Past, Current, and Future Perspectives on Transplanting Acute Kidney Injury Kidneys
Abstract
1. Introduction
1.1. Historical Context
1.2. Ongoing Underutilization of AKI Kidneys
1.3. Current AKI Kidney Practices
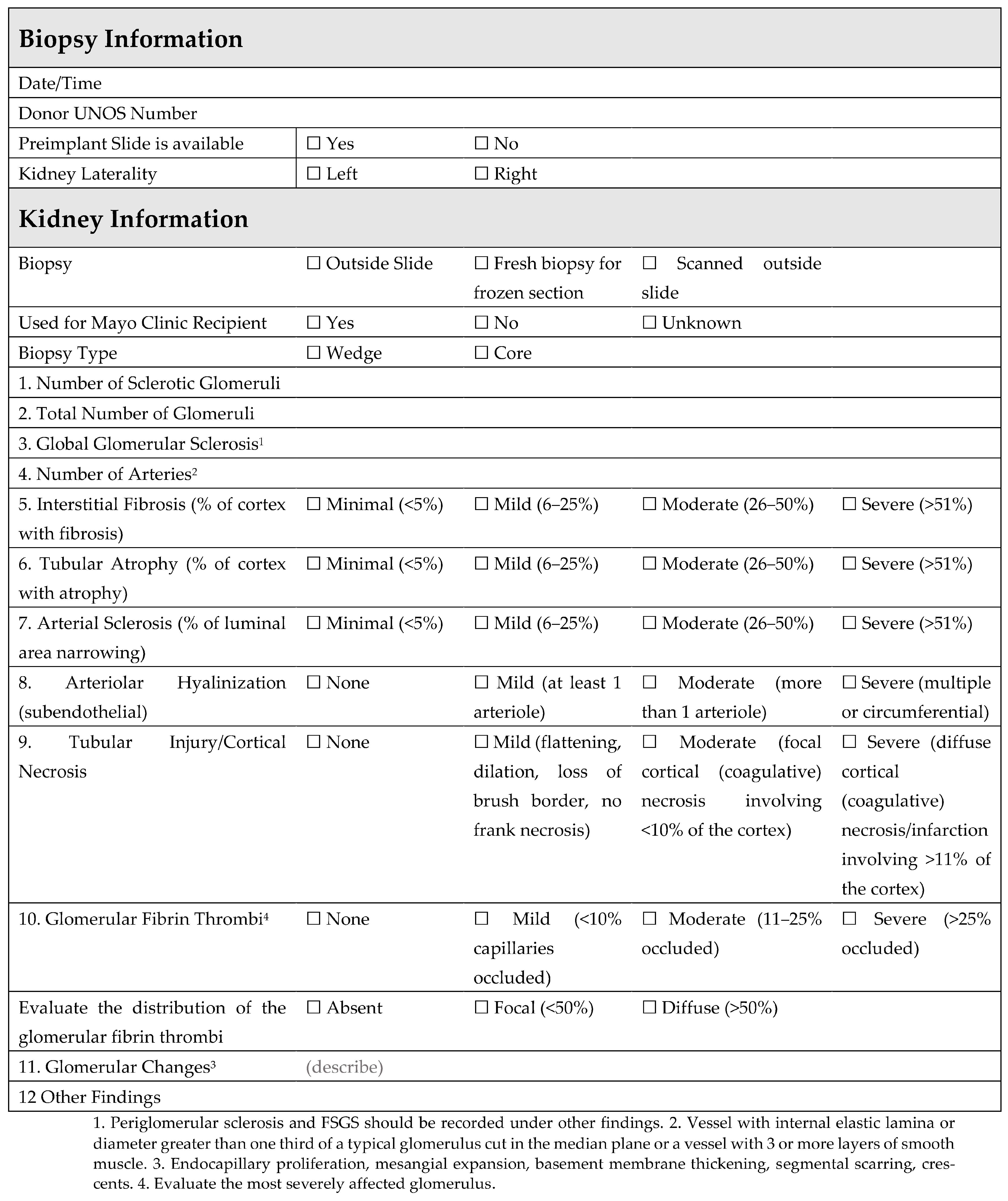
1.4. Role of Procurement Biopsies for AKI Kidneys
1.5. Preservation Methods for AKI Kidneys: Static Cold Storage vs. Machine Perfusion
1.6. Duration of DGF
1.7. DGF Management
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmisano, A.; Gandolfini, I.; Delsante, M.; Cantarelli, C.; Fiaccadori, E.; Cravedi, P.; Maggiore, U. Acute Kidney Injury (AKI) before and after Kidney Transplantation: Causes, Medical Approach, and Implications for the Long-Term Outcomes. J. Clin. Med. 2021, 10, 1484. [Google Scholar]
- de Mendonça, A.; Vincent, J.L.; Suter, P.M.; Moreno, R.; Dearden, N.M.; Antonelli, M.; Takala, J.; Sprung, C.; Cantraine, F. Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000, 26, 915–921. [Google Scholar] [CrossRef]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef]
- Lentine, K.L.; Smith, J.M.; Hart, A.; Miller, J.; Skeans, M.A.; Larkin, L.; Robinson, A.; Gauntt, K.; Israni, A.K.; Hirose, R.; et al. OPTN/SRTR 2020 Annual Data Report: Kidney. Am. J. Transplant. 2022, 22, 21–136. [Google Scholar]
- Boffa, C.; van de Leemkolk, F.; Curnow, E.; Homan van der Heide, J.; Gilbert, J.; Sharples, E.; Ploeg, R.J. Transplantation of Kidneys from Donors with Acute Kidney Injury: Friend or Foe? Am. J. Transplant. 2017, 17, 411–419. [Google Scholar] [CrossRef]
- Heilman, R.L.; Smith, M.L.; Kurian, S.M.; Huskey, J.; Batra, R.K.; Chakkera, H.A.; Katariya, N.N.; Khamash, H.; Moss, A.; Salomon, D.R.; et al. Transplanting Kidneys from Deceased Donors with Severe Acute Kidney Injury. Am. J. Transplant. 2015, 15, 2143–2151. [Google Scholar]
- Heilman, R.L.; Smith, M.L.; Smith, B.H.; Kumar, A.; Srinivasan, A.; Huskey, J.L.; Khamash, H.A.; Jadlowiec, C.C.; Mathur, A.K.; Moss, A.A.; et al. Long-term Outcomes Following Kidney Transplantation from Donors with Acute Kidney Injury. Transplantation 2019, 103, e263. [Google Scholar] [CrossRef]
- Jadlowiec, C.C.; Heilman, R.L.; Smith, M.L.; Khamash, H.A.; Huskey, J.L.; Harbell, J.; Reddy, K.S.; Moss, A.A. Transplanting kidneys from donation after cardiac death donors with acute kidney injury. Am. J. Transplant. 2020, 20, 864–869. [Google Scholar] [CrossRef]
- Dube, G.K.; Brennan, C.; Husain, S.A.; Crew, R.J.; Chiles, M.C.; Cohen, D.J.; Mohan, S. Outcomes of kidney transplant from deceased donors with acute kidney injury and prolonged cold ischemia time—A retrospective cohort study. Transpl. Int. 2019, 32, 646–657. [Google Scholar] [CrossRef]
- Liu, C.; Hall, I.E.; Mansour, S.; Thiessen Philbrook, H.R.; Jia, Y.; Parikh, C.R. Association of Deceased Donor Acute Kidney Injury with Recipient Graft Survival. JAMA Netw. Open 2020, 3, e1918634. [Google Scholar] [CrossRef]
- Yu, K.; King, K.; Husain, S.A.; Dube, G.K.; Stevens, J.S.; Ratner, L.E.; Cooper, M.; Parikh, C.R.; Mohan, S. Kidney nonprocurement in solid organ donors in the United States. Am. J. Transplant. 2020, 20, 3413–3425. [Google Scholar] [CrossRef]
- Cooper, M.; Formica, R.; Friedewald, J.; Hirose, R.; O’Connor, K.; Mohan, S.; Schold, J.; Axelrod, D.; Pastan, S. Report of National Kidney Foundation Consensus Conference to Decrease Kidney Discards. Clin. Transplant. 2019, 33, e13419. [Google Scholar] [CrossRef]
- Lentine, K.L.; Naik, A.S.; Schnitzler, M.A.; Randall, H.; Wellen, J.R.; Kasiske, B.L.; Marklin, G.; Brockmeier, D.; Cooper, M.; Xiao, H.; et al. Variation in use of procurement biopsies and its implications for discard of deceased donor kidneys recovered for transplantation. Am. J. Transplant. 2019, 19, 2241–2251. [Google Scholar] [CrossRef]
- Jadlowiec, C.C.; Hanna, W.A.; Ninan, J.; Ryan, M.S.; Das, D.M.; Smith, M.; Khamash, H.; Mathur, A.K.; Singer, A.; Moss, A.; et al. Transplant outcomes using kidneys from high KDPI acute kidney injury donors. Clin. Transplant. 2021, 35, e14279. [Google Scholar] [CrossRef]
- Establish Minimum Kidney Donor Criteria to Require Biopsy. Available online: https://optn.transplant.hrsa.gov/media/v2zfd4xp/policy-notice_est-min-kid-donor-crit-for-bpsy_kid.pdf (accessed on 10 April 2023).
- King, K.L.; Husain, S.A.; Perotte, A.; Adler, J.T.; Schold, J.D.; Mohan, S. Deceased donor kidneys allocated out of sequence by organ procurement organizations. Am. J. Transplant. 2022, 22, 1372–1381. [Google Scholar] [CrossRef]
- Garner, M.; Jay, C.L.; Sharda, B.; Webb, C.; Farney, A.C.; Orlando, G.; Rogers, J.; Reeves-Daniel, A.; Mena-Gutierrez, A.; Sakhovskaya, N.; et al. Long-term outcomes of kidney transplantation from deceased donors with terminal acute kidney injury: Single center experience and literature review. Clin. Transplant. 2023, 37, e14886. [Google Scholar] [CrossRef]
- Budhiraja, P.; Heilman, R.L.; Jadlowiec, C.C.; Smith, M.L.; Ryan, M.S.; Khamash, H.A.; Kodali, L.; Moss, A.A.; Mathur, A.K.; Reddy, K.S. Successful outcomes with transplanting kidneys from deceased donors with acute kidney injuryon temporary renal replacement therapy. Clin. Transplant. 2021, 35, e14465. [Google Scholar] [CrossRef]
- Heilman, R.L.; Smith, M.L.; Smith, B.H.; Qaqish, I.; Khamash, H.; Singer, A.L.; Kaplan, B.; Reddy, K.S. Progression of Interstitial Fibrosis during the First Year after Deceased Donor Kidney Transplantation among Patients with and without Delayed Graft Function. Clin. J. Am. Soc. Nephrol. 2016, 11, 2225–2232. [Google Scholar] [CrossRef]
- Jiao, B.; An, C.; Du, H.; Tran, M.; Wang, P.; Zhou, D.; Wang, Y. STAT6 Deficiency Attenuates Myeloid Fibroblast Activation and Macrophage Polarization in Experimental Folic Acid Nephropathy. Cells 2021, 10, 3057. [Google Scholar] [CrossRef]
- An, C.; Jiao, B.; Du, H.; Tran, M.; Zhou, D.; Wang, Y. Myeloid PTEN deficiency aggravates renal inflammation and fibrosis in angiotensin II-induced hypertension. J. Cell Physiol. 2022, 237, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Campenot, E.; Chiles, M.C.; Santoriello, D.; Bland, E.; Crew, R.J.; Rosenstiel, P.; Dube, G.; Batal, I.; Radhakrishnan, J.; et al. Association between Reperfusion Renal Allograft Biopsy Findings and Transplant Outcomes. J. Am. Soc. Nephrol. 2017, 28, 3109–3117. [Google Scholar] [CrossRef]
- Kosmoliaptsis, V.; Salji, M.; Bardsley, V.; Chen, Y.; Thiru, S.; Griffiths, M.H.; Copley, H.C.; Saeb-Parsy, K.; Bradley, J.A.; Torpey, N.; et al. Baseline donor chronic renal injury confers the same transplant survival disadvantage for DCD and DBD kidneys. Am. J. Transplant. 2015, 15, 754–763. [Google Scholar] [CrossRef]
- De Vusser, K.; Lerut, E.; Kuypers, D.; Vanrenterghem, Y.; Jochmans, I.; Monbaliu, D.; Pirenne, J.; Naesens, M. The predictive value of kidney allograft baseline biopsies for long-term graft survival. J. Am. Soc. Nephrol. 2013, 24, 1913–1923. [Google Scholar]
- Lu, A.D.; Desai, D.; Myers, B.D.; Dafoe, D.C.; Alfrey, E.J. Severe glomerular sclerosis is not associated with poor outcome after kidney transplantation. Am. J. Surg. 2000, 180, 470–474. [Google Scholar] [CrossRef]
- Kayler, L.K.; Mohanka, R.; Basu, A.; Shapiro, R.; Randhawa, P.S. Correlation of histologic findings on preimplant biopsy with kidney graft survival. Transpl. Int. 2008, 21, 892–898. [Google Scholar] [CrossRef]
- Pokorná, E.; Vítko, S.; Chadimová, M.; Schück, O.; Ekberg, H. Proportion of glomerulosclerosis in procurement wedge renal biopsy cannot alone discriminate for acceptance of marginal donors. Transplantation 2000, 69, 36–43. [Google Scholar] [CrossRef]
- Edwards, E.B.; Posner, M.P.; Maluf, D.G.; Kauffman, H.M. Reasons for non-use of recovered kidneys: The effect of donor glomerulosclerosis and creatinine clearance on graft survival. Transplantation 2004, 77, 1411–1415. [Google Scholar]
- Muruve, N.A.; Steinbecker, K.M.; Luger, A.M. Are wedge biopsies of cadaveric kidneys obtained at procurement reliable? Transplantation 2000, 69, 2384–2388. [Google Scholar] [CrossRef]
- Liapis, H.; Gaut, J.P.; Klein, C.; Bagnasco, S.; Kraus, E.; Farris, A.B., III; Honsova, E.; Perkowska-Ptasinska, A.; David, D.; Goldberg, J.; et al. Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am. J. Transplant. 2017, 17, 140–150. [Google Scholar] [CrossRef]
- Standardize Kidney Biopsy Reporting and Data Collection. Available online: https://optn.transplant.hrsa.gov/policies-bylaws/public-comment/standardize-kidney-biopsy-reporting-and-data-collection/ (accessed on 3 July 2023).
- Batra, R.K.; Heilman, R.L.; Smith, M.L.; Thomas, L.F.; Khamash, H.A.; Katariya, N.N.; Hewitt, W.R.; Singer, A.; Mathur, A.K.; Huskey, J.; et al. Rapid Resolution of Donor-Derived Glomerular Fibrin Thrombi After Deceased Donor Kidney Transplantation. Am. J. Transplant. 2016, 16, 1015–1020. [Google Scholar] [CrossRef]
- Choudhary, D.; Sharma, A.; Singh, S.; Kenwar, D.B.; Walker Minz, R.; Singh Kohli, H.; Nada, R.; Wangkheimayum, S.; Jain, K.; Patil, S.S. Application of Ex Vivo Normothermic Machine Perfusion in Deceased Donors with Acute Kidney Injury with Successful Renal Transplantation: A Preliminary Experience. Transpl. Direct. 2022, 8, e1391. [Google Scholar]
- Bouari, S.; Rijkse, E.; Hosgood, S.A.; Minnee, R.C. Ex situ machine perfusion in kidney transplantation. Artif. Organs. 2022, 46, 173–176. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.; Squifflet, J.P.; van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Thompson, E.R.; Ibrahim, I.K.; Bates, L.; Talbot, D.; Wilson, C.H. Hypothermic machine perfusion is superior to static cold storage in deceased donor kidney transplantation: A meta-analysis. Clin. Transplant. 2020, 34, e13814. [Google Scholar] [CrossRef]
- Gasteiger, S.; Berchtold, V.; Bösmüller, C.; Dostal, L.; Ulmer, H.; Bogensperger, C.; Resch, T.; Rudnicki, M.; Neuwirt, H.; Oberhuber, R.; et al. A Retrospective Propensity Score Matched Analysis Reveals Superiority of Hypothermic Machine Perfusion over Static Cold Storage in Deceased Donor Kidney Transplantation. J. Clin. Med. 2020, 9, 2311. [Google Scholar] [CrossRef]
- Goggins, M.O.; Gaynor, J.J.; Mattiazzi, A.; Figueiro, J.; Burke, G.; Ciancio, G.; Guerra, G. Machine Perfusion Allows Use of High KDPI and Prolonged Cold Ischemia Time in Deceased Donors: A Single Center Effort to Decrease Discard Rates. Am. J. Transplant. 2019, 19 (Suppl. S3). Available online: https://atcmeetingabstracts.com/abstract/machine-perfusion-allows-use-of-high-kdpi-and-prolonged-cold-ischemia-time-in-deceased-donors-a-single-center-effort-to-decrease-discard-rates/ (accessed on 12 April 2023).
- De Deken, J.; Kocabayoglu, P.; Moers, C. Hypothermic machine perfusion in kidney transplantation. Curr. Opin. Organ Transplant. 2016, 21, 294–300. [Google Scholar] [CrossRef]
- Brat, A.; de Vries, K.M.; van Heurn, E.W.E.; Huurman, V.A.L.; de Jongh, W.; Leuvenink, H.G.D.; van Zuilen, A.D.; Haase-Kromwijk, B.J.J.M.; de Jonge, J.; Berger, S.P.; et al. Hypothermic Machine Perfusion as a National Standard Preservation Method for Deceased Donor Kidneys. Transplantation 2022, 106, 1043–1050. [Google Scholar] [CrossRef]
- Ciancio, G.; Gaynor, J.J.; Sageshima, J.; Chen, L.; Roth, D.; Kupin, W.; Guerra, G.; Tueros, L.; Zarak, A.; Hanson, L.; et al. Favorable Outcomes with Machine Perfusion and Longer Pump Times in Kidney Transplantation: A Single-Center, Observational Study. Transplantation 2010, 90, 882. [Google Scholar] [CrossRef]
- Guerra, G. Kidney Transplant Success: Strategic Organ Selection/Optimization and Patient Selection—To Infinity and Beyond; Mayo Clinic Arizona: Phoenix, AZ, USA, 2023. [Google Scholar]
- Liu, C.; Alasfar, S.; Reese, P.P.; Mohan, S.; Doshi, M.D.; Hall, I.E.; Thiessen Philbrook, H.; Jia, Y.; Stewart, D.; Parikh, C.R. Trends in the procurement and discard of kidneys from deceased donors with acute kidney injury. Am. J. Transplant. 2022, 22, 898–908. [Google Scholar] [CrossRef]
- Chandak, P.; Phillips, B.L.; Uwechue, R.; Thompson, E.; Bates, L.; Ibrahim, I.; Sewpaul, A.; Figueiredo, R.; Olsburgh, J.; Hosgood, S.; et al. Dissemination of a novel organ perfusion technique: Ex vivo normothermic perfusion of deceased donor kidneys. Artif. Organs. 2019, 43, E308–E319. [Google Scholar] [PubMed]
- Rijkse, E.; de Jonge, J.; Kimenai, H.J.A.N.; Hoogduijn, M.J.; de Bruin, R.W.F.; van den Hoogen, M.W.F.; IJzermans, J.N.M.; Minnee, R.C. Safety and feasibility of 2 h of normothermic machine perfusion of donor kidneys in the Eurotransplant Senior Program. BJS Open 2021, 5, zraa024. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; von Horn, C.; Gallinat, A.; Kaths, M.; Kribben, A.; Treckmann, J.; Paul, A. First-in-man controlled rewarming and normothermic perfusion with cell-free solution of a kidney prior to transplantation. Am. J. Transplant. 2020, 20, 1192–1195. [Google Scholar] [CrossRef]
- Mazilescu, L.I.; Urbanellis, P.; Kim, S.J.; Goto, T.; Noguchi, Y.; Konvalinka, A.; Reichman, T.W.; Sayed, B.A.; Mucsi, I.; Lee, J.Y.; et al. Normothermic Ex Vivo Kidney Perfusion for Human Kidney Transplantation: First North American Results. Transplantation 2022, 106, 1852. [Google Scholar] [PubMed]
- Elliott, T.R.; Nicholson, M.L.; Hosgood, S.A. Normothermic kidney perfusion: An overview of protocols and strategies. Am. J. Transplant. 2021, 21, 1382–1390. [Google Scholar] [PubMed]
- Pinnelas, R.; Kobashigawa, J.A. Ex vivo normothermic perfusion in heart transplantation: A review of the TransMedics® Organ Care System. Future Cardiol. 2022, 18, 5–15. [Google Scholar]
- Liew, B.; Nasralla, D.; Iype, S.; Pollok, J.M.; Davidson, B.; Raptis, D.A. Liver transplant outcomes after ex vivo machine perfusion: A meta-analysis. Br. J. Surg. 2021, 108, 1409–1416. [Google Scholar]
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic Ex Vivo Lung Perfusion in Clinical Lung Transplantation. N. Engl. J. Med. 2011, 364, 1431–1440. [Google Scholar] [CrossRef]
- Nguyen, M. Early Outcomes and Hospital Resource Utilization After Liver Transplantation: The Impact of Normothermic Mechanical Perfusion in a High Volume U.S. DCD Transplant Center. Hepatology 2022, 76. Available online: https://www.aasld.org/the-liver-meeting/early-outcomes-and-hospital-resource-utilization-after-liver-transplantation (accessed on 11 April 2023).
- Hydrick, T.C.; Zhang, C.; Ruch, B.; Wagler, J.; Kumm, K.; Harbell, J.W.; Hewitt, W.R.; Jadlowiec, C.C.; Katariya, N.N.; Moss, A.A.; et al. Declining Medicare reimbursement in abdominal transplantation from 2000 to 2021. Surgery 2023, 173, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, M.L.; Hosgood, S.A. Renal Transplantation After Ex Vivo Normothermic Perfusion: The First Clinical Study. Am. J. Transplant. 2013, 13, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Budhiraja, P.; Reddy, K.S.; Butterfield, R.J.; Jadlowiec, C.C.; Moss, A.A.; Khamash, H.A.; Kodali, L.; Misra, S.S.; Heilman, R.L. Duration of delayed graft function and its impact on graft outcomes in deceased donor kidney transplantation. BMC Nephrol. 2022, 23, 154. [Google Scholar] [CrossRef]
- Lim, W.H.; Johnson, D.W.; Teixeira-Pinto, A.; Wong, G. Association Between Duration of Delayed Graft Function, Acute Rejection, and Allograft Outcome After Deceased Donor Kidney Transplantation. Transplantation 2019, 103, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.L.; Ibrahim, M.; Greenhall, G.H.B.; Mumford, L.; Dorling, A.; Callaghan, C.J. Effect of delayed graft function on longer-term outcomes after kidney transplantation from donation after circulatory death donors in the United Kingdom: A national cohort study. Am. J. Transplant. 2021, 21, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.T.; Chen, C.B.; Yuan, X.P.; Wang, C.X. Impact of acute kidney injury in donors on renal graft survival: A systematic review and Meta-Analysis. Ren. Fail. 2018, 40, 649–656. [Google Scholar] [CrossRef]
- Yarlagadda, S.G.; Coca, S.G.; Formica, R.N., Jr.; Poggio, E.D.; Parikh, C.R. Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2009, 24, 1039–1047. [Google Scholar] [CrossRef]
- Ravindra, K.V.; Sanoff, S.; Vikraman, D.; Zaaroura, A.; Nanavati, A.; Sudan, D.; Irish, W. Lymphocyte depletion and risk of acute rejection in renal transplant recipients at increased risk for delayed graft function. Am. J. Transplant. 2019, 19, 781–789. [Google Scholar] [CrossRef]
- Jadlowiec, C.C.; Hippen, B.; Gill, J.; Heilman, R.; Stewart, D.; Reddy, K.S.; Mohan, S.; Wiseman, A.; Cooper, M. Current opinions on DGF management practices: A survey of the United States and Canada. Clin. Transplant. 2023, 37, e14949. [Google Scholar] [CrossRef]
- Muth, B.L.; Astor, B.C.; Turk, J.; Mohamed, M.; Parajuli, S.; Kaufman, D.B.; Mandelbrot, D.A.; Djamali, A. Outpatient Management of Delayed Graft Function Is Associated with Reduced Length of Stay Without an Increase in Adverse Events. Am. J. Transplant. 2016, 16, 1604–1611. [Google Scholar] [CrossRef]
- Kim, D.W.; Tsapepas, D.; King, K.L.; Husain, S.A.; Corvino, F.A.; Dillon, A.; Wang, W.; Mayne, T.J.; Mohan, S. Financial impact of delayed graft function in kidney transplantation. Clin. Transplant. 2020, 34, e14022. [Google Scholar] [CrossRef] [PubMed]
- Jadlowiec, C.C.; Frasco, P.; Macdonough, E.; Wagler, J.; Das, D.; Budhiraja, P.; Mathur, A.K.; Katariya, N.; Reddy, K.; Khamash, H.; et al. Association of DGF and Early Readmissions on Outcomes Following Kidney Transplantation. Transpl. Int. 2022, 35, 10849. [Google Scholar] [CrossRef] [PubMed]
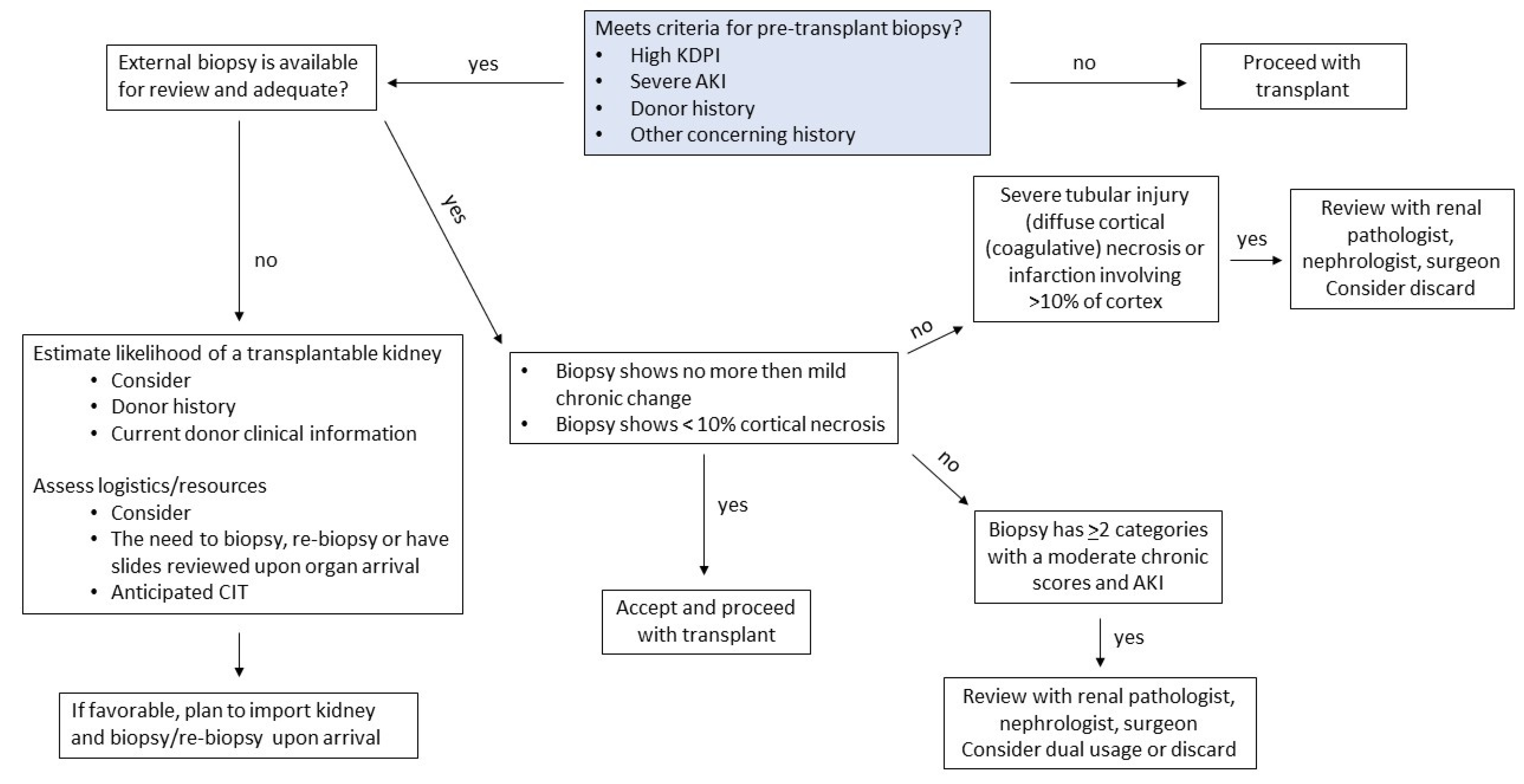

| Organ Procurement Organizations Must Make a Reasonable Effort to Ensure That a Procurement Kidney Biopsy Is Performed for All Donors Meeting Any of the Following Criteria, Excluding Donors Less than 18 Years Old: |
|---|
|
|
|
|
|
| Study | Study Design | Risk of DGF | Duration of DGF | PNF | Acute Rejection | Graft Survival | Economic Burden |
|---|---|---|---|---|---|---|---|
| Moers, 2009 [36] | Paired RCT | ↓ | ↓ | ↔ | ↔ | ↑ | - |
| Tingle, 2020 [37] | Meta-analysis of HMP vs. SCS RCT’s | ↓ | - | ↔ | - | ↑ | ↓ |
| Gasteiger, 2020 [38] | Retrospective propensity score-matched analysis | ↓ | - | - | - | ↔ | - |
| Brat, 2021 [41] | Prospective HMP arm, historical retrospective SCS arm | ↓ | ↓ | - | - | ↔ | - |
| DGF Management |
|---|
Inpatient
|
Outpatient
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punukollu, R.; Ryan, M.; Misra, S.; Budhiraja, P.; Ohara, S.; Kumm, K.; Guerra, G.; Reddy, K.S.; Heilman, R.; Jadlowiec, C.C. Past, Current, and Future Perspectives on Transplanting Acute Kidney Injury Kidneys. Clin. Pract. 2023, 13, 944-958. https://doi.org/10.3390/clinpract13040086
Punukollu R, Ryan M, Misra S, Budhiraja P, Ohara S, Kumm K, Guerra G, Reddy KS, Heilman R, Jadlowiec CC. Past, Current, and Future Perspectives on Transplanting Acute Kidney Injury Kidneys. Clinics and Practice. 2023; 13(4):944-958. https://doi.org/10.3390/clinpract13040086
Chicago/Turabian StylePunukollu, Rachana, Margaret Ryan, Suman Misra, Pooja Budhiraja, Stephanie Ohara, Kayla Kumm, Giselle Guerra, Kunam S. Reddy, Raymond Heilman, and Caroline C. Jadlowiec. 2023. "Past, Current, and Future Perspectives on Transplanting Acute Kidney Injury Kidneys" Clinics and Practice 13, no. 4: 944-958. https://doi.org/10.3390/clinpract13040086
APA StylePunukollu, R., Ryan, M., Misra, S., Budhiraja, P., Ohara, S., Kumm, K., Guerra, G., Reddy, K. S., Heilman, R., & Jadlowiec, C. C. (2023). Past, Current, and Future Perspectives on Transplanting Acute Kidney Injury Kidneys. Clinics and Practice, 13(4), 944-958. https://doi.org/10.3390/clinpract13040086








