Literature
Abstract
1. Introduction
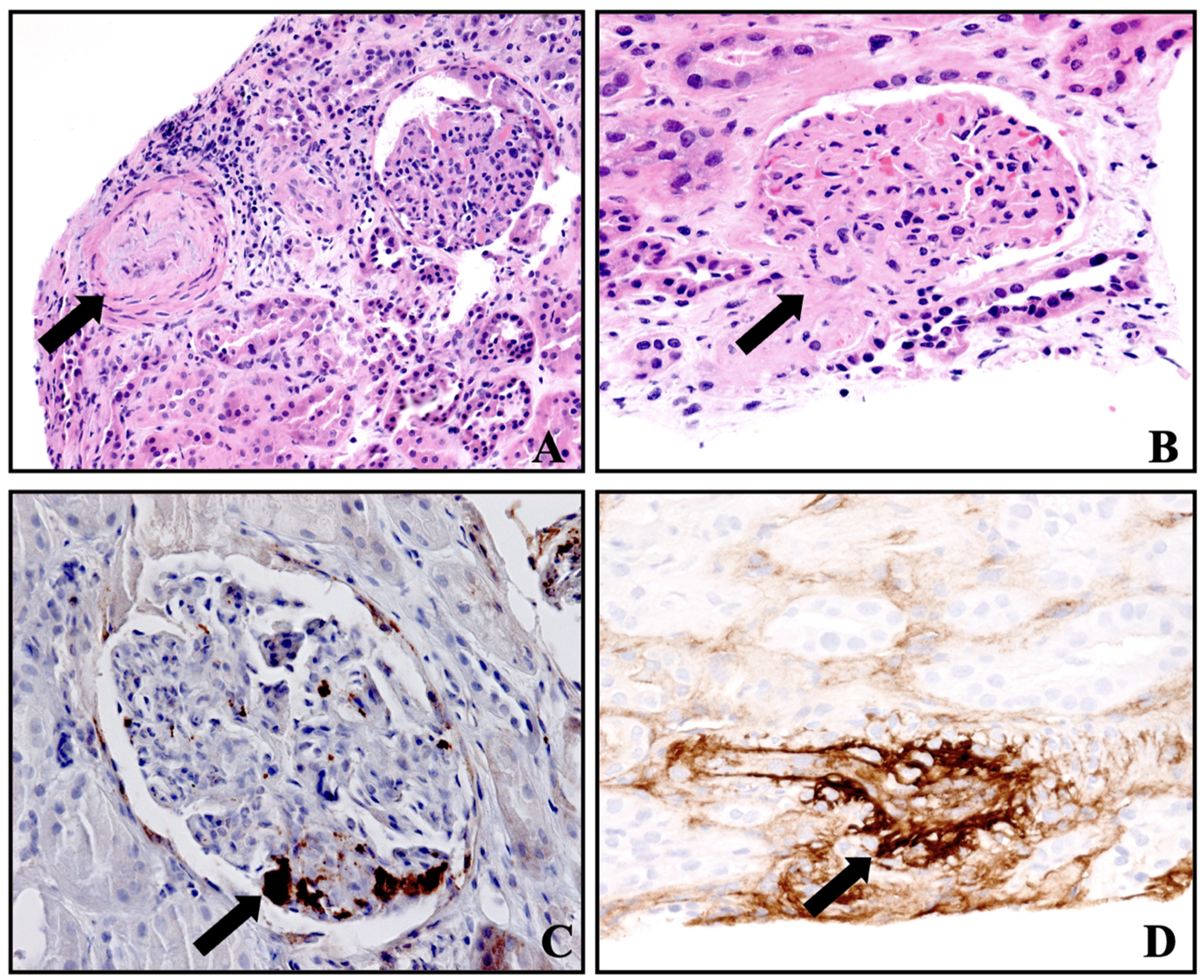
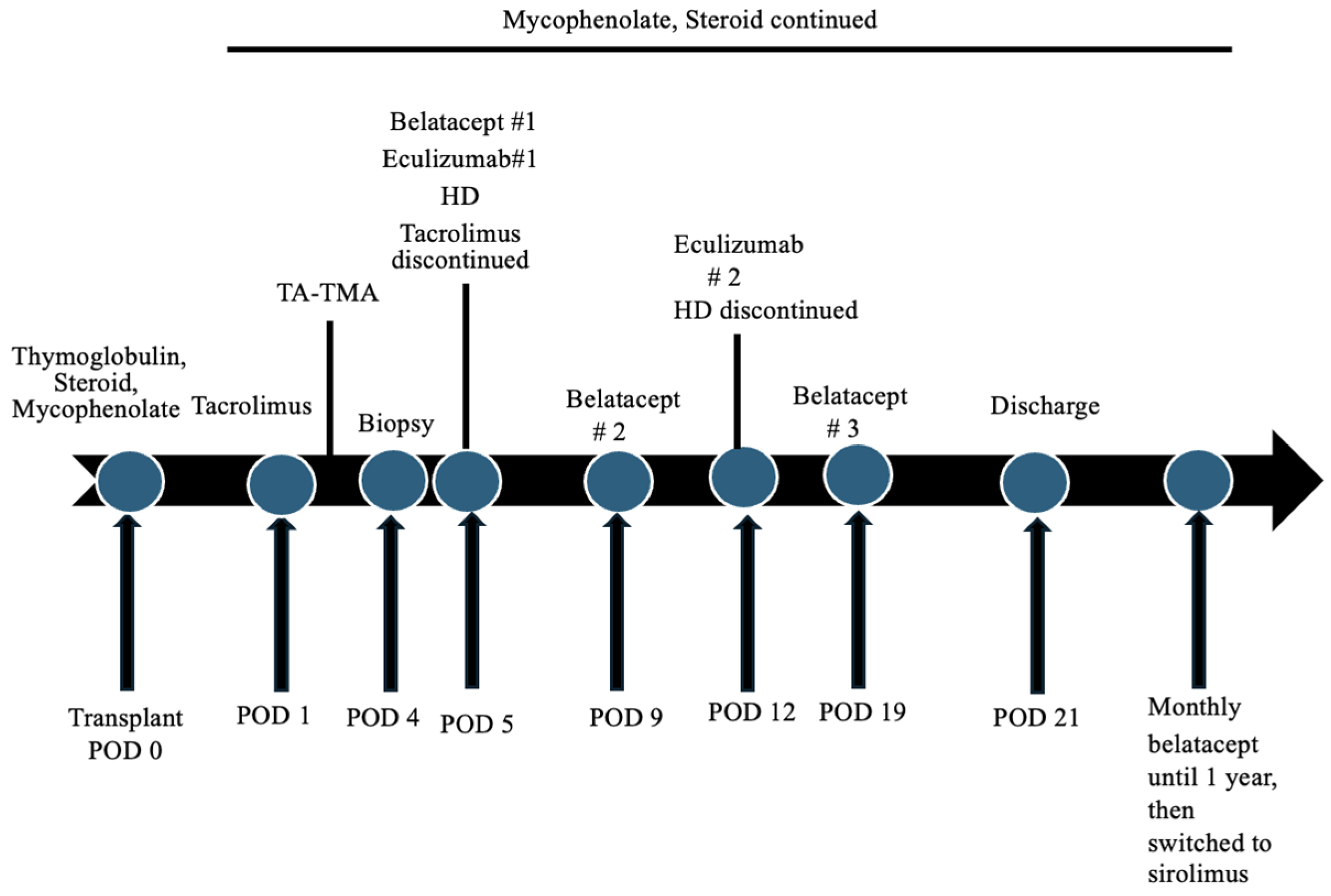
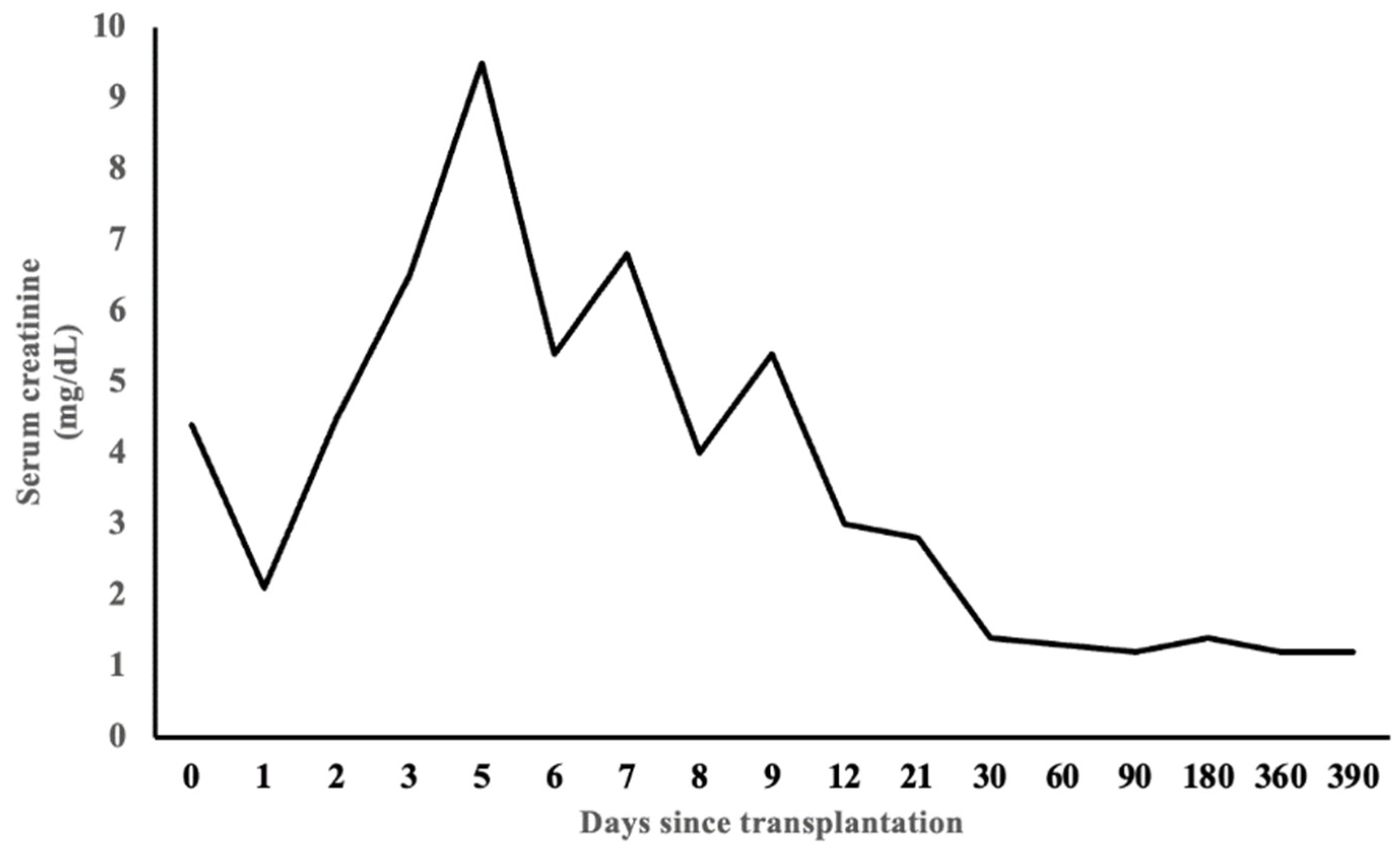
2. Case Presentation
3. Discussion
Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trimarchi, H.M.; Truong, L.D.; Brennan, S.; Gonzalez, J.M.; Suki, W.N. FK506-associated thrombotic microangiopathy: Report of two cases and review of the literature. Transplantation 1999, 67, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Ashman, N.; Chapagain, A.; Dobbie, H.; Raftery, M.J.; Sheaff, M.T.; Yaqoob, M.M. Belatacept as maintenance immunosuppression for postrenal transplant de novo drug-induced thrombotic microangiopathy. Am. J. Transplant. 2009, 9, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Bren, A.; Pajek, J.; Grego, K.; Buturovic, J.; Ponikvar, R.; Lindic, J.; Knap, B.; Vizjak, A.; Ferluga, D.; Kandus, A. Follow-up of kidney graft recipients with cyclosporine-associated hemolytic-uremic syndrome and thrombotic microangiopathy. Transplant. Proc. 2005, 37, 1889–1891. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C.; Agodoa, L.Y.; Yuan, C.M.; Abbott, K.C. Thrombotic microangiopathy after renal transplantation in the United States. Am. J. Kidney Dis. 2003, 42, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Merola, J.; Yoo, P.S.; Schaub, J.; Smith, J.D.; Rodriguez-Davalos, M.I.; Tichy, E.; Mulligan, D.C.; Asch, W.; Formica, R.; Kashgarian, M.; et al. Belatacept and Eculizumab for treatment of calcineurin inhibitor-induced thrombotic microangiopathy after kidney transplantation: Case report. Transplant. Proc. 2016, 48, 3106–3108. [Google Scholar] [CrossRef] [PubMed]
- Safa, K.; Logan, M.S.; Batal, I.; Gabardi, S.; Rennke, H.G.; Abdi, R. Eculizumab for drug-induced de novo posttransplantation thrombotic microangiopathy: A case report. Clin. Nephrol. 2015, 83, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, V.; Parasuraman, R.; Shah, V.; Vera, E.; Venkat, K.K. Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. Am. J. Transplant. 2003, 3, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Karpe, K.M.; Talaulikar, G.S.; Walters, G.D. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst. Rev. 2017, 7, CD006750. [Google Scholar] [CrossRef] [PubMed]
- Archdeacon, P.; Dixon, C.; Belen, O.; Albrecht, R.; Meyer, J. Summary of the US FDA approval of belatacept. Am. J. Transplant. 2012, 12, 554–562. [Google Scholar] [CrossRef]
- Larsen, C.P.; Pearson, T.C.; Adams, A.B.; Tso, P.; Shirasugi, N.; Strobert, E.; Anderson, D.; Cowan, S.; Price, K.; Naemura, J.; et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am. J. Transplant. 2005, 5, 443–453. [Google Scholar] [CrossRef]
- Masson, P.; Henderson, L.; Chapman, J.R.; Craig, J.C.; Webster, A.C. Belatacept for kidney transplant recipients. Cochrane Database Syst. Rev. 2014, 24, CD010699. [Google Scholar] [CrossRef] [PubMed]
- Blew, K.H.; Chua, A.; Foreman, J.; Gbadegesin, R.; Jackson, A.; Nagaraj, S.; Sadun, R.; Wigfall, D.; Kirk, A.D.; Chambers, E.T. Tailored use of belatacept in adolescent kidney transplantation. Am. J. Transplant. 2020, 20, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Garro, R.; Winterberg, P.; Liverman, R.; Serluco, A.; Warshaw, B.; George, R. Outcomes and experience with belatacept in pediatric kidney transplantation [abstract]. Am. J. Transplant. 2020, 20 (Suppl. S3). Available online: https://atcmeetingabstracts.com/abstract/outcomes-and-experience-with-belatacept-in-pediatric-kidney-transplantation/ (accessed on 3 November 2023).
- Lerch, C.; Kanzelmeyer, N.K.; Ahlenstiel-Grunow, T.; Froede, K.; Kreuzer, M.; Drube, J.; Verboom, M.; Pape, L. Belatacept after kidney transplantation in adolescents: A retrospective study. Transpl. Int. 2017, 30, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Israni, A.K.; Danovitch, G. Long-term survival after kidney transplantation. N. Engl. J. Med. 2021, 385, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Gaston, R.S. Chronic calcineurin inhibitor nephrotoxicity: Reflections on an evolving paradigm. Clin. J. Am. Soc. Nephrol. 2009, 4, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Charpentier, B.; Vanrenterghem, Y.; Rostaing, L.; Bresnahan, B.; Darji, P.; Massari, P.; Mondragon-Ramirez, G.A.; Agarwal, M.; Di Russo, G.; et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am. J. Transplant. 2010, 10, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Durrbach, A.; Pestana, J.M.; Pearson, T.; Vincenti, F.; Garcia, V.D.; Campistol, J.; Rial, M.d.C.; Florman, S.; Block, A.; Di Russo, G.; et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am. J. Transplant. 2010, 10, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Larsen, C.P.; Alberu, J.; Bresnahan, B.; Garcia, V.D.; Kothari, J.; Lang, P.; Urrea, E.M.; Massari, P.; Mondragon-Ramirez, G.; et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am. J. Transplant. 2012, 12, 210–217. [Google Scholar] [CrossRef]
- Pestana, J.O.; Grinyo, J.M.; Vanrenterghem, Y.; Becker, T.; Campistol, J.M.; Florman, S.; Garcia, V.D.; Kamar, N.; Lang, P.; Manfro, R.C.; et al. Three-year outcomes from BENEFIT-EXT: A phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am. J. Transplant. 2012, 12, 630–639. [Google Scholar] [CrossRef]
- Vincenti, F.; Rostaing, L.; Grinyo, J.; Rice, K.; Steinberg, S.; Gaite, L.; Moal, M.C.; Mondragon-Ramirez, G.A.; Kothari, J.; Polinsky, M.S.; et al. Belatacept and long-term outcomes in kidney transplantation. N. Engl. J. Med. 2016, 374, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Darres, A.; Ulloa, C.; Brakemeier, S.; Garrouste, C.; Bestard, O.; Del Bello, A.; Sberro Soussan, R.; Dürr, M.; Budde, K.; Legendre, C.; et al. Conversion to belatacept in maintenance kidney transplant patients: A retrospective multicenter European study. Transplantation 2018, 102, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Schulte, K.; Vollmer, C.; Klasen, V.; Bräsen, J.H.; Püchel, J.; Borzikowsky, C.; Kunzendorf, U.; Feldkamp, T. Late conversion from tacrolimus to a belatacept-based immunosuppression regime in kidney transplant recipients improves renal function, acid-base derangement and mineral-bone metabolism. J. Nephrol. 2017, 30, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Najjar, R.; Peng, A.; Choi, J.; Lim, K.; Vo, A.; Jordan, S.C.; Huang, E. Outcomes of conversion from calcineurin inhibitor to belatacept-based immunosuppression in HLA-sensitized kidney transplant recipients. Transplantation 2020, 104, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Kirk, A.D.; Guasch, A.; Xu, H.; Cheeseman, J.; Mead, S.I.; Ghali, A.; Mehta, A.K.; Wu, D.; Gebel, H.; Bray, R.; et al. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. Am. J. Transplant. 2014, 14, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, A.; Dharnidharka, V.R.; Feig, D.I.; Warshaw, B.L.; Perera, V.; Murthy, B.; Roberts, M.E.; Polinsky, M.S.; Ettenger, R.B. Phase I study of single-dose pharmacokinetics and pharmacodynamics of belatacept in adolescent kidney transplant recipients. Am. J. Transplant. 2019, 19, 1218–1223. [Google Scholar] [CrossRef]
- Garg, N.; Rennke, H.G.; Pavlakis, M.; Zandi-Nejad, K. De novo thrombotic microangiopathy after kidney transplantation. Transplant. Rev. 2018, 32, 58–68. [Google Scholar] [CrossRef]
- Le Clech, A.; Simon-Tillaux, N.; Provôt, F.; Delmas, Y.; Vieira-Martins, P.; Limou, S.; Halimi, J.M.; Le Quintrec, M.; Lebourg, L.; Grangé, S.; et al. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int. 2019, 95, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Young, J.A.; Pallas, C.R.; Knovich, M.A. Transplant-associated thrombotic microangiopathy: Theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 2021, 56, 1805–1817. [Google Scholar] [CrossRef]
- Schwimmer, J.; Nadasdy, T.A.; Spitalnik, P.F.; Kaplan, K.L.; Zand, M.S. De novo thrombotic microangiopathy in renal transplant recipients: A comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am. J. Kidney Dis. 2003, 41, 471–479. [Google Scholar] [CrossRef]
- Satoskar, A.A.; Pelletier, R.; Adams, P.; Nadasdy, G.M.; Brodsky, S.; Pesavento, T.; Henry, M.; Nadasdy, T. De novo thrombotic microangiopathy in renal allograft biopsies-role of antibody-mediated rejection. Am. J. Transplant. 2010, 10, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Cavero, T.; Rabasco, C.; López, A.; Román, E.; Ávila, A.; Sevillano, Á.; Huerta, A.; Rojas-Rivera, J.; Fuentes, C.; Blasco, M.; et al. Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol. Dial. Transplant. 2017, 32, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Meehan, S.M.; Kremer, J.; Ali, F.N.; Curley, J.; Marino, S.; Chang, A.; Kadambi, P.V. Thrombotic microangiopathy and peritubular capillary C4d expression in renal allograft biopsies. Clin. J. Am. Soc. Nephrol. 2011, 6, 395–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petr, V.; Hruba, P.; Kollar, M.; Krejci, K.; Safranek, R.; Stepankova, S.; Dedochova, J.; Machova, J.; Zieg, J.; Slatinska, J.; et al. Rejection-associated phenotype of de novo thrombotic microangiopathy represents a risk for premature graft loss. Transplant. Direct. 2021, 7, e779. [Google Scholar] [CrossRef] [PubMed]
- Lanfranco, L.; Joly, M.; Del Bello, A.; Esposito, L.; Cognard, N.; Perrin, P.; Moulin, B.; Kamar, N.; Caillard, S. Eculizumab for thrombotic microangiopathy associated with antibody-mediated rejection after ABO-incompatible kidney transplantation. Case Rep. Transplant. 2017, 2017, 3197042. [Google Scholar] [CrossRef] [PubMed]
- Sahin, G.; Akay, O.M.; Bal, C.; Yalcin, A.U.; Gulbas, Z. The effect of calcineurin inhibitors on endothelial and platelet function in renal transplant patients. Clin. Nephrol. 2011, 76, 218–225. [Google Scholar] [PubMed]
- Tomasiak, M.; Rusak, T.; Gacko, M.; Stelmach, H. Cyclosporine enhances platelet procoagulant activity. Nephrol. Dial. Transplant. 2007, 22, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Palma, L.M.P.; Sridharan, M.; Sethi, S. Complement in secondary thrombotic microangiopathy. Kidney Int. Rep. 2021, 6, 11–23. [Google Scholar] [CrossRef]
- Chua, J.S.; Baelde, H.J.; Zandbergen, M.; Wilhelmus, S.; van Es, L.A.; de Fijter, J.W.; Bruijn, J.A.; Bajema, I.M.; Cohen, D. Complement factor C4d Is a common denominator in thrombotic microangiopathy. J. Am. Soc. Nephrol. 2015, 26, 2239–2247. [Google Scholar] [CrossRef]
- Bhutani, G.; Leung, N.; Said, S.M.; Valeri, A.M.; Astor, B.C.; Fidler, M.E.; Alexander, M.P.; Cornell, L.D.; Nasr, S.H. The prevalence and clinical outcomes of microangiopathic hemolytic anemia in patients with biopsy-proven renal thrombotic microangiopathy. Am. J. Hematol. 2022, 97, E426–E429. [Google Scholar] [CrossRef]
- Le Quintrec, M.; Lionet, A.; Kamar, N.; Karras, A.; Barbier, S.; Buchler, M.; Fakhouri, F.; Provost, F.; Fridman, W.H.; Thervet, E.; et al. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am. J. Transplant. 2008, 8, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Praga, M.; Rodríguez de Córdoba, S. Secondary atypical hemolytic uremic syndromes in the era of complement blockade. Kidney Int. 2019, 95, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
| Investigations | Initial Values | Values at Discharge | Normal Values |
|---|---|---|---|
| WBC count | 10.2 × 109/L | 6.2 × 109/L | 4–11 × 109/L |
| Hemoglobin | 7.3 g/dL | 12.1 g/dL | 10.5–13.5 g/dL |
| Platelet count | 35 × 109/L | 310 × 109/L | 150–450 × 109/L |
| LDH | 1452 IU/L | 247 IU/L | 135–225 IU/L |
| Haptoglobin | <30 mg/dL | 110 mg/dL | 40–215 mg/dL |
| Peripheral smear | Few schistocytes | ||
| Direct antiglobulin test, C3 and IgG | Negative | ||
| ADAMTS13 activity | 80% | >60% | |
| C3 complement | 104 mg/dL | 87–200 mg/dL | |
| C4 complement | 18 mg/dL | 13–50 mg/dL | |
| SC5B-9 level | 480 ng/mL | ≤244 ng/mL | |
| Genetic testing for atypical HUS | Negative for complement mutations or deficiencies | ||
| Serum sodium | 141 mmol/L | 140 mmol/L | 135–145 mmol/L |
| Serum potassium | 4.3 mmol/L | 4.2 mmol/L | 3.5–4.5 mmol/L |
| Serum bicarbonate | 23 mmol/L | 22 mmol/L | 22–30 mmol/L |
| BUN | 126 mg/dL | 52 mg/dL | 6–21 mg/dL |
| Serum creatinine | 9.5 mg/dL | 2.8 mg/dL | 0.20–0.43 mg/dL |
| Serum calcium | 8.3 mg/dL | 10.4 mg/dL | 8.4–10.2 mg/dL |
| Serum phosphorus | 5.8 mg/dL | 4.4 mg/dL | 4.3–6.8 mg/dL |
| Serum albumin | 3 g/dL | 4.1 g/dL | 3.5–5.2 g/dL |
| EBV DNA PCR, CMV DNA PCR, BK virus DNA PCR | Negative | ||
| Urinalysis | 2 RBC/hpf, 2 WBC/hpf, trace proteinuria, negative nitrites and leukocytes, pH 7, specific gravity 1.025 | ||
| Renal transplant sonogram |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, R.; Clapp, W.; Upadhyay, K. Safety and Efficacy of Very Early Conversion to Belatacept in Pediatric Kidney Transplantation with Transplant-Associated Thrombotic Microangiopathy: Case Study and Review of Literature. Clin. Pract. 2024, 14, 882-891. https://doi.org/10.3390/clinpract14030069
Acharya R, Clapp W, Upadhyay K. Safety and Efficacy of Very Early Conversion to Belatacept in Pediatric Kidney Transplantation with Transplant-Associated Thrombotic Microangiopathy: Case Study and Review of Literature. Clinics and Practice. 2024; 14(3):882-891. https://doi.org/10.3390/clinpract14030069
Chicago/Turabian StyleAcharya, Ratna, William Clapp, and Kiran Upadhyay. 2024. "Safety and Efficacy of Very Early Conversion to Belatacept in Pediatric Kidney Transplantation with Transplant-Associated Thrombotic Microangiopathy: Case Study and Review of Literature" Clinics and Practice 14, no. 3: 882-891. https://doi.org/10.3390/clinpract14030069
APA StyleAcharya, R., Clapp, W., & Upadhyay, K. (2024). Safety and Efficacy of Very Early Conversion to Belatacept in Pediatric Kidney Transplantation with Transplant-Associated Thrombotic Microangiopathy: Case Study and Review of Literature. Clinics and Practice, 14(3), 882-891. https://doi.org/10.3390/clinpract14030069






