Evaluating Nutritional Risk Factors for Delirium in Intensive-Care-Unit Patients: Present Insights and Prospects for Future Research
Abstract
1. Introduction
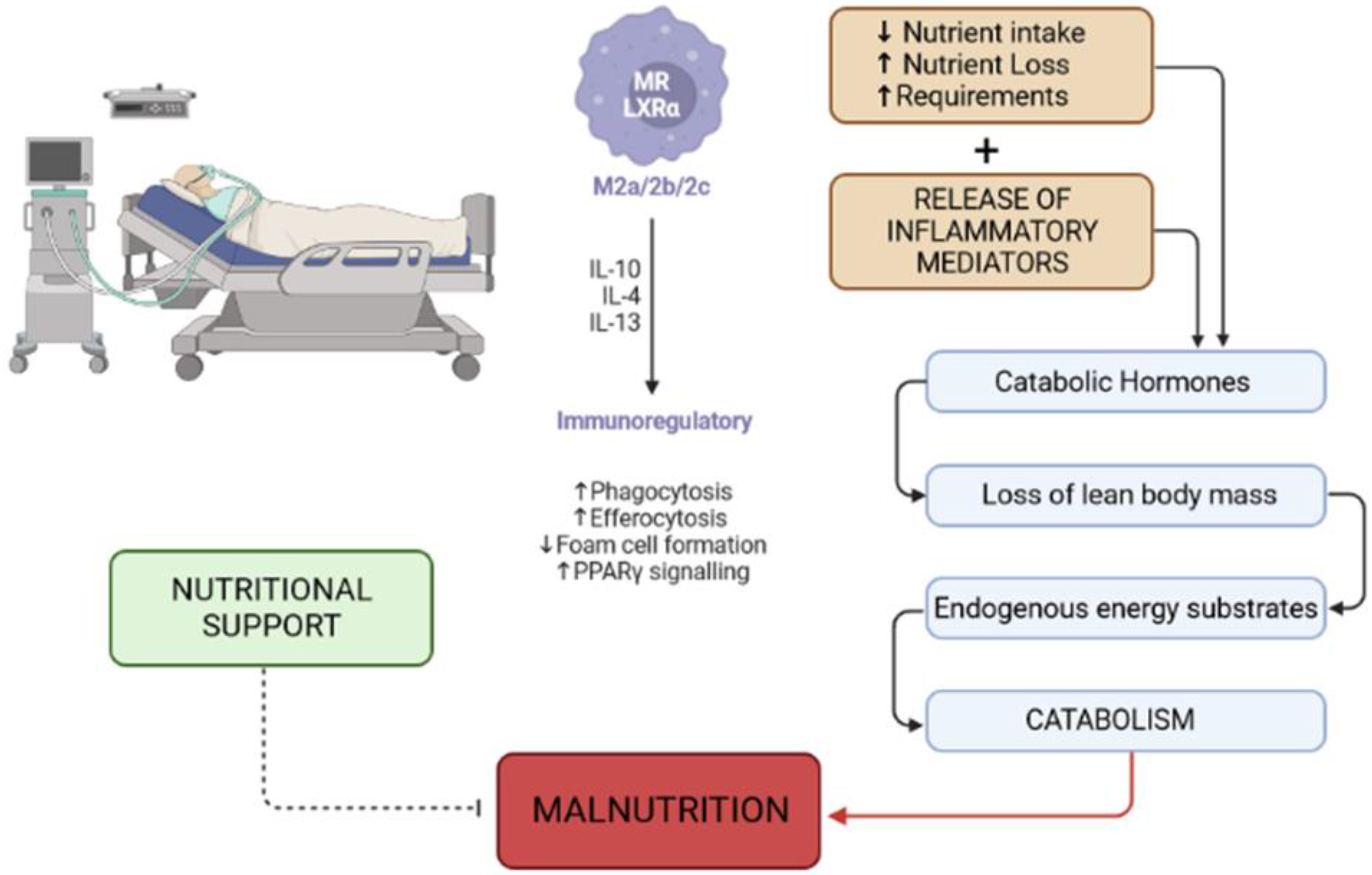
2. Causes of Malnutrition in the Critically Ill Patient
3. Nutritional Screening and Assessment
Bioelectrical Impedance Analysis
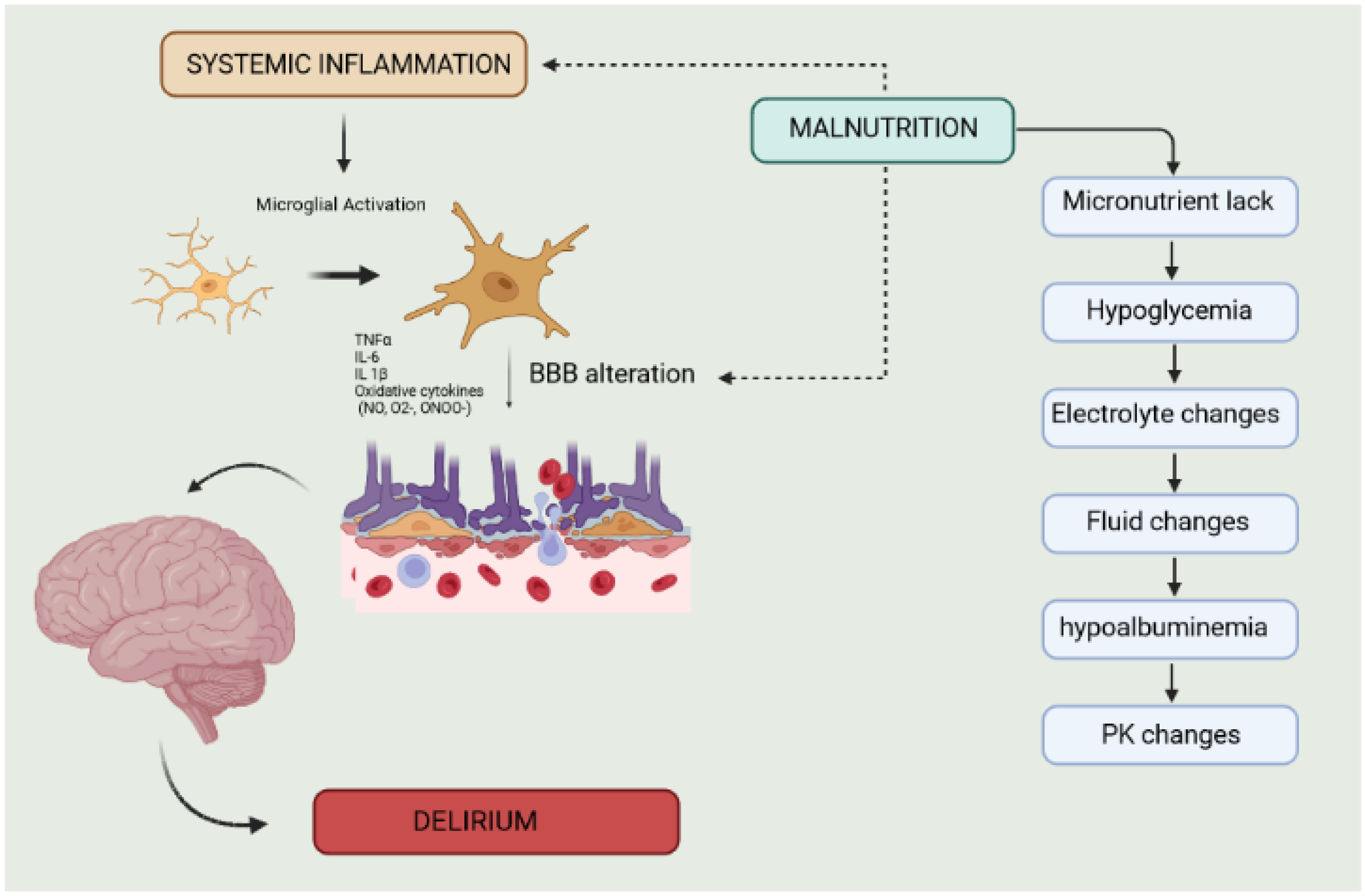
4. Malnutrition and Delirium
4.1. Alterations in Body Fluid Composition
4.2. Electrolyte Imbalances
4.3. Changes in Drug Metabolism
4.4. Deficiency of Trace Elements
4.5. Metabolic Alterations
4.6. Neuroinflammation
4.7. Malnutrition and Anemia
5. Clinical and Research Perspectives
5.1. Clinical Perspectives
5.2. Research Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diagnostic and Statistical Manual of Mental Disorders: DSM-5TM, 5th ed.; Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013; p. xliv947. ISBN 978-0-89042-554-1.
- Mehta, S.; Cook, D.; Devlin, J.W.; Skrobik, Y.; Meade, M.; Fergusson, D.; Herridge, M.; Steinberg, M.; Granton, J.; Ferguson, N.; et al. Prevalence, Risk Factors, and Outcomes of Delirium in Mechanically Ventilated Adults. Crit. Care Med. 2015, 43, 557–566. [Google Scholar] [CrossRef]
- Girard, T.D.; Thompson, J.L.; Pandharipande, P.P.; Brummel, N.E.; Jackson, J.C.; Patel, M.B.; Hughes, C.G.; Chandrasekhar, R.; Pun, B.T.; Boehm, L.M.; et al. Clinical Phenotypes of Delirium during Critical Illness and Severity of Subsequent Long-Term Cognitive Impairment: A Prospective Cohort Study. Lancet Respir. Med. 2018, 6, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, E.R. Delirium in Hospitalized Older Adults. N. Engl. J. Med. 2017, 377, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.N.; Raeburn, C.D.; Tran, Z.V.; Brenner, L.A.; Moss, M. Motor Subtypes of Postoperative Delirium in Older Adults. Arch. Surg. 2011, 146, 295–300. [Google Scholar] [CrossRef]
- Collet, M.O.; Caballero, J.; Sonneville, R.; Bozza, F.A.; Nydahl, P.; Schandl, A.; Wøien, H.; Citerio, G.; van den Boogaard, M.; Hästbacka, J.; et al. Prevalence and Risk Factors Related to Haloperidol Use for Delirium in Adult Intensive Care Patients: The Multinational AID-ICU Inception Cohort Study. Intensive Care Med. 2018, 44, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Minden, S.L.; Carbone, L.A.; Barsky, A.; Borus, J.F.; Fife, A.; Fricchione, G.L.; Orav, E.J. Predictors and Outcomes of Delirium. Gen. Hosp. Psychiatry 2005, 27, 209–214. [Google Scholar] [CrossRef]
- Dasgupta, M.; Dumbrell, A.C. Preoperative Risk Assessment for Delirium after Noncardiac Surgery: A Systematic Review. J. Am. Geriatr. Soc. 2006, 54, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Pun, B.T.; Balas, M.C.; Barnes-Daly, M.A.; Thompson, J.L.; Aldrich, J.M.; Barr, J.; Byrum, D.; Carson, S.S.; Devlin, J.W.; Engel, H.J.; et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit. Care Med. 2019, 47, 3–14. [Google Scholar] [CrossRef]
- Webber, C.; Watt, C.L.; Bush, S.H.; Lawlor, P.G.; Talarico, R.; Tanuseputro, P. The Occurrence and Timing of Delirium in Acute Care Hospitalizations in the Last Year of Life: A Population-Based Retrospective Cohort Study. Palliat. Med. 2020, 34, 1067–1077. [Google Scholar] [CrossRef]
- Aiello, G.; Cuocina, M.; La Via, L.; Messina, S.; Attaguile, G.A.; Cantarella, G.; Sanfilippo, F.; Bernardini, R. Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 435. [Google Scholar] [CrossRef]
- Sampson, E.L.; Raven, P.R.; Ndhlovu, P.N.; Vallance, A.; Garlick, N.; Watts, J.; Blanchard, M.R.; Bruce, A.; Blizard, R.; Ritchie, C.W. A Randomized, Double-Blind, Placebo-Controlled Trial of Donepezil Hydrochloride (Aricept) for Reducing the Incidence of Postoperative Delirium after Elective Total Hip Replacement. Int. J. Geriatr. Psychiatry 2007, 22, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Vidán, M.T.; Sánchez, E.; Alonso, M.; Montero, B.; Ortiz, J.; Serra, J.A. An Intervention Integrated into Daily Clinical Practice Reduces the Incidence of Delirium during Hospitalization in Elderly Patients. J. Am. Geriatr. Soc. 2009, 57, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.M.; Ramazani, N.; Greene, N.; Bruse, L. Review of Postoperative Delirium in Geriatric Patients After Hip Fracture Treatment. Geriatr. Orthop. Surg. Rehabil. 2022, 13, 21514593211058947. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, K. Risk Factors for Postoperative Delirium Identified. Am. J. Nurs. 2022, 122, 58. [Google Scholar] [CrossRef] [PubMed]
- Rosted, E.; Prokofieva, T.; Sanders, S.; Schultz, M. Serious Consequences of Malnutrition and Delirium in Frail Older Patients. J. Nutr. Gerontol. Geriatr. 2018, 37, 105–116. [Google Scholar] [CrossRef]
- Peng, C.; Wang, M.; Geng, Y.; Ke, J.; Dong, P.; Qin, J.; Zhong, D. Risk Factors for Postoperative Delirium in ICU Patients with Severe Illness Based on Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2022, 11, 309–320. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN Guidelines on Definitions and Terminology of Clinical Nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Stollings, J.L.; Kotfis, K.; Chanques, G.; Pun, B.T.; Pandharipande, P.P.; Ely, E.W. Delirium in Critical Illness: Clinical Manifestations, Outcomes, and Management. Intensive Care Med. 2021, 47, 1089–1103. [Google Scholar] [CrossRef]
- Hassen, T.A.; Pearson, S.; Cowled, P.A.; Fitridge, R.A. Preoperative Nutritional Status Predicts the Severity of the Systemic Inflammatory Response Syndrome (SIRS) Following Major Vascular Surgery. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 696–702. [Google Scholar] [CrossRef]
- Saunders, J.; Smith, T. Malnutrition: Causes and Consequences. Clin. Med. 2010, 10, 624–627. [Google Scholar] [CrossRef]
- Prado, C.M.; Ford, K.L.; Gonzalez, M.C.; Murnane, L.C.; Gillis, C.; Wischmeyer, P.E.; Morrison, C.A.; Lobo, D.N. Nascent to Novel Methods to Evaluate Malnutrition and Frailty in the Surgical Patient. JPEN J. Parenter. Enter. Nutr. 2023, 47 (Suppl. S1), S54–S68. [Google Scholar] [CrossRef]
- Kubota, T.; Shoda, K.; Konishi, H.; Okamoto, K.; Otsuji, E. Nutrition Update in Gastric Cancer Surgery. Ann. Gastroenterol. Surg. 2020, 4, 360–368. [Google Scholar] [CrossRef]
- Schneider, S.M.; Veyres, P.; Pivot, X.; Soummer, A.-M.; Jambou, P.; Filippi, J.; van Obberghen, E.; Hébuterne, X. Malnutrition Is an Independent Factor Associated with Nosocomial Infections. Br. J. Nutr. 2004, 92, 105–111. [Google Scholar] [CrossRef]
- Rai, J.; Gill, S.S.; Kumar, B.R.J.S. The Influence of Preoperative Nutritional Status in Wound Healing after Replacement Arthroplasty. Orthopedics 2002, 25, 417–421. [Google Scholar] [CrossRef]
- Unizony, S.; Menendez, M.E.; Rastalsky, N.; Stone, J.H. Inpatient Complications in Patients with Giant Cell Arteritis: Decreased Mortality and Increased Risk of Thromboembolism, Delirium and Adrenal Insufficiency. Rheumatology 2015, 54, 1360–1368. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy of Nutrition and Dietetics Malnutrition Work Group; A.S.P.E.N. Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus Statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics Recommended for the Identification and Documentation of Adult Malnutrition (Undernutrition). J. Acad. Nutr. Diet. 2012, 112, 730–738. [Google Scholar] [CrossRef]
- Preiser, J.-C.; Ichai, C.; Orban, J.-C.; Groeneveld, A.B.J. Metabolic Response to the Stress of Critical Illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.R.; Spencer-Segal, J.L. Glucocorticoids and the Brain after Critical Illness. Endocrinology 2021, 162, bqaa242. [Google Scholar] [CrossRef] [PubMed]
- McCowen, K.C.; Malhotra, A.; Bistrian, B.R. Stress-Induced Hyperglycemia. Crit. Care Clin. 2001, 17, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Plank, L.D.; Hill, G.L. Sequential Metabolic Changes Following Induction of Systemic Inflammatory Response in Patients with Severe Sepsis or Major Blunt Trauma. World J. Surg. 2000, 24, 630–638. [Google Scholar] [CrossRef]
- Wolfe, R.R. The Underappreciated Role of Muscle in Health and Disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute Skeletal Muscle Wasting in Critical Illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Krogh-Madsen, R.; Plomgaard, P.; Akerstrom, T.; Møller, K.; Schmitz, O.; Pedersen, B.K. Effect of Short-Term Intralipid Infusion on the Immune Response during Low-Dose Endotoxemia in Humans. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E371–E379. [Google Scholar] [CrossRef]
- Hill, A.; Elke, G.; Weimann, A. Nutrition in the Intensive Care Unit-A Narrative Review. Nutrients 2021, 13, 2851. [Google Scholar] [CrossRef]
- Stumpf, F.; Keller, B.; Gressies, C.; Schuetz, P. Inflammation and Nutrition: Friend or Foe? Nutrients 2023, 15, 1159. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Wickel, J.; Brunkhorst, F.M.; Geis, C. Sepsis-Associated Encephalopathy: From Delirium to Dementia? J. Clin. Med. 2020, 9, 703. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN Practical Guideline: Clinical Nutrition in Surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Hill, A.; Goetzenich, A.; Marx, G.; Stoppe, C. Role of Nutrition Support in Cardiac Surgery Patient—An Overview. Anasthesiol. Intensivmed. Notfallmed Schmerzther. 2018, 53, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying Critically Ill Patients Who Benefit the Most from Nutrition Therapy: The Development and Initial Validation of a Novel Risk Assessment Tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis–Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- The BIA Compendium—Data-Input.De. Available online: https://www.yumpu.com/en/document/view/13012439/the-bia-compendium-data-inputde (accessed on 30 November 2023).
- Bosy-Westphal, A.; Danielzik, S.; Dörhöfer, R.-P.; Piccoli, A.; Müller, M.J. Patterns of Bioelectrical Impedance Vector Distribution by Body Mass Index and Age: Implications for Body-Composition Analysis. Am. J. Clin. Nutr. 2005, 82, 60–68. [Google Scholar] [CrossRef]
- Lima, J.; Eckert, I.; Gonzalez, M.C.; Silva, F.M. Prognostic Value of Phase Angle and Bioelectrical Impedance Vector in Critically Ill Patients: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. 2022, 41, 2801–2816. [Google Scholar] [CrossRef]
- Thanapholsart, J.; Khan, E.; Lee, G.A. A Current Review of the Uses of Bioelectrical Impedance Analysis and Bioelectrical Impedance Vector Analysis in Acute and Chronic Heart Failure Patients: An Under-Valued Resource? Biol. Res. Nurs. 2023, 25, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Kaushik, M.; Valle, R.; Aspromonte, N.; Peacock, W.F. Diagnosis and Management of Fluid Overload in Heart Failure and Cardio-Renal Syndrome: The “5B” Approach. Semin. Nephrol. 2012, 32, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Uszko-Lencer, N.H.M.K.; Bothmer, F.; van Pol, P.E.J.; Schols, A.M.W.J. Measuring Body Composition in Chronic Heart Failure: A Comparison of Methods. Eur. J. Heart Fail. 2006, 8, 208–214. [Google Scholar] [CrossRef]
- Kong, D.; Luo, W.; Zhu, Z.; Sun, S.; Zhu, J. Factors Associated with Post-Operative Delirium in Hip Fracture Patients: What Should We Care. Eur. J. Med. Res. 2022, 27, 40. [Google Scholar] [CrossRef]
- Palakshappa, J.A.; Hough, C.L. How We Prevent and Treat Delirium in the ICU. Chest 2021, 160, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Reintam-Blaser, A.; Calder, P.C.; Casaer, M.; Hiesmayr, M.J.; Mayer, K.; Montejo, J.C.; Pichard, C.; Preiser, J.-C.; van Zanten, A.R.H.; et al. Monitoring Nutrition in the ICU. Clin. Nutr. 2019, 38, 584–593. [Google Scholar] [CrossRef]
- Finfer, S.; Myburgh, J.; Bellomo, R. Intravenous Fluid Therapy in Critically Ill Adults. Nat. Rev. Nephrol. 2018, 14, 541–557. [Google Scholar] [CrossRef]
- Cascella, M.; Muzio, M.R.; Bimonte, S.; Cuomo, A.; Jakobsson, J.G. Postoperative Delirium and Postoperative Cognitive Dysfunction: Updates in Pathophysiology, Potential Translational Approaches to Clinical Practice and Further Research Perspectives. Minerva Anestesiol. 2018, 84, 246–260. [Google Scholar] [CrossRef]
- Sanford, A.M.; Flaherty, J.H. Do Nutrients Play a Role in Delirium? Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 45–50. [Google Scholar] [CrossRef]
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A Multidisciplinary Consensus on Dehydration: Definitions, Diagnostic Methods and Clinical Implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Adan, A. Cognitive Performance and Dehydration. J. Am. Coll. Nutr. 2012, 31, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, S.J.; Nandyala, S.V.; Marquez-Lara, A.; Oglesby, M.; Patel, A.A.; Singh, K. Incidence and Risk Factors for Postoperative Delirium after Lumbar Spine Surgery. Spine 2013, 38, 1790–1796. [Google Scholar] [CrossRef]
- Koster, S.; Oosterveld, F.G.J.; Hensens, A.G.; Wijma, A.; van der Palen, J. Delirium after Cardiac Surgery and Predictive Validity of a Risk Checklist. Ann. Thorac. Surg. 2008, 86, 1883–1887. [Google Scholar] [CrossRef]
- van der Mast, R.C.; Huyse, F.J.; Rosier, P.F.W.M. Guideline ‘Delirium’. Ned. Tijdschr. Geneeskd. 2005, 149, 1027–1032. [Google Scholar]
- Caplan, J.P.; Chang, G. Refeeding Syndrome as an Iatrogenic Cause of Delirium: A Retrospective Pilot Study. Psychosomatics 2010, 51, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Central Nervous System: A Conductor Orchestrating Metabolic Regulations Harmed by Both Hyperglycaemia and Hypoglycaemia. Diabetes Metab. 2010, 36 (Suppl. S3), S31–S38. [Google Scholar] [CrossRef] [PubMed]
- Sevuk, U.; Baysal, E.; Ay, N.; Altas, Y.; Altindag, R.; Yaylak, B.; Alp, V.; Demirtas, E. Relationship between Cobalamin Deficiency and Delirium in Elderly Patients Undergoing Cardiac Surgery. Neuropsychiatr. Dis. Treat. 2015, 11, 2033–2039. [Google Scholar] [CrossRef]
- Vahdat Shariatpanahi, M.; Velayati, A.; Jamalian, S.A.; Babevaynejad, M.; Vahdat Shariatpanahi, Z. The Relationship between Serum Cobalamin, Folic Acid, and Homocysteine and the Risk of Post-Cardiac Surgery Delirium. Neuropsychiatr. Dis. Treat. 2019, 15, 1413–1419. [Google Scholar] [CrossRef]
- Eikelenboom, P.; Hoogendijk, W.J. Do Delirium and Alzheimer’s Dementia Share Specific Pathogenetic Mechanisms? Dement. Geriatr. Cogn. Disord. 1999, 10, 319–324. [Google Scholar] [CrossRef]
- Hung, K.-C.; Wang, L.-K.; Lin, Y.-T.; Yu, C.-H.; Chang, C.-Y.; Sun, C.-K.; Chen, J.-Y. Association of Preoperative Vitamin D Deficiency with the Risk of Postoperative Delirium and Cognitive Dysfunction: A Meta-Analysis. J. Clin. Anesth. 2022, 79, 110681. [Google Scholar] [CrossRef]
- Park, J.E.; Shin, T.G.; Jo, I.J.; Jeon, K.; Suh, G.Y.; Park, M.; Won, H.; Chung, C.R.; Hwang, S.Y. Impact of Vitamin C and Thiamine Administration on Delirium-Free Days in Patients with Septic Shock. J. Clin. Med. 2020, 9, 193. [Google Scholar] [CrossRef]
- Voigt, K.; Kontush, A.; Stuerenburg, H.-J.; Muench-Harrach, D.; Hansen, H.-C.; Kunze, K. Decreased Plasma and Cerebrospinal Fluid Ascorbate Levels in Patients with Septic Encephalopathy. Free Radic. Res. 2002, 36, 735–739. [Google Scholar] [CrossRef]
- Florez, C.M.; Lukankin, V.; Sugumar, S.; McGinn, R.; Zhang, Z.J.; Zhang, L.; Carlen, P.L. Hypoglycemia-Induced Alterations in Hippocampal Intrinsic Rhythms: Decreased Inhibition, Increased Excitation, Seizures and Spreading Depression. Neurobiol. Dis. 2015, 82, 213–225. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Chen, K.S.; Elzinga, S.E.; Feldman, E.L. Diabetes and Dementia: Clinical Perspective, Innovation, Knowledge Gaps. J. Diabetes Complicat. 2022, 36, 108333. [Google Scholar] [CrossRef]
- Zhang, D.-F.; Su, X.; Meng, Z.-T.; Cui, F.; Li, H.-L.; Wang, D.-X.; Li, X.-Y. Preoperative Severe Hypoalbuminemia Is Associated with an Increased Risk of Postoperative Delirium in Elderly Patients: Results of a Secondary Analysis. J. Crit. Care 2018, 44, 45–50. [Google Scholar] [CrossRef]
- Uchikado, H.; Akiyama, H.; Kondo, H.; Ikeda, K.; Tsuchiya, K.; Kato, M.; Oda, T.; Togo, T.; Iseki, E.; Kosaka, K. Activation of Vascular Endothelial Cells and Perivascular Cells by Systemic Inflammation-an Immunohistochemical Study of Postmortem Human Brain Tissues. Acta Neuropathol. 2004, 107, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hernandes, M.S. Sepsis-Associated Encephalopathy and Blood-Brain Barrier Dysfunction. Inflammation 2021, 44, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Tian, M.; Deng, S.; Li, J.; Yang, M.; Gao, J.; Pei, X.; Wang, Y.; Tan, J.; Zhao, F.; et al. The Key Drivers of Brain Injury by Systemic Inflammatory Responses after Sepsis: Microglia and Neuroinflammation. Mol. Neurobiol. 2023, 60, 1369–1390. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, K.; Xiao, Q.; Hou, R.; Pan, X.; Zhu, X. Central Role of Microglia in Sepsis-Associated Encephalopathy: From Mechanism to Therapy. Front. Immunol. 2022, 13, 929316. [Google Scholar] [CrossRef] [PubMed]
- Heimberger, A.B.; Lukas, R.V. The Kynurenine Pathway Implicated in Patient Delirium: Possible Indications for Indoleamine 2,3 Dioxygenase Inhibitors. J. Clin. Investig. 2023, 133, e164577. [Google Scholar] [CrossRef]
- Kudoh, A.; Takase, H.; Katagai, H.; Takazawa, T. Postoperative Interleukin-6 and Cortisol Concentrations in Elderly Patients with Postoperative Confusion. Neuroimmunomodulation 2005, 12, 60–66. [Google Scholar] [CrossRef]
- Castro, L.V.G.; Gonçalves-de-Albuquerque, C.F.; Silva, A.R. Polarization of Microglia and Its Therapeutic Potential in Sepsis. Int. J. Mol. Sci. 2022, 23, 4925. [Google Scholar] [CrossRef]
- De Backer, D.; Aissaoui, N.; Cecconi, M.; Chew, M.S.; Denault, A.; Hajjar, L.; Hernandez, G.; Messina, A.; Myatra, S.N.; Ostermann, M.; et al. How Can Assessing Hemodynamics Help to Assess Volume Status? Intensive Care Med. 2022, 48, 1482–1494. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Dezio, V.; Amelio, P.; Genoese, G.; Franchi, F.; Messina, A.; Robba, C.; Noto, A. Inferior Vena Cava Distensibility from Subcostal and Trans-Hepatic Imaging Using Both M-Mode or Artificial Intelligence: A Prospective Study on Mechanically Ventilated Patients. Intensive Care Med. Exp. 2023, 11, 40. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.; Teboul, J.-L. Passive Leg Raising for Predicting Fluid Responsiveness: A Systematic Review and Meta-Analysis. Intensive Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, W.; Gu, J.; Sun, Y.; Ye, X.; Qiu, W.; Su, C.; Zhang, S.; Ye, W. Risk Factors for Postoperative Delirium in Patients after Coronary Artery Bypass Grafting: A Prospective Cohort Study. J. Crit. Care 2015, 30, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Xu, D.-J.; Wei, X.-J.; Chang, H.-T.; Xu, G.-H. Electrolyte Disorders and Aging: Risk Factors for Delirium in Patients Undergoing Orthopedic Surgeries. BMC Psychiatry 2016, 16, 418. [Google Scholar] [CrossRef]
- Ali, I.; Bazzar, A.; Hussein, N.; Sahhar, E. Potential Drug-Drug Interactions in ICU Patients: A Retrospective Study. Drug Metab. Pers. Ther. 2020, 35, 20200114. [Google Scholar] [CrossRef] [PubMed]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Razzaque, M.S. Vitamin D and Phosphate Interactions in Health and Disease. Adv. Exp. Med. Biol. 2022, 1362, 37–46. [Google Scholar] [CrossRef] [PubMed]
- De Waele, E.; Malbrain, M.L.N.G.; Spapen, H. Nutrition in Sepsis: A Bench-to-Bedside Review. Nutrients 2020, 12, 395. [Google Scholar] [CrossRef]
- Zhong, X.; Jiao, H.; Zhao, D.; Teng, J. Association between Serum Albumin Levels and Paroxysmal Atrial Fibrillation by Gender in a Chinese Population: A Case–Control Study. BMC Cardiovasc. Disord. 2022, 22, 387. [Google Scholar] [CrossRef]
- Cichon, B.; Fabiansen, C.; Iuel-Brockdorf, A.-S.; Yaméogo, C.W.; Ritz, C.; Christensen, V.B.; Filteau, S.; Briend, A.; Michaelsen, K.F.; Friis, H. Impact of Food Supplements on Hemoglobin, Iron Status, and Inflammation in Children with Moderate Acute Malnutrition: A 2 × 2 × 3 Factorial Randomized Trial in Burkina Faso. Am. J. Clin. Nutr. 2018, 107, 278–286. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
- Maldonado, J.R. Acute Brain Failure: Pathophysiology, Diagnosis, Management, and Sequelae of Delirium. Crit. Care Clin. 2017, 33, 461–519. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, J.; Ma, D. Postoperative Delirium: Perioperative Assessment, Risk Reduction, and Management. Br. J. Anaesth. 2020, 125, 492–504. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Huang, H.; Gao, J.; Zhou, J.; Chu, H.-C. Hemoglobin Concentration and Post-Operative Delirium in Elderly Patients Undergoing Femoral Neck Fracture Surgery. Front. Med. 2021, 8, 780196. [Google Scholar] [CrossRef]
- de Souza, T.L.; Azzolin, K.d.O.; Fernandes, V.R. Multiprofessional care for delirium patients in intensive care: Integrative review. Rev. Gaucha Enferm. 2018, 39, e20170157. [Google Scholar] [CrossRef][Green Version]
- Magny, E.; Le Petitcorps, H.; Pociumban, M.; Bouksani-Kacher, Z.; Pautas, É.; Belmin, J.; Bastuji-Garin, S.; Lafuente-Lafuente, C. Predisposing and Precipitating Factors for Delirium in Community-Dwelling Older Adults Admitted to Hospital with This Condition: A Prospective Case Series. PLoS ONE 2018, 13, e0193034. [Google Scholar] [CrossRef] [PubMed]
- Kukolja, J.; Kuhn, J. SOP: Treatment of Delirium. Neurol. Res. Pract. 2021, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.K.; Craig, L.E.; Yong, S.Q.; Siddiqi, N.; Teale, E.A.; Woodhouse, R.; Barugh, A.J.; Shepherd, A.M.; Brunton, A.; Freeman, S.C.; et al. Non-Pharmacological Interventions for Preventing Delirium in Hospitalised Non-ICU Patients. Cochrane Database Syst. Rev. 2021, 7, CD013307. [Google Scholar] [CrossRef] [PubMed]
- Ringaitienė, D.; Gineitytė, D.; Vicka, V.; Žvirblis, T.; Šipylaitė, J.; Irnius, A.; Ivaškevičius, J.; Kačergius, T. Impact of Malnutrition on Postoperative Delirium Development after on Pump Coronary Artery Bypass Grafting. J. Cardiothorac. Surg. 2015, 10, 74. [Google Scholar] [CrossRef]
- Mazzola, P.; Ward, L.; Zazzetta, S.; Broggini, V.; Anzuini, A.; Valcarcel, B.; Brathwaite, J.S.; Pasinetti, G.M.; Bellelli, G.; Annoni, G. Association Between Preoperative Malnutrition and Postoperative Delirium After Hip Fracture Surgery in Older Adults. J. Am. Geriatr. Soc. 2017, 65, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Dunne, S.S.; Coffey, J.C.; Konje, S.; Gasior, S.; Clancy, C.C.; Gulati, G.; Meagher, D.; Dunne, C.P. Biomarkers in Delirium: A Systematic Review. J. Psychosom. Res. 2021, 147, 110530. [Google Scholar] [CrossRef]
- Tripp, B.A.; Dillon, S.T.; Yuan, M.; Asara, J.M.; Vasunilashorn, S.M.; Fong, T.G.; Metzger, E.D.; Inouye, S.K.; Xie, Z.; Ngo, L.H.; et al. Targeted Metabolomics Analysis of Postoperative Delirium. Sci. Rep. 2021, 11, 1521. [Google Scholar] [CrossRef]
- Vasunilashorn, S.M.; Ngo, L.H.; Chan, N.Y.; Zhou, W.; Dillon, S.T.; Otu, H.H.; Inouye, S.K.; Wyrobnik, I.; Kuchel, G.A.; McElhaney, J.E.; et al. Development of a Dynamic Multi-Protein Signature of Postoperative Delirium. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 261–268. [Google Scholar] [CrossRef]
- Vasunilashorn, S.M.; Lunardi, N.; Newman, J.C.; Crosby, G.; Acker, L.; Abel, T.; Bhatnagar, S.; Cunningham, C.; de Cabo, R.; Dugan, L.; et al. Preclinical and Translational Models for Delirium: Recommendations for Future Research from the NIDUS Delirium Network. Alzheimers Dement. 2023, 19, 2150–2174. [Google Scholar] [CrossRef]
| Parameter | Notes | Findings |
|---|---|---|
| BCM | All cells that influence metabolism (e.g., muscle, internal organs, nervous system) | ↑ good training status, intracellular water retention |
| ↓ malnutrition, cachexia, dehydration | ||
| BCM % | The percentage of BCM in FFM which measures individual nutritional status and physical fitness level | ↑good training status |
| ↓ malnutrition | ||
| EC | Mainly extracellular water | ↑ extracellular water retention (e.g., edema) |
| ↓ extracellular loss of water (e.g., diuretics) | ||
| FFM | No body fat tissues. It consists of BCM and ECM | ↓ elderly, chronic diseases |
| FM | It is indirectly determined from body weight minus FFM | |
| Phase angle | Key indicator of cell membrane function | |
| TBW | ↑ high portion of the muscle, water retention (e.g., edema) | |
| ↓ small portion of muscle, dehydration/insufficient fluid intake |
| Research Topic | Proposed Strategy(ies) |
|---|---|
| The impact of early enteral feeding on the incidence and severity of clinical manifestations. | Analysis of large datasets through AI methods. |
| Efficacy of nutritional supplementation in preventing delirium in at-risk patients. | AI-based strategies; multicentric investigations; EBM studies. |
| Investigations in specific patient populations. | For example, those with sepsis, traumatic brain injury, or acute respiratory distress syndrome. |
| Gender differences and age groups | Retrospective cohort studies to examine the association between pre-existing conditions, medications, and the incidence of delirium in male and female patients across different age categories. Meta-analyses and systematic reviews. AI-based strategies. |
| Relationship between specific nutrients and the development of delirium in critically ill patients. | For example, vitamin B12 or omega-3 fatty acids. |
| Potential benefits of parenteral nutrition on delirium incidence in ICU patients who are unable to tolerate enteral feeding. | Clinical trial (e.g., prospective investigations). |
| The role of individualized nutrition plans on delirium prevention and management. | Clinical trial (e.g., prospective investigations). Nutritional assessment (e.g., BIA) |
| The potential association between delirium and markers of malnutrition. | Serum albumin levels or prealbumin. |
| The impact of different routes of feeding (enteral vs. parenteral) on the development of delirium. | Retrospective analyses on large datasets. |
| The role of nutritional interventions in reducing the duration of delirium. | AI studies; EBM analyses; clinical trials |
| The impact of regular hydration and electrolyte management on the incidence and severity of delirium. | AI studies; EBM analyses; clinical trials |
| Assessment of the impact of nutritional interventions on cognitive outcomes in those who have experienced delirium. | Long-time follow-up studies. |
| Multiprofessional approaches. | Nurse-led nutritional interventions in preventing delirium and improving outcomes. |
| Predictive biomarkers. | Target metabolomic analysis. |
| Preclinical research. | Development of reliable animal models. |
| Artificial Intelligence Approach | Applications | Aims |
|---|---|---|
| Machine learning (ML) algorithms | Development of predictive models on datasets comprising patient demographics, medical history, medications, and vital signs. | Delirium risk (e.g., phenotypes) |
| ML algorithms | Predictive ML-based models on datasets including patient data, nutritional history, metabolic rates, and response to interventions. | Tailored nutritional plans, optimizing recovery, and minimizing complications |
| Computer vision (CV) and ML | Use of CV for analyzing patient images to estimate body composition changes, and ML for processing nutritional intake records, lab values, and patient-reported outcomes. | To assess and predict nutritional status. |
| Deep learning approaches | Development of models that continuously analyze real-time data, triggering alerts when abnormal patterns are detected. | Automated surveillance systems can be useful to ensure timely responses and interventions. |
| Natural language processing | Analysis of a vast amount of literature for extracting insights. | Extensive literature review. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccirillo, A.; Perri, F.; Vittori, A.; Ionna, F.; Sabbatino, F.; Ottaiano, A.; Cascella, M. Evaluating Nutritional Risk Factors for Delirium in Intensive-Care-Unit Patients: Present Insights and Prospects for Future Research. Clin. Pract. 2023, 13, 1577-1592. https://doi.org/10.3390/clinpract13060138
Piccirillo A, Perri F, Vittori A, Ionna F, Sabbatino F, Ottaiano A, Cascella M. Evaluating Nutritional Risk Factors for Delirium in Intensive-Care-Unit Patients: Present Insights and Prospects for Future Research. Clinics and Practice. 2023; 13(6):1577-1592. https://doi.org/10.3390/clinpract13060138
Chicago/Turabian StylePiccirillo, Arianna, Francesco Perri, Alessandro Vittori, Franco Ionna, Francesco Sabbatino, Alessandro Ottaiano, and Marco Cascella. 2023. "Evaluating Nutritional Risk Factors for Delirium in Intensive-Care-Unit Patients: Present Insights and Prospects for Future Research" Clinics and Practice 13, no. 6: 1577-1592. https://doi.org/10.3390/clinpract13060138
APA StylePiccirillo, A., Perri, F., Vittori, A., Ionna, F., Sabbatino, F., Ottaiano, A., & Cascella, M. (2023). Evaluating Nutritional Risk Factors for Delirium in Intensive-Care-Unit Patients: Present Insights and Prospects for Future Research. Clinics and Practice, 13(6), 1577-1592. https://doi.org/10.3390/clinpract13060138









