Abstract
Background: The global pandemic caused by the coronavirus disease 2019 (COVID-19) resulted in many deaths from fulminant respiratory failure. Chronic obstructive pulmonary disease (COPD) is the leading cause of morbidity and mortality worldwide. There has been great concern regarding the impact of COPD on the COVID-19 illness. Methods: Data from the Philippine CORONA study were analyzed to determine the association of COPD and COVID-19 in terms of mortality, disease severity, respiratory failure, mechanical ventilation, and lengths of stay in the intensive care unit (ICU) and hospital. Results: A total of 10,881 patients were included in this study, and 156 (1.4%) patients had been diagnosed with COPD. A majority of COVID-19 patients with COPD had other existing comorbidities: hypertension, diabetes mellitus, chronic cardiac disease, and chronic kidney disease. COPD patients were 2.0× more likely to present with severe to critical COVID-19 disease. COVID-19 patients with COPD in our study have a 1.7× increased mortality, 1.6× increased respiratory failure, and 2.0× increased risk for ICU admission. Smokers with COVID-19 were 1.8× more likely to present with more severe disease and have a 1.9× increased mortality. Conclusion: Our study supports the growing evidence that COPD among COVID-19 patients is a risk factor for higher mortality, more severe form of COVID-19, higher ICU admission, and higher respiratory failure needing ventilatory support.
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been responsible for the global coronavirus disease 2019 (COVID-19) pandemic. This highly transmissible disease can cause a wide range of symptoms from mild viral illness to fulminant respiratory failure and death [1]. As of September 2023, there has been 695,358,466 cases worldwide and 6,915,923 deaths [2].
Multiple descriptive studies have been made regarding the effect of pulmonary co-morbidities on the COVID 19 disease severity and outcomes [3,4,5,6,7,8]. At least one co-morbidity is present in 20–51% of COVID-19 patients, including hypertension and diabetes [3]. In the Philippines, several papers have reported on the presence of co-morbidities conferred poorer clinical outcomes (increased risk of mortality, respiratory failure, and the need for ICU admission) among Filipino COVID-19 patients [9,10,11].
The Global Initiative for Chronic Obstructive Lung Disease in its 2023 document defines chronic obstructive pulmonary disease (COPD) as a heterogeneous lung condition with long term respiratory symptoms due to abnormalities in the airways and/or alveoli that results in persistent, often progressive, airflow limitation [12]. To date, smoking tobacco remains the main environmental exposure that leads to the pathology of COPD. COPD is the leading cause of morbidity and mortality worldwide [12].
As COVID-19 is a predominantly respiratory infection, there has been great concern regarding patients with COPD and their susceptibility in acquiring the SARS-CoV-2 virus and risk for developing severe disease. It is an established fact that viral infections can cause COPD exacerbations that can lead to poor clinical outcomes.
There is growing evidence on the impact of COPD as a co-morbidity in COVID-19 infection worldwide. To date there have been no published data from the Philippine population. This study aimed to determine and compare the outcomes of COVID-19 patients with COPD co-morbidity against those without in terms of COVID-19 severity, respiratory failure, ICU admission, and length of ICU stay.
2. Materials and Methods
2.1. Study Design, Data Collection, Sampling, and Definition of Cohorts
We performed an analysis of data from the Philippine CORONA (COVID-19 Outcomes: a Retrospective Study of Neurological Manifestations and Associated Symptoms) Study with and without COPD co-morbidity. The Philippine CORONA Study is a nationwide, multicenter, comparative, retrospective, cohort study involving admitted patients with COVID-19 [13]. This was the largest Philippine study of COVID-19 to date involving 10,881 patients. COVID-19 cases were identified from the census of all participating institutions. Pertinent data were obtained through a review of medical records and encoded using electronic data collection form using Epi Info Software (V.7.2.2.16). The original cohort was approved by the individual institutional review and research boards of the hospital sites and the Single Joint Research Ethics Board of the Department of Health of the Philippines (see complete list below).
2.2. Outcome Variables
In this study, the following effect of COPD on the following outcomes were measured: (a) mortality, (b) the severity of COVID-19 based on Philippine COVID-19 Living Guidelines [14] (defined as the worst COVID classification of severity throughout the admission: mild–moderate—presence of mild pneumonia or absence of pneumonia; severe—presence of dyspnea, respiratory rate above 30 breaths per minute, oxygen saturations < 93% or more than 50% lung involvement on radiologic imaging within 24–48 h; critical—presence of respiratory failure, shock, or multiorgan dysfunction), (c) respiratory failure defined as the use of ventilatory support, (d) intensive care unit (ICU) admission, (e) the length of ICU stay, (f) the length of hospital stay, and (g) ventilator days for those placed on mechanical ventilation. In this analysis, we also explored the association of smoking with COVID-19 severity and mortality.
2.3. Statistical Analysis
The participants’ baseline characteristics and clinical outcomes were presented using descriptive statistics. Numerical variables that were normally distributed as assessed by the Shapiro–Wilk test for normality were presented as mean and standard deviation and as median and interquartile range (IQR), if otherwise. Categorical variables were described as count and proportion. Comparative analysis of baseline characteristics and clinical outcomes were performed between two groups: with COPD and without COPD. Student’s t test for variables was used to determine significant differences in the mean/median/mean rank of the different numerical variables between the two groups for normally distributed data, while the Mann–Whitney U test was performed for non-normally distributed variables. The heterogeneity of the proportions of the different categorical variables between the two groups were determined by chi-square test or Fisher exact test.
The associations between COPD and the different dichotomous and count outcome variables of interest were determined by binary logistic and Poisson regression, respectively. The logistic and Poisson regression models were adjusted for the age, sex, history of smoking, and comorbidities. In addition, the Poisson regression model was adjusted for the effect-measure modifier COVID-19 severity at nadir for lengths of hospital and ICU stay. Survival analysis was performed for the time-to-event data of mortality, respiratory failure, and admission to the intensive care unit (ICU). The time-to-event were right-censored on time-to-discharge as the exit from the time-at-risk among those who have not experienced the event, i.e., mortality, respiratory failure, or admission to ICU during their hospital stay. The associations between having COPD and the different time-to-event outcome variables of interest, adjusting for the age, sex, history of smoking, and comorbidities, were determined by a log-rank test of equality of survivor function. A cutoff of p-value < 0.05 identifies COPD and smoking status as significant predictors of the different outcomes of interest. Kaplan–Meier curves were constructed to visualize the survival curves of COPD versus non-COPD patients for the different time-to-event outcome variables.
3. Results
A total of 10,881 patients were included in the study, with 156 (1.4%) patients having been diagnosed with COPD. Table 1 presents the clinicodemographic characteristics of patients included in the study. COVID-19 patients with COPD were from the older age group 60 years old and above (78.8%, p < 0.001), predominantly male (84.0%, p < 0.001) and had a significant smoking history (52.5%, p < 0.001). A majority of COVID-19 patients with COPD had other existing co-morbidities. The most common of which were hypertension (66.7%, p < 0.001), diabetes mellitus (39.1%, p < 0.001), chronic cardiac disease (18.6%, p < 0.001), and chronic kidney disease (10.9%, p = 0.004). Many of the COVID-19 patients with COPD reported a history of a previous stroke (6.4% p = 0.026) and of neurodegenerative disorders (1.9%, p = 0.025).

Table 1.
Clinicodemographic characteristics stratified according to presence of COPD.
COVID-19 patients with COPD presented more often with fever, cough, dyspnea, and sputum production compared to those without COPD diagnosis. They also tend to present with new onset neurologic symptoms manifesting as altered mental state (8.3%, p = 0.035) or encephalopathy (12.1%, p = 0.001).
The COVID-19 patients with COPD in our study received a higher proportion of COVID-19 treatments namely glucocorticoids (55.8%, p < 0.001), tocilizumab (15.4%, p = 0.011), anti-virals (29.5%, p < 0.001), anti-bacterials (96.2%, p < 0.001), and other therapies (48.1%, p = 0.001) compared to those without COPD.
Table 2 summarizes the clinical outcomes of COVID-19 patients stratified according to presence of COPD diagnosis. COPD patients more commonly present with severe (38.1%, p < 0.001) or critical (33.6%, p < 0.001) COVID-19 compared to those without COPD. Figure 1 shows the Kaplan–Meier Curves on mortality, the development of respiratory failure and ICU admission. Our study showed that COVID-19 patients with COPD have higher rates of in-hospital mortality (Hazard ratio (HR) = 1.52, 95% CI (1.17, 1.97), p = 0.02), increased risk for developing respiratory failure predominantly from acute respiratory distress syndrome (HR = 2.90, 95% CI (2.24, 3.76), p < 0.001), and greater ICU admission rates (HR = 2.56, 95% CI (3.56, 4.17), p < 0.001), These suggests that COPD is a significant risk factor for mortality, the development of respiratory failure, and ICU admission for COVID-19 patients.

Table 2.
Clinical outcomes of COVID-19 patients stratified according to COPD.
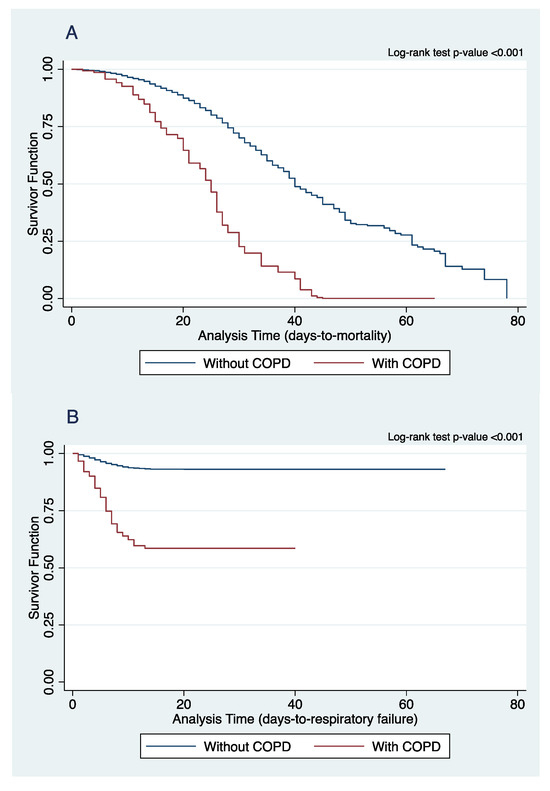
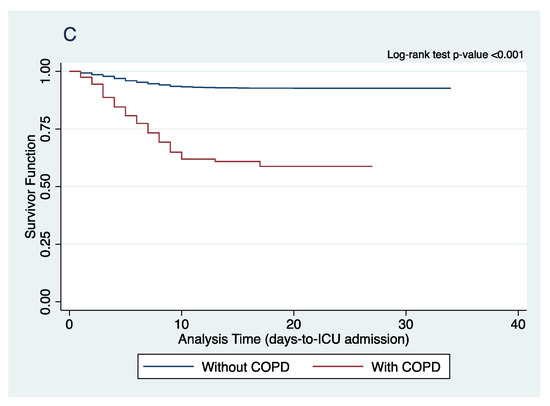
Figure 1.
Comparison of Kaplan–Meier curves of (A) in-hospital mortality, (B) respiratory failure, and (C) ICU admission between COPD and non-COPD COVID-19 patients.
On further analysis, however, there was no sufficient evidence to conclude any significant difference in time to the development of respiratory failure, the duration of mechanical ventilation, time to ICU admission, and the length of ICU stay between COPD and non-COPD patients.
The association of having COPD diagnosis with different outcomes of interest in COVID-19 is shown in Table 3. Our COPD patients with COVID-19 were 2.0 times more likely to present as severe to critical COVID. They were also 1.7 times more likely to experience in-hospital mortality, 1.6 times more likely to have respiratory failure during the course of their admission and 2.0 times more likely to be admitted in the ICU. COPD COVID-19 patients have been found to also have 37% decreased odds of full/partial neurological improvement compared to patients without COPD.

Table 3.
Association of COPD with the different clinical outcomes.
4. Discussion
Our study showed a small proportion of admitted COVID-19 patients had COPD diagnosis (1.4%). This was lower than the 7-11% reported in other studies [15,16]. This, however, does not mean that COPD patients are at a lower risk for contracting the disease. The cases in this study occurred during the early part of the pandemic when COVID-19 restrictions on mobility were heightened. Many of the COPD patients have been shielded from potential COVID-19 exposures because of these measures. The propensity to acquire COVID-19 among COPD patients in various studies have been confounded by these as well. Several reasons have been postulated as to the low prevalence of COPD among COVID-19 patients: usage of inhaled corticosteroids or bronchodilators, the already diseased state of the lungs may not be conducive for the SARS-CoV-2 to establish COVID-19, the presence of mucous plugs in large and small airways may hinder the virus from reaching the alveoli [17]. This under-representation may also be due to their pre-existing poor prognosis and decisions to continue with palliative care [18].
Our study highlighted that COPD patients who get admitted for COVID-19 have 3.4 times increased mortality and are 4.3 times likely to experience a severe or critical form of the disease that requires ventilatory support. This was also true in a study on 8395 patients, but not among patients with asthma, suggesting that the severity of the COVID-19 may be due to intrinsic immunological factors, such as type 2 inflammation [8]. Our study also showed increased health care utilization among COPD patients with COVID-19: 4.4 times likelihood of ICU admission, anti-inflammatory therapy (steroids and tocilizumab), antiviral, and antibacterial use. Furthermore, our study showed that COPD patients with COVID-19 have a higher incidence of neurologic symptoms and poor neurologic recovery.
The reason behind the poor outcomes of COVID-19 among COPD patients continues to be investigated. Multiple factors have been postulated. First, COPD patients and smokers may be predisposed to SARS-CoV-2 infection. Studies have demonstrated that the gene expression for ACE-2 in bronchial epithelial cells from COPD patients is significantly elevated compared to control subjects [6,19]. This increased expression of the virus receptor may allow for the faster spread of the virus into the distal airways and alveoli, leading to progression from to a more severe COVID-19 pneumonia [20].
Another potential reason is that many COPD patients have poor lung function, small airway disease and emphysema resulting in significantly reduced respiratory functional reserve to cope with the intrapulmonary shunting created by a superimposed pneumonia or pulmonary vascular thromboembolic events observed in COVID-19 [19]. Increased intrapulmonary shunting has been associated with worse outcomes, including mortality [21].
Innate immune responses to viruses in COPD has been shown to be impaired as well. There are defective interferon responses to SARS-CoV-2 and this has been linked to an increased risk of severe COVID-19 [5]. COPD patients have colonizing pathogenic bacteria in their airways during the stable state. This colonization can cause secondary bacterial infections following respiratory viral infections. Bacterial infection is common among COVID-19 patients and leads to worse outcomes. This may be due to reduced antimicrobial responses during viral infections including decreased bacterial phagocytosis by alveolar macrophages and decreased antimicrobial peptide release [7].
The presence of comorbidities is also associated with more severe COVID-19 [5,9,10,11,22,23,24,25,26,27,28,29,30]. This was also seen in our patients and can potentially explain the poor outcomes.
The manifestations of COPD exacerbation and COVID-19 among COPD patients may be difficult to distinguish. A high index of suspicion and vigilance is recommended to ensure early diagnosis and access to life saving COVID-19 treatment modalities.
Our study had several limitations. Our data collection did not differentiate different types of ventilatory support like invasive ventilation, high-flow nasal oxygen therapy, and non-invasive ventilation. As the diagnosis of COPD was based on medical history, there was no data on COPD severity, the use of maintenance medication and adherence, as well as lung function. These details may provide further insight into which subset or phenotype of COPD may have a poorer prognosis for COVID-19. Furthermore, our data on smoking did not differentiate current from previous smokers. It is interesting to know if smoking cessation has an impact on COVID-19 severity as well. We therefore recommend interested researchers to include these data in future research endeavors.
5. Conclusions
This study on Filipino patients with COVID-19 supports the growing evidence that COPD patients developing COVID-19 are at higher risk mortality, at having severe and critical forms of COVID-19, at higher likelihood of needing ICU admission, and have higher chances of developing respiratory failure needing ventilatory support.
Author Contributions
Conceptualization, R.D.G.J., M.C.C.S., A.I.E. and V.M.M.A.; methodology, R.D.G.J., M.C.C.S., A.I.E. and V.M.M.A.; data curation, M.C.C.S.; formal analysis, A.B.A.J., M.B.D.F.D. and E.Q.V.III; interpretation of data, A.B.A.J., M.B.D.F.D. and E.Q.V.III; writing—original draft, A.B.A.J. and M.B.D.F.D.; writing—review, R.D.G.J., A.B.A.J. and M.B.D.F.D.; editing, R.D.G.J., A.I.E. and V.M.M.A.; study supervision, R.D.G.J., M.C.C.S., A.I.E. and V.M.M.A.; and final approval of the version to be published, R.D.G.J., A.B.A.J., M.B.D.F.D., M.C.C.S., E.Q.V.III, A.I.E. and V.M.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Our protocol was approved and endorsed by the local institutional review boards (code): Asian Hospital and Medical Center, Muntinlupa City (2020-010-A); Baguio General Hospital and Medical Center, Baguio City (BGHMC-ERC-2020–13); Cagayan Valley Medical Center, Tuguegarao City (2020–314-01 SJREB); Capitol Medical Center, Quezon City; Cardinal Santos Medical Center, San Juan City (CSMC REC 2020–020); Chong Hua Hospital, Cebu City (IRB 2420–04); De La Salle Medical and Health Sciences Institute, Cavite (2020–23-02-A); Dr. Jose N. Rodriguez Memorial and Sanitarium Hospital, Caloocan City (2020–314-01 SJREB); East Avenue Medical Center, Quezon City (EAMC IERB 2020-38); Jose B. Lingad Memorial Regional Hospital, City of San Fernando, Pampanga (2020–314-01 SJREB); Jose R. Reyes Memorial Medical Center, Manila (2020–314-01 SJREB); Lung Center of the Philippines, Quezon City (LCP-CT-010–2020); Makati Medical Center, Makati City (MMC IRB 2020–054); Manila Doctors Hospital, Manila (MDH IRB 2020–006); Medical Center Manila, Manila (MMERC 2020–09); Northern Mindanao Medical Center, Cagayan de Oro City (025–2020); Quirino Memorial Medical Center, Quezon City (QMMC REB GCS 2020–28); Ospital ng Makati, Makati City (2020–314-01 SJREB); Philippine General Hospital, Manila (2020–314-01 SJREB); Philippine Heart Center, Quezon City (2020–314-01 SJREB); Research Institute for Tropical Medicine, Muntinlupa City (RITM IRB 2020–16); San Lazaro Hospital, Manila (2020–314-01 SJREB); San Juan De Dios Educational Foundation Inc. Hospital, Pasay City (SJRIB 2020–0006); Single Joint Research Ethics Board of the DOH, Philippines (SJREB-2020–24); Southern Isabela Medical Center, Santiago City (2020–03); Southern Philippines Medical Center, Davao City (P20062001); St. Luke’s Medical Center, Quezon City (SL-20116); St. Luke’s Medical Center, Bonifacio Global City, Taguig City (SL-20116); The Medical City, Pasig City; University of Santo Tomas Hospital, Manila (UST-REC-2020–04-071-MD); University of the East Ramon Magsaysay Memorial Medical Center, Inc., Quezon City (0835/E/2020/063); Veterans Memorial Medical Center, Quezon City (VMMC-2020–025); and Vicente Sotto Memorial Medical Center, Cebu City (VSMMC-REC-O-2020–048).
Informed Consent Statement
Acquiring informed consent was not needed for this study since the design was a retrospective cohort study employing medical chart review and the data obtained for this study were completely anonymized.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://www.worldometers.info/coronavirus/. (accessed on 16 September 2023).
- Song, J.; Zeng, M.; Wang, H.; Qin, C.; Hou, H.Y.; Sun, Z.Y.; Xu, S.P.; Wang, G.P.; Guo, C.L.; Deng, Y.K.; et al. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy 2021, 76, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Uruma, Y.; Manabe, T.; Fujikura, Y.; Iikura, M.; Hojo, M.; Kudo, K. Effect of asthma, COPD, and ACO on COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0276774. [Google Scholar]
- Bonato, M.; Semenzato, U.; Tinè, M.; Bazzan, M.; Damin, M.; Biondini, D.; Casara, A.; Romagnoli, M.; Turato, G.; Cosio, M.G.; et al. Risk factors for development and severity of COVID-19 in COPD patients. Front. Med. 2021, 8, 714570. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Niikura, M.; Yang, C.W.; Sin, D.D. COVID-19 and COPD. Eur. Respir. J. 2020, 56, 2002108. [Google Scholar] [CrossRef]
- Singh, D.; Mathioudakis, A.G.; Higham, A. Chronic obstructive pulmonary disease and COVID-19: Interrelationships. Curr. Opin. Pulm. Med. 2022, 28, 76–83. [Google Scholar] [CrossRef]
- Liu, Y.; Rajeevan, H.; Simonov, M.; Lee, S.; Wilson, F.P.; Desir, G.V.; Vinetz, J.M.; Yan, X.; Wang, Z.; Clark, B.J.; et al. Differences in mortality among patients with asthma and COPD hospitalized with COVID-19. J. Allergy Clin. Immunol. Pract. 2023. [Google Scholar]
- Espiritu, A.I.; Sucaldito, M.S.F.P.; Ona, D.I.D.; Apor, A.D.A.O.; Sy, M.C.C.; Anlacan, V.M.M.; Jamora, R.D.G. Clinical outcomes in COVID-19 among patients with hypertension in the Philippine CORONA Study. Eur. J. Med. Res. 2023, 28, 62. [Google Scholar] [CrossRef]
- Espiritu, A.I.; Larrazabal, R.B.; Sy, M.C.C.; Villanueva, E.Q.; Anlacan, V.M.M.; Jamora, R.D.G. Outcomes and risk factors of patients with COVID-19 and cancer (ONCORONA): Findings from the Philippine CORONA Study. Front. Oncol. 2022, 12, 857076. [Google Scholar] [CrossRef]
- Espiritu, A.I.; Chiu, H.H.C.; Sy, M.C.C.; Anlacan, V.M.M.; The Philippine CORONA Study Group; Jamora, R.D.G. The outcomes of patients with diabetes mellitus in The Philippine CORONA Study. Sci. Rep. 2021, 11, 24436. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. Available online: https://goldcopd.org (accessed on 1 May 2023).
- Espiritu, A.I.; Sy, M.C.C.; Anlacan, V.M.M.; Jamora, R.D.G.; Philippine CORONA Study Group Investigators. COVID-19 Outcomes of 10,881 patients: Retrospective Study of Neurological symptoms and Associated manifestations (Philippine CORONA Study). J. Neural Transm. 2021, 128, 1687–1703. [Google Scholar]
- Philippine Society of Microbiology and Infectious Diseases. Philippine COVID-19 Living Recommendations. Available online: https://www.psmid.org/philippine-covid-19-living-recommendations-3/ (accessed on 22 October 2023).
- Gerayeli, F.V.; Milne, S.; Cheung, C.; Li, X.; Yang, C.W.T.; Tam, A.; Choi, L.H.; Bae, A.; Sin, D.D. COPD and the risk of poor outcomes in COVID-19: A systematic review and meta-analysis. EClinicalMedicine 2021, 33, 100789. [Google Scholar]
- Montiel-Lopez, F.; Rodríguez-Ramírez, D.; Miranda-Márquez, M.C.; Cassou-Martínez, M.; Perea-Gutiérrez, H.; Hernández-Pérez, A.; Martínez Gómez, M.L.; Sansores, R.H.; Hernández-Zenteno, R.; Pérez-Padilla, R.; et al. Prevalence, attitude, knowledge, and risk perception towards COVID-19 in COPD patients associated to biomass exposure. Int. J. Environ. Health Res. 2023, 33, 170–179. [Google Scholar]
- Yong, S.J. Diseased lungs may hinder COVID-19 development: A possible reason for the low prevalence of COPD in COVID-19 patients. Med. Hypothesis 2021, 153, 110628. [Google Scholar]
- Olloquequi, J. COVID-19 susceptibility in chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2020, 50, e13382. [Google Scholar]
- Motoc, N.Ș.; Făgărășan, I.; Urda-Cîmpean, A.E.; Todea, D.A. Prognosis predictive markers in patients with chronic obstructive pulmonary disease and COVID-19. Diagnostics 2023, 13, 2597. [Google Scholar] [PubMed]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [PubMed]
- Kotwica, A.; Knights, H.; Mayor, N.; Russell-Jones, E.; Dassios, T.; Russell-Jones, D. Intrapulmonary shunt measured by bedside pulse oximetry predicts worse outcomes in severe COVID-19. Eur. Respir. J. 2021, 57, 2003841. [Google Scholar] [CrossRef]
- Pranata, R.; Lim, M.A.; Huang, I.; Raharjo, S.B.; Lukito, A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis and meta-regression. J. Renin Angiotensin Aldosterone Sys. 2020, 21, 1470320320926899. [Google Scholar]
- Liu, T.L.; Woodward, J.M.; Kowalkowski, M.; Taylor, Y.J.; Gutnik, B.; Mangieri, D.A. Assessing healthcare outcomes among patients with dementia requiring hospitalization for COVID-19: An observational study. J. Am. Geriatr. Soc. 2023, 71, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Lobanova, I.; Suri, M.F.K.; Naqvi, S.H.; French, B.R.; et al. Acute ischemic stroke and COVID-19: An analysis of 27676 patients. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Lim, M.A.; Yonas, E.; Vania, R.; Lukito, A.A.; Siswanto, B.B.; Meyer, M. Body mass index and outcome in patients with COVID-19: A dose-response meta-analysis. Diabetes Metab. 2021, 47, 101178. [Google Scholar] [CrossRef] [PubMed]
- Gholamalizadeh, M.; Attari, M.; Mousavi, M.; Shekari, S.; Salimi, Z.; Rajabi Harsini, A.; Zeinolabedin, M.; Barzkar, A.; Mahmoudi, Z.; Alami, F.; et al. The association between obesity with treatment duration, ICU length of stay and risk of death in critically ill patients with COVID-19. Endocrinol. Diabetes Metab. 2023, e458. [Google Scholar] [CrossRef]
- Patel, B.; Chapman, S.A.; Neumann, J.T.; Visaria, A.; Ogungbe, O.; Wen, S.; Khodaverdi, M.; Makwana, P.; Singh, J.A.; Sokos, G.; et al. Outcomes of patients with active cancers and pre-existing cardiovascular diseases infected with SARS-CoV-2. Res. Sq. 2023, 9, 36. [Google Scholar] [CrossRef]
- Bode, B.; Garrett, V.; Messler, J.; McFarland, R.; Crowe, J.; Booth, R.; Klonoff, D.C. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020, 14, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.L.; Woodruff, R.C.; Nagavedu, K.; Fearrington, J.; Rolka, D.B.; Twentyman, E.; Carton, T.W.; Puro, J.; Denson, J.L.; Kappelman, M.D.; et al. Association between hypertension and diabetes control and COVID-19 severity: National Patient-Centered Clinical Research Network, United States, March 2020 to February 2022. J. Am. Heart Assoc. 2023, e030240. [Google Scholar]
- Hägglöf, E.; Bell, M.; Zettersten, E.; Engerström, L.; Larsson, E. Long-term survival after intensive care for COVID-19: A nationwide cohort study of more than 8000 patients. Ann. Intensive Care 2023, 13, 76. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).