Inducing Cough Reflex by Capsaicin Spray Stimulation in Patients with Acquired Brain Injury: A Preliminary Test and Proof of Concept
Abstract
:1. Introduction
2. Methods
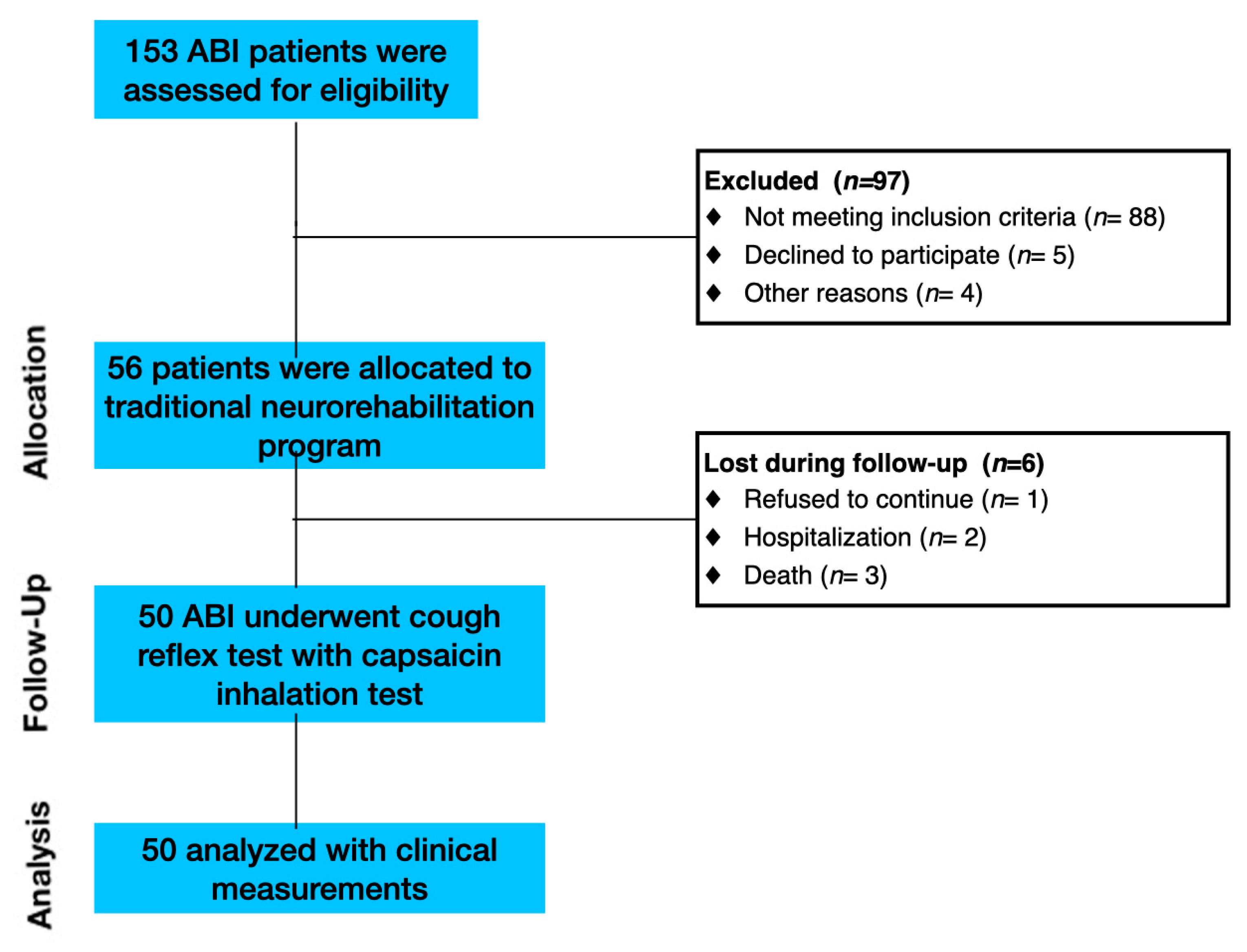
2.1. Participants
2.2. Design and Procedure
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Cough Reflex Test
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontana, G.A.; Pantaleo, T.; Lavorini, F.; Benvenuti, F.; Gangemi, S. Defective motor control of coughing in parkinson’s disease. Am. J. Respir. Crit. Care Med. 1998, 158, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Hadjikoutis, S.; Wiles, C.; Eccles, R. Cough in motor neuron disease: A review of mechanisms. QJM Int. J. Med. 1999, 92, 487–494. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hoshino, M.; Okayama, K.; Sekizawa, K.; Sasaki, H. Swallowing and cough reflexes after onset of stroke. Chest 1994, 105, 1623. [Google Scholar] [CrossRef]
- Belal, E.S.; Selim, S.; Fotouh, A.M.A.; Mohammad, A. Detection of airway protective level of the cough reflex in acute stroke patients. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 21. [Google Scholar] [CrossRef]
- Ward, K.; Seymour, J.; Steier, J.; Jolley, C.J.; Polkey, M.I.; Kalra, L.; Moxham, J. Acute ischaemic hemispheric stroke is associated with im-pairment of reflex in addition to voluntary cough. Eur. Respir. J. 2010, 36, 138–390. [Google Scholar] [CrossRef]
- Watts, S.A.; Tabor, L.; Plowman, E.K. To cough or not to cough? examining the potential utility of cough testing in the clinical evaluation of swallowing. Curr. Phys. Med. Rehabil. Rep. 2016, 4, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.; Hernandez, E.G.; Ang, A.; Hiew, S.; Macrae, P. A systematic review of methods of citric acid cough reflex testing. Pulm. Pharmacol. Ther. 2019, 58, 101827. [Google Scholar] [CrossRef]
- Mai, Y.; Fang, L.; Zhong, S.; de Silva, S.D.S.H.; Chen, R.; Lai, K. Methods for assessing cough sensitivity. J. Thorac. Dis. 2020, 12, 5224–5237. [Google Scholar] [CrossRef]
- Boulet, L.-P.; Coeytaux, R.R.; McCrory, D.C.; French, C.T.; Birring, S.S.; Smith, J.; Diekemper, R.L.; Rubin, B.; Irwin, R.S.; Adams, T.M.; et al. Tools for assessing outcomes in studies of chronic cough. Chest 2015, 147, 804–814. [Google Scholar] [CrossRef]
- Lai, K.; Shen, H.; Zhou, X.; Qiu, Z.; Cai, S.; Huang, K.; Wang, Q.; Wang, C.; Lin, J.; Hao, C.; et al. Clinical Practice Guidelines for Diagnosis and Management of Cough—Chinese Thoracic Society (CTS) Asthma Consortium. J. Thorac. Dis. 2018, 10, 6314–6351. [Google Scholar] [CrossRef]
- Mazzone, S.B. An Overview of the Sensory Receptors Regulating Cough. Cough 2005, 1, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Morice, A. Inhalation Cough Challenge in the Investigation of the Cough Reflex and Antitussives. Pulm. Pharmacol. 1996, 9, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Morice, A.H.; Fontana, G.A.; Belvisi, M.G.; Birring, S.S.; Chung, K.F.; Dicpinigaitis, P.V.; Kastelik, J.A.; McGarvey, L.P.; Smith, J.A.; Tatar, M.; et al. ERS Guidelines on the Assessment of Cough. Eur. Respir. J. 2007, 29, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, P.V. Review: Effect of drugs on human cough reflex sensitivity to inhaled capsaicin. Cough 2012, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Lüthi-Müller, E.; Kool, J.; Mylius, V.; Diesener, P. A New Therapeutic Approach for Dystussia and Atussia in Neurogenic Dysphagia: Effect of Aerosolized Capsaicin on Peak Cough Flow. Dysphagia 2022, 37, 1814–1821. [Google Scholar] [CrossRef]
- Borders, J.C.; Curtis, J.A.; Sevitz, J.S.; Vanegas-Arroyave, N.; Troche, M.S. Immediate Effects of Sensorimotor Training in Airway Protection (smTAP) on Cough Outcomes in Progressive Supranuclear Palsy: A Feasibility Study. Dysphagia 2022, 37, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Hegland, K.W.; Troche, M.; Brandimore, A.; Okun, M.; Davenport, P.W. Comparison of Two Methods for Inducing Reflex Cough in Patients With Parkinson’s Disease, With and Without Dysphagia. Dysphagia 2015, 31, 66–73. [Google Scholar] [CrossRef]
- Chao, W.; You-Qin, M.; Hong, C.; Hai-Ying, Z.; Li, Y.; Su-Xue, J.; Lan, X.; Zhong, W. Effect of Capsaicin Atomization on Cough and Swallowing Function in Patients With Hemorrhagic Stroke: A Randomized Controlled Trial. J. Speech Lang. Hear. Res. 2023, 66, 503–512. [Google Scholar] [CrossRef]
- Trapl, M.; Enderle, P.; Nowotny, M.; Teuschl, Y.; Matz, K.; Dachenhausen, A.; Brainin, M. Dysphagia bedside screening for acute-stroke patients: The gugging swallowing screen. Stroke 2007, 38, 2948–2952. [Google Scholar] [CrossRef]
- Enrichi, C.; Zanetti, C.; Gregorio, C.; Koch, I.; Vio, A.; Palmer, K.; Meneghello, F.; Piccione, F.; Battel, I. The assessment of the peak of reflex cough in subjects with acquired brain injury and tracheostomy and healthy controls. Respir. Physiol. Neurobiol. 2019, 274, 103356. [Google Scholar] [CrossRef]
- Mazzone, S.B.; Undem, B.J.; Horn, C.C.; Ardell, J.L.; Fisher, L.E.; Bautista, T.G.; Leech, J.; Farrell, M.J.; Xu, F.; Zhang, C.; et al. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol. Rev. 2016, 96, 975–1024. [Google Scholar] [CrossRef]
- Nishino, T. Physiological and pathophysiological implications of upper airway reflexes in humans. Jpn. J. Physiol. 2000, 50, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, P.V.; Alva, R.V. Safety of capsaicin cough challenge testing. Chest 2005, 128, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Imoto, Y.; Kojima, A.; Osawa, Y.; Sunaga, H.; Fujieda, S. Cough reflex induced by capsaicin inhalation in patients with dysphagia. Acta Oto-Laryngol. 2011, 131, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Kurokawa, R.; Akamatsu, T.; Fukumitsu, K.; Fukuda, S.; Ito, Y.; Takeda, N.; Nishiyama, H.; Ito, K.; Tajiri, T.; et al. Decreased capsaicin cough reflex sensitivity predicts hospitalisation due to COPD. BMJ Open Respir. Res. 2023, 10, e001283. [Google Scholar] [CrossRef]

| ABI without Dysphagia (n = 26) | ABI with Dysphagia (n = 24) | p-Value | |
|---|---|---|---|
| Age (years) | 59.1 ± 18.3 | 51.6 ± 17.4 | t = 1.46; n.s. |
| Gender (% Male) | 17 (65%) | 15 (63%) | X2 = 0.8; n.s. |
| Length of Stay in ICU (days) | 24.2 ± 15.3 | 37.1 ± 17.7 | t = 2.4 p < 0.02 |
| Side of Lesion | |||
| Left % | 19 (73%) | 7 (29%) | X2 = 10.1; p < 0.006 |
| Right % | 3 (12%) | 10 (42%) | |
| Bilateral % | 4 (15%) | 7 (29%) | |
| Etiology | |||
| Vascular % | 22 (84%) | 13 (54%) | X2 = 5.1; p = 0.06 |
| Traumatic % | 2 (8%) | 8 (33%) | |
| Others % | 2 (8%) | 3 (13%) | |
| Route of Feeding (%) | |||
| Oral feeding | 24 (92%) | 8 (33%) | X2 = 6.4; p < 0.04 |
| nasogastric tubes | 2 (8%) | 5 (21%) | |
| PEG | 0 (0%) | 11 (46%) | |
| % Tracheostomy (yes) | 1 (4%) | 23 (96%) | X2 = 98.1; p < 0.001 |
| % Infratentorial Lesion (yes) | 5 (19%) | 5 (21%) | n.s. |
| Barthel Index at admission | 13.2 ± 20.1 | 2.7 ± 9.4 | t = 2.89; p < 0.006 |
| Barthel Index at discharge | 56.3 ± 33.3 | 28.3 ± 35.1 | t = 2.34; p < 0.02 |
| GUSS scale | 17.7 ± 2.5 | 8.9 ± 4.2 | t = 8.98; p < 0.001 |
| Healthy Controls (n = 150) | ABI without Dysphagia (n = 26) | ABI with Dysphagia (n = 24) | p-Level | |
|---|---|---|---|---|
| % response at 1° Administration | 104 (69%) | 20 (77%) | 12 (50%) | X2 = 8.3; p= 0.21 |
| % response at 2° Administration | 14 (9%) | 4 (15%) | 5 (21%) | |
| % response at 3° Administration | 8 (5%) | 0 (0%) | 4 (16%) | |
| Absence of response | 24 (16%) | 2 (8%) | 3 (13%) | |
| Cough Reflex Trigger time (s) | 12.2 ± 13.2 | 12.1 ± 7.5 | 23.8 ± 20.1 | F = 3.42; p = 0.049 |
| Cough Intensity | 1.3 ± 0.5 1 [0–3] | 1.3 ± 0.6 1 [0–3] | 1.5 ± 0.6 1 [0–3] | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spezzano, L.; Cortese, M.D.; Quintieri, M.; Pignolo, L.; Tonin, P.; Lucca, F.L.; Tomaiuolo, F.; Calabrò, R.S.; Morone, G.; Cerasa, A. Inducing Cough Reflex by Capsaicin Spray Stimulation in Patients with Acquired Brain Injury: A Preliminary Test and Proof of Concept. Clin. Pract. 2023, 13, 1603-1611. https://doi.org/10.3390/clinpract13060140
Spezzano L, Cortese MD, Quintieri M, Pignolo L, Tonin P, Lucca FL, Tomaiuolo F, Calabrò RS, Morone G, Cerasa A. Inducing Cough Reflex by Capsaicin Spray Stimulation in Patients with Acquired Brain Injury: A Preliminary Test and Proof of Concept. Clinics and Practice. 2023; 13(6):1603-1611. https://doi.org/10.3390/clinpract13060140
Chicago/Turabian StyleSpezzano, Luisa, Maria Daniela Cortese, Maria Quintieri, Loris Pignolo, Paolo Tonin, Francesca Lucia Lucca, Francesco Tomaiuolo, Rocco Salvatore Calabrò, Giovanni Morone, and Antonio Cerasa. 2023. "Inducing Cough Reflex by Capsaicin Spray Stimulation in Patients with Acquired Brain Injury: A Preliminary Test and Proof of Concept" Clinics and Practice 13, no. 6: 1603-1611. https://doi.org/10.3390/clinpract13060140
APA StyleSpezzano, L., Cortese, M. D., Quintieri, M., Pignolo, L., Tonin, P., Lucca, F. L., Tomaiuolo, F., Calabrò, R. S., Morone, G., & Cerasa, A. (2023). Inducing Cough Reflex by Capsaicin Spray Stimulation in Patients with Acquired Brain Injury: A Preliminary Test and Proof of Concept. Clinics and Practice, 13(6), 1603-1611. https://doi.org/10.3390/clinpract13060140








