Association between Family History of Breast Cancer and Breast Density in Saudi Premenopausal Women Participating in Mammography Screening
Abstract
1. Introduction
2. Materials and Methods
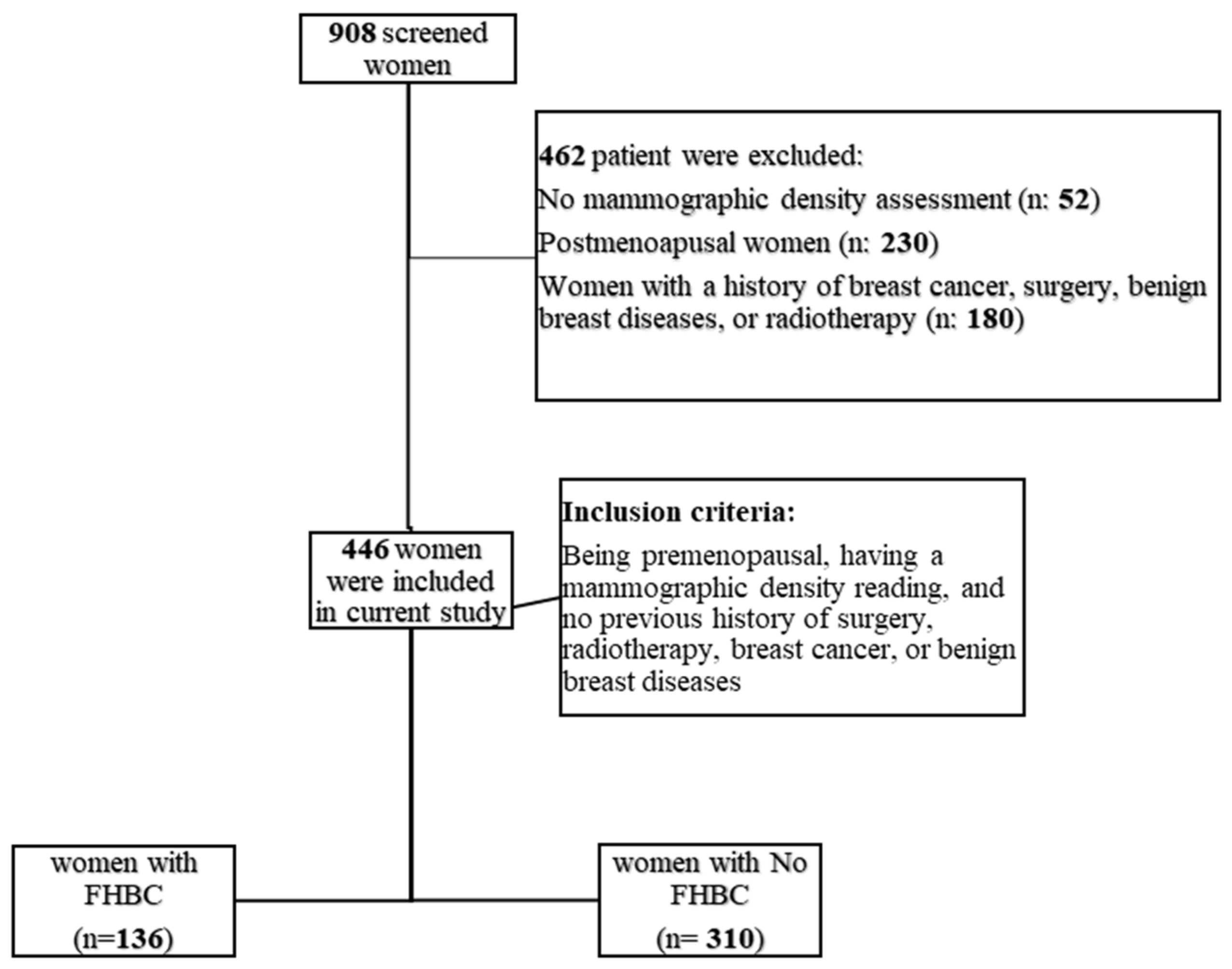
2.1. Study Population
2.2. Covariates
2.3. Mammographic Density Assessment
2.4. Statistical Analysis
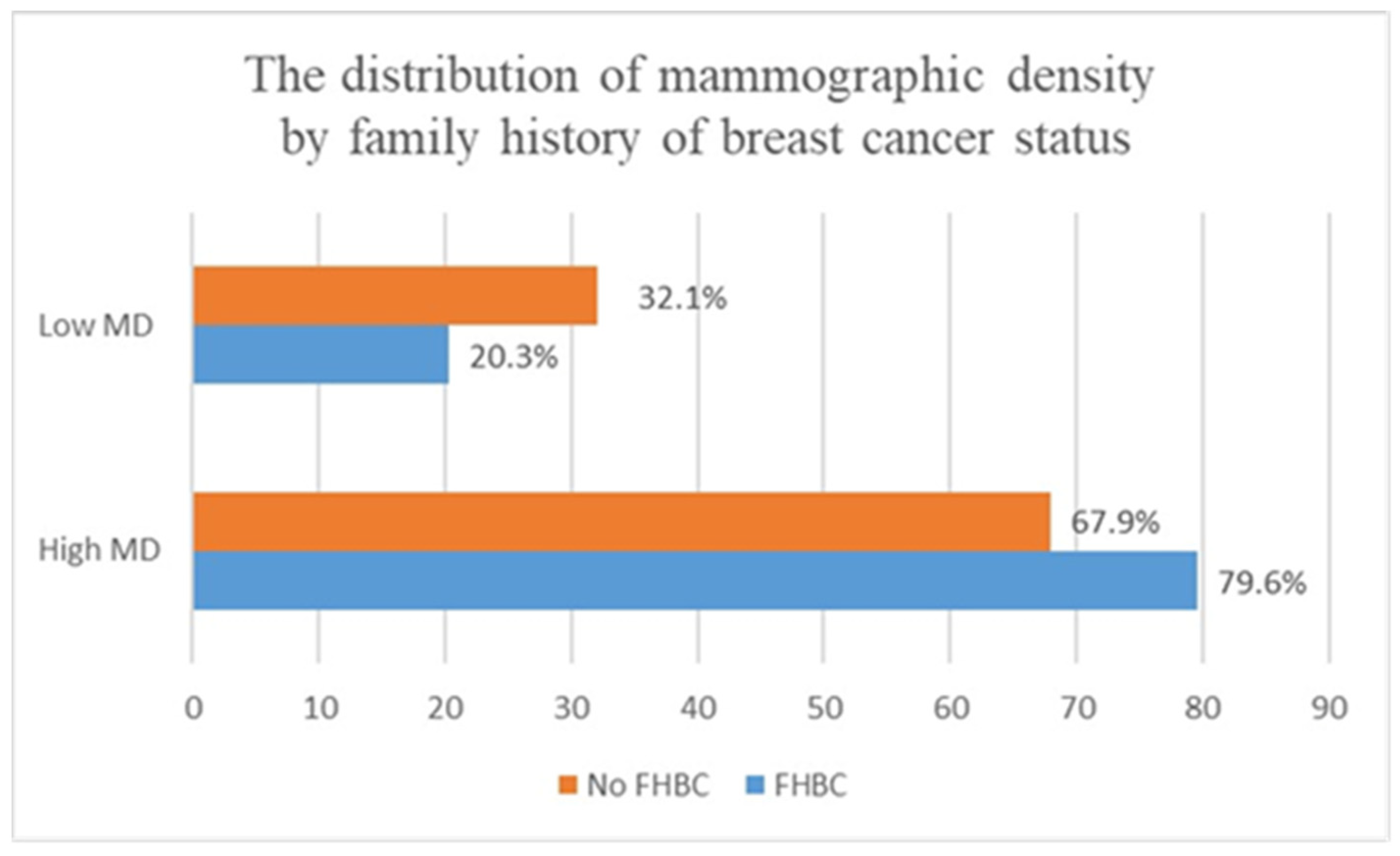
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brewer, H.R.; Jones, M.E.; Schoemaker, M.J.; Ashworth, A.; Swerdlow, A.J. Family history and risk of breast cancer: An analysis accounting for family structure. Breast Cancer Res. Treat. 2017, 165, 193–200. [Google Scholar] [CrossRef]
- Barnard, M.E.; Boeke, C.E.; Tamimi, R.M. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim. Biophys. Acta 2015, 1856, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, F.T.H.; van Asselt, A.A.; Dorrius, M.D.; Greuter, M.J.W.; de Bock, G.H. Mammographic breast density and the risk of breast cancer: A systematic review and meta-analysis. Breast 2022, 66, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Graff, R.E.; Ursin, G.; Santos Silva, I.D.; McCormack, V.; Baglietto, L.; Vachon, C.; Bakker, M.F.; Giles, G.G.; Chia, K.S.; et al. Mammographic density phenotypes and risk of breast cancer: A meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju078. [Google Scholar] [CrossRef]
- Bahcall, O. Common variation and heritability estimates for breast, ovarian and prostate cancers. Nat. Genet. 2013, 10, 304. [Google Scholar] [CrossRef]
- Michailidou, K.; Lindström, S.; Dennis, J.; Beesley, J.; Hui, S.; Kar, S.; Lemaçon, A.; Soucy, P.; Glubb, D.; Rostamianfar, A.; et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017, 551, 92–94. [Google Scholar] [CrossRef]
- Fernandez-Navarro, P.; Pita, G.; Santamariña, C.; Moreno, M.P.; Vidal, C.; Miranda-García, J.; Ascunce, N.; Casanova, F.; Collado-García, F.; Herráez, B.; et al. Association analysis between breast cancer genetic variants and mammographic density in a large population-based study (Determinants of Density in Mammographies in Spain) identifies susceptibility loci in TOX3 gene. Eur. J. Cancer 2013, 49, 474–481. [Google Scholar] [CrossRef]
- Mariapun, S.; Ho, W.K.; Kang, P.C.; Li, J.; Lindström, S.; Yip, C.H.; Teo, S.H. Variants in 6q25.1 Are Associated with Mammographic Density in Malaysian Chinese Women. Cancer Epidemiol. Biomark. Prev. 2016, 25, 327–333. [Google Scholar] [CrossRef]
- Darcey, E.; McCarthy, N.; Moses, E.K.; Saunders, C.; Cadby, G.; Stone, J. Is Mammographic Breast Density an Endophenotype for Breast Cancer? Cancers 2021, 13, 3916. [Google Scholar] [CrossRef]
- Ali, K.A.S.; Fateh, S.M. Mammographic breast density status in women aged more than 40 years in Sulaimaniyah, Iraq: A cross-sectional study. J. Int. Med. Res. 2022, 50, 3000605221139712. [Google Scholar] [CrossRef]
- Crest, A.B.; Aiello, E.J.; Anderson, M.L.; Buist, D.S. Varying levels of family history of breast cancer in relation to mammographic breast density (United States). Cancer Causes Control. CCC 2006, 17, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Moore, J.X.; Colditz, G.A.; Toriola, A.T. Family History of Breast Cancer and Mammographic Breast Density in Premenopausal Women. JAMA Netw. Open 2022, 5, e2148983. [Google Scholar] [CrossRef] [PubMed]
- Ziv, E.; Shepherd, J.; Smith-Bindman, R.; Kerlikowske, K. Mammographic Breast Density and Family History of Breast Cancer. JNCI J. Natl. Cancer Inst. 2003, 95, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Gu, R.; Hu, Y.; Liu, F.; Yun, M.; Xiao, Q.; Wu, M.; Liu, Q.; Su, F. Influence of factors on mammographic density in premenopausal Chinese women. Eur. J. Cancer Prev. 2016, 25, 306–311. [Google Scholar] [CrossRef]
- Boyd, N.F.; Martin, L.J.; Bronskill, M.; Yaffe, M.J.; Duric, N.; Minkin, S. Breast tissue composition and susceptibility to breast cancer. J. Natl. Cancer Inst. 2010, 102, 1224–1237. [Google Scholar] [CrossRef]
- Seely, J.M.; Peddle, S.E.; Yang, H.; Chiarelli, A.M.; McCallum, M.; Narasimhan, G.; Zakaria, D.; Earle, C.C.; Fung, S.; Bryant, H.; et al. Breast Density and Risk of Interval Cancers: The Effect of Annual Versus Biennial Screening Mammography Policies in Canada. Can. Assoc. Radiol. J. 2022, 73, 90–100. [Google Scholar] [CrossRef]
- Nazari, S.S.; Mukherjee, P. An overview of mammographic density and its association with breast cancer. Breast Cancer 2018, 25, 259–267. [Google Scholar] [CrossRef]
- Maskarinec, G.; Nakamura, K.L.; Woolcott, C.G.; Conroy, S.M.; Byrne, C.; Nagata, C.; Ursin, G.; Vachon, C.M. Mammographic density and breast cancer risk by family history in women of white and Asian ancestry. Cancer Causes Control. CCC 2015, 26, 621–626. [Google Scholar] [CrossRef]
- Ellison-Loschmann, L.; McKenzie, F.; Highnam, R.; Cave, A.; Walker, J.; Jeffreys, M. Age and Ethnic Differences in Volumetric Breast Density in New Zealand Women: A Cross-Sectional Study. PLoS ONE 2013, 8, e70217. [Google Scholar] [CrossRef]
- Mariapun, S.; Li, J.; Yip, C.H.; Taib, N.A.; Teo, S.H. Ethnic differences in mammographic densities: An Asian cross-sectional study. PLoS ONE 2015, 10, e0117568. [Google Scholar] [CrossRef]
- McLean, K.; Darcey, E.; Cadby, G.; Lund, H.; Pilkington, L.; Redfern, A.; Thompson, S.; Saunders, C.; Wylie, E.; Stone, J. The distribution and determinants of mammographic density measures in Western Australian aboriginal women. Breast Cancer Res. 2019, 21, 33. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Baker, J.L.; Bihrmann, K.; Vejborg, I.; Sørensen, T.I.A.; Lynge, E. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: A register-based cohort study. Breast Cancer Res. 2014, 16, R4. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.M.; Mostafa, S.; Salem, D.; ElHatw, A.M.; Mokhtar, S.M.; Wessam, R.; Fakhry, S. Body mass index, breast density, and the risk of breast cancer development in relation to the menopausal status; results from a population-based screening program in a native African-Arab country. Acta Radiol. Open 2022, 11, 20584601221111704. [Google Scholar] [CrossRef]
- Burton, A.; Maskarinec, G.; Perez-Gomez, B.; Vachon, C.; Miao, H.; Lajous, M.; López-Ridaura, R.; Rice, M.; Pereira, A.; Garmendia, M.L.; et al. Mammographic density and ageing: A collaborative pooled analysis of cross-sectional data from 22 countries worldwide. PLoS Med. 2017, 14, e1002335. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Boyd, N.F. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: Hypotheses based on epidemiological evidence. Breast Cancer Res. 2008, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Gierach, G.L.; Loud, J.T.; Chow, C.K.; Prindiville, S.A.; Eng-Wong, J.; Soballe, P.W.; Giambartolomei, C.; Mai, P.L.; Galbo, C.E.; Nichols, K.; et al. Mammographic density does not differ between unaffected BRCA1/2 mutation carriers and women at low-to-average risk of breast cancer. Breast Cancer Res. Treat. 2010, 123, 245–255. [Google Scholar] [CrossRef]
- Girard, E.; Eon-Marchais, S.; Olaso, R.; Renault, A.L.; Damiola, F.; Dondon, M.G.; Barjhoux, L.; Goidin, D.; Meyer, V.; Le Gal, D.; et al. Familial breast cancer and DNA repair genes: Insights into known and novel susceptibility genes from the GENESIS study, and implications for multigene panel testing. Int. J. Cancer 2019, 144, 1962–1974. [Google Scholar] [CrossRef]
- Sieh, W.; Rothstein, J.H.; Klein, R.J.; Alexeeff, S.E.; Sakoda, L.C.; Jorgenson, E.; McBride, R.B.; Graff, R.E.; McGuire, V.; Achacoso, N.; et al. Identification of 31 loci for mammographic density phenotypes and their associations with breast cancer risk. Nat. Commun. 2020, 11, 5116. [Google Scholar] [CrossRef]
- DeFilippis, R.A.; Chang, H.; Dumont, N.; Rabban, J.T.; Chen, Y.Y.; Fontenay, G.V.; Berman, H.K.; Gauthier, M.L.; Zhao, J.; Hu, D.; et al. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov. 2012, 2, 826–839. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Reeves, G.K.; Travis, R.C.; Alberg, A.J.; Barricarte, A.; Berrino, F.; Krogh, V.; Sieri, S.; Brinton, L.A.; et al. Sex hormones and risk of breast cancer in premenopausal women: A collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013, 14, 1009–1019. [Google Scholar] [CrossRef]
- Begg, C.B.; Zabor, E.C.; Bernstein, J.L.; Bernstein, L.; Press, M.F.; Seshan, V.E. A conceptual and methodological framework for investigating etiologic heterogeneity. Stat. Med. 2013, 32, 5039–5052. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Weedon, M.N.; Green, H.D.; Mallabar-Rimmer, B.; Harrison, J.W.; Wood, A.R.; Ruth, K.S.; Tyrrell, J.; Wright, C.F. Influence of family history on penetrance of hereditary cancers in a population setting. EClinicalMedicine 2023, 64, 102159. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Scott, C.G.; Fasching, P.A.; Hall, P.; Tamimi, R.M.; Li, J.; Stone, J.; Apicella, C.; Odefrey, F.; Gierach, G.L.; et al. Common breast cancer susceptibility variants in LSP1 and RAD51L1 are associated with mammographic density measures that predict breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Thompson, D.J.; Dos Santos Silva, I.; Scott, C.; Tamimi, R.M.; Lindstrom, S.; Kraft, P.; Hazra, A.; Li, J.; Eriksson, L.; et al. Novel Associations between Common Breast Cancer Susceptibility Variants and Risk-Predicting Mammographic Density Measures. Cancer Res. 2015, 75, 2457–2467. [Google Scholar] [CrossRef]
- Xu, J.; Olusola, G.; Footman, A.; Hansen, N.; Cheriyan, A.M.; Koganti, K.; Reddy, V.; Yezdani, S.; Eddy, V.; De’smond, H.; et al. A Provocative Molecular Link between Mammographic Density and BRCA1-loss associated TNBC. Int. J. Hum. Genet. Genet. Disord. 2019, 1, 1–8. [Google Scholar] [PubMed]
- Alsowiyan, A.A.; Almotyri, H.M.; Alolayan, N.S.; Alissa, L.I.; Almotyri, B.H.; AlSaigh, S.H. Breast cancer knowledge and awareness among females in Al-Qassim Region, Saudi Arabia in 2018. J. Fam. Med. Prim. Care 2020, 9, 1712–1718. [Google Scholar] [CrossRef]
- Aloufi, A.S.; AlNaeem, A.N.; Almousa, A.S.; Hashem, A.M.; Malik, M.A.; Altahan, F.M.; Elsharkawi, M.M.; Almasar, K.A.; ElMahdy, M.H.; Squires, S.E.; et al. Mammographic breast density and breast cancer risk in the Saudi population: A case-control study using visual and automated methods. Br. J. Radiol. 2022, 95, 20211197. [Google Scholar] [CrossRef]
- Albeshan, S.M.; Hossain, S.Z.; Mackey, M.G.; Peat, J.K.; Al Tahan, F.M.; Brennan, P.C. Preliminary investigation of mammographic density among women in Riyadh: Association with breast cancer risk factors and implications for screening practices. Clin. Imaging 2019, 54, 138–147. [Google Scholar] [CrossRef]
- Ekpo, E.U.; McEntee, M.F. Measurement of breast density with digital breast tomosynthesis—A systematic review. Br. J. Radiol. 2014, 87, 20140460. [Google Scholar] [CrossRef]
| Population Characteristics | FHBC (n = 136) | No FHBC (n = 310) | p Value | |
|---|---|---|---|---|
| BMI median (IQR) | 28.44 (25.53–32.13) | 28.51 (25.43–32.35) | 0.8 | |
| Parity | Has children n (%) | 121 (89.60) | 277 (90.22) | 0.84 |
| Has no children n (%) | 14 (13.37) | 30 (9.77) | ||
| Missing n (%) | 1 (0.73) | 3 (0.96) | ||
| Breast feeding | Breastfed n (%) | 114 (84.4) | 253 (82.9) | 0.6 |
| Never breastfed n (%) | 21 (15.5) | 52 (17) | ||
| Missing n (%) | 1 (0.73) | 5 (1.61) | ||
| Age median (IQR) | 44 (42–47) | 44 (41–46) | 0.1 | |
| OCP administration | Use n (%) | 78 (57.7) | 156 (551.3) | 0.2 |
| Never use n (%) | 57 (42.2) | 148 (48.6) | ||
| Missing n (%) | 1 (0.73) | 6 (1.93) | ||
| Presence of FHBC | Presence of High Mammographic Density Crude OR (95% CI) | Presence of High Mammographic Density Adjusted OR (95% CI) a |
|---|---|---|
| Women without FHBC (n = 310) | 1 (Reference) | 1 (Reference) |
| Women with FHBC (n = 136) | 1.84 (1.12–3.02), p = 0.01 | 1.87 (1.14–3.08), p = 0.01 |
| Presence of FHBC | Presence of High Mammographic Density Crude OR (95% CI) | Presence of High Mammographic Density Adjusted OR (95% CI) a |
|---|---|---|
| No (n = 423) | 1 (Reference) | 1 (Reference) |
| Yes (n = 28) | 5.5 (1.29–23.6), p = 0.02 | 5.6 (1.3–24.1), p = 0.02 |
| FHBC (Sister) | Presence of High Mammographic Density Crude OR (95% CI) | Presence of High Mammographic Density Adjusted OR (95% CI) a |
|---|---|---|
| No (n = 438) | 1 (Reference) | 1 (Reference) |
| Yes (n = 13) | 2.31 (0.5–10.5), p = 0.27 | 2.34 (0.51–10.75), p = 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanbayti, I.H.; Alzahrani, M.A.; Yeslam, Y.O.; Habib, N.H.; Hadadi, I.; Almaimoni, Y.; Alahmadi, A.; Ekpo, E.U. Association between Family History of Breast Cancer and Breast Density in Saudi Premenopausal Women Participating in Mammography Screening. Clin. Pract. 2024, 14, 164-172. https://doi.org/10.3390/clinpract14010013
Kanbayti IH, Alzahrani MA, Yeslam YO, Habib NH, Hadadi I, Almaimoni Y, Alahmadi A, Ekpo EU. Association between Family History of Breast Cancer and Breast Density in Saudi Premenopausal Women Participating in Mammography Screening. Clinics and Practice. 2024; 14(1):164-172. https://doi.org/10.3390/clinpract14010013
Chicago/Turabian StyleKanbayti, Ibrahem Hussain, Mayada A. Alzahrani, Yara O. Yeslam, Noora H. Habib, Ibrahim Hadadi, Yousef Almaimoni, Adnan Alahmadi, and Ernest U. Ekpo. 2024. "Association between Family History of Breast Cancer and Breast Density in Saudi Premenopausal Women Participating in Mammography Screening" Clinics and Practice 14, no. 1: 164-172. https://doi.org/10.3390/clinpract14010013
APA StyleKanbayti, I. H., Alzahrani, M. A., Yeslam, Y. O., Habib, N. H., Hadadi, I., Almaimoni, Y., Alahmadi, A., & Ekpo, E. U. (2024). Association between Family History of Breast Cancer and Breast Density in Saudi Premenopausal Women Participating in Mammography Screening. Clinics and Practice, 14(1), 164-172. https://doi.org/10.3390/clinpract14010013







