Global Prevalence and Associated Clinical Markers of Thrombocytopenia in People Living with HIV: Evidence from Meta-Analysis
Abstract
1. Introduction
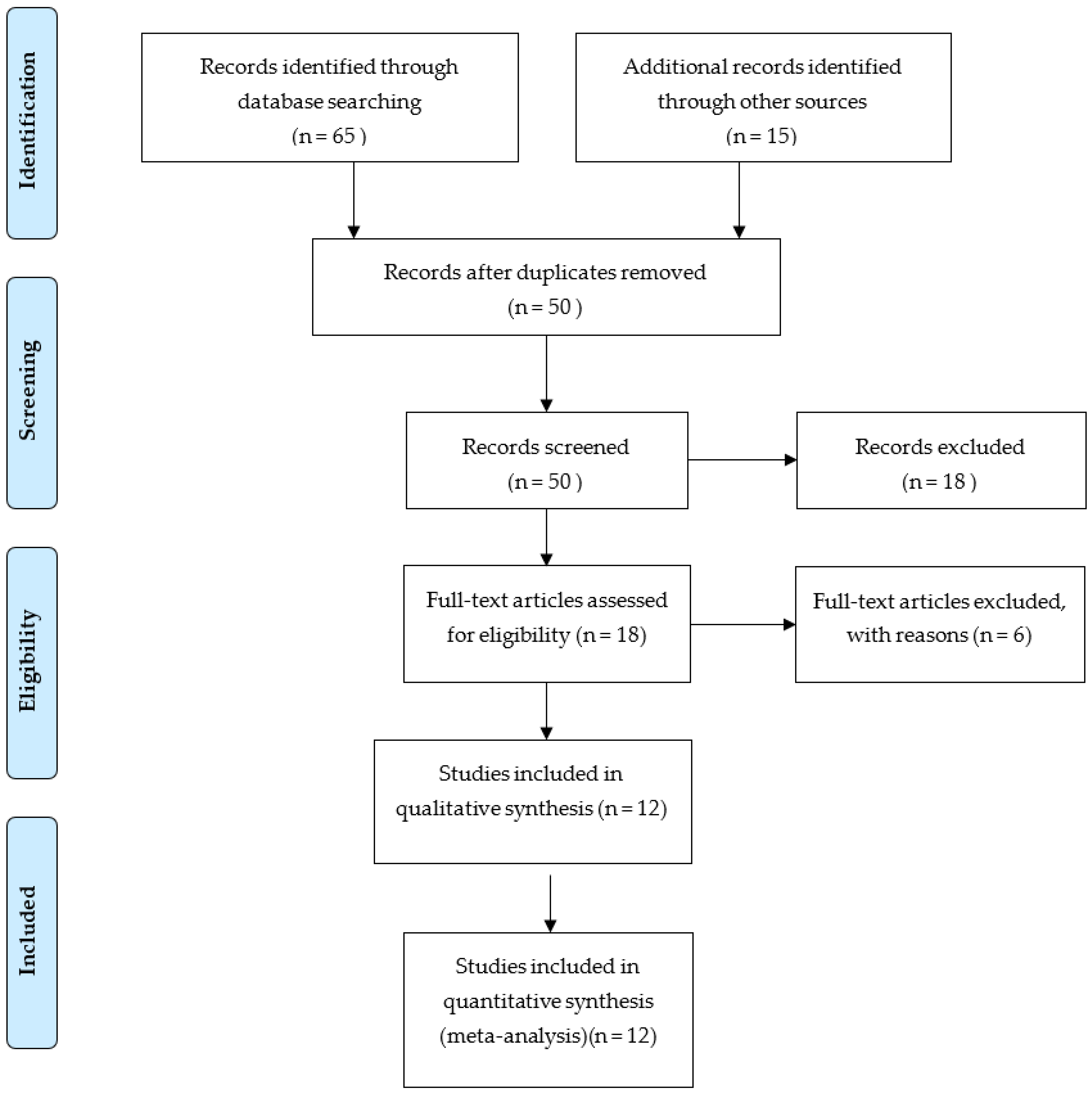
2. Methods
2.1. Search Techniques and Criteria for Selection
2.2. Statistical Analysis
2.3. Quality Assessment
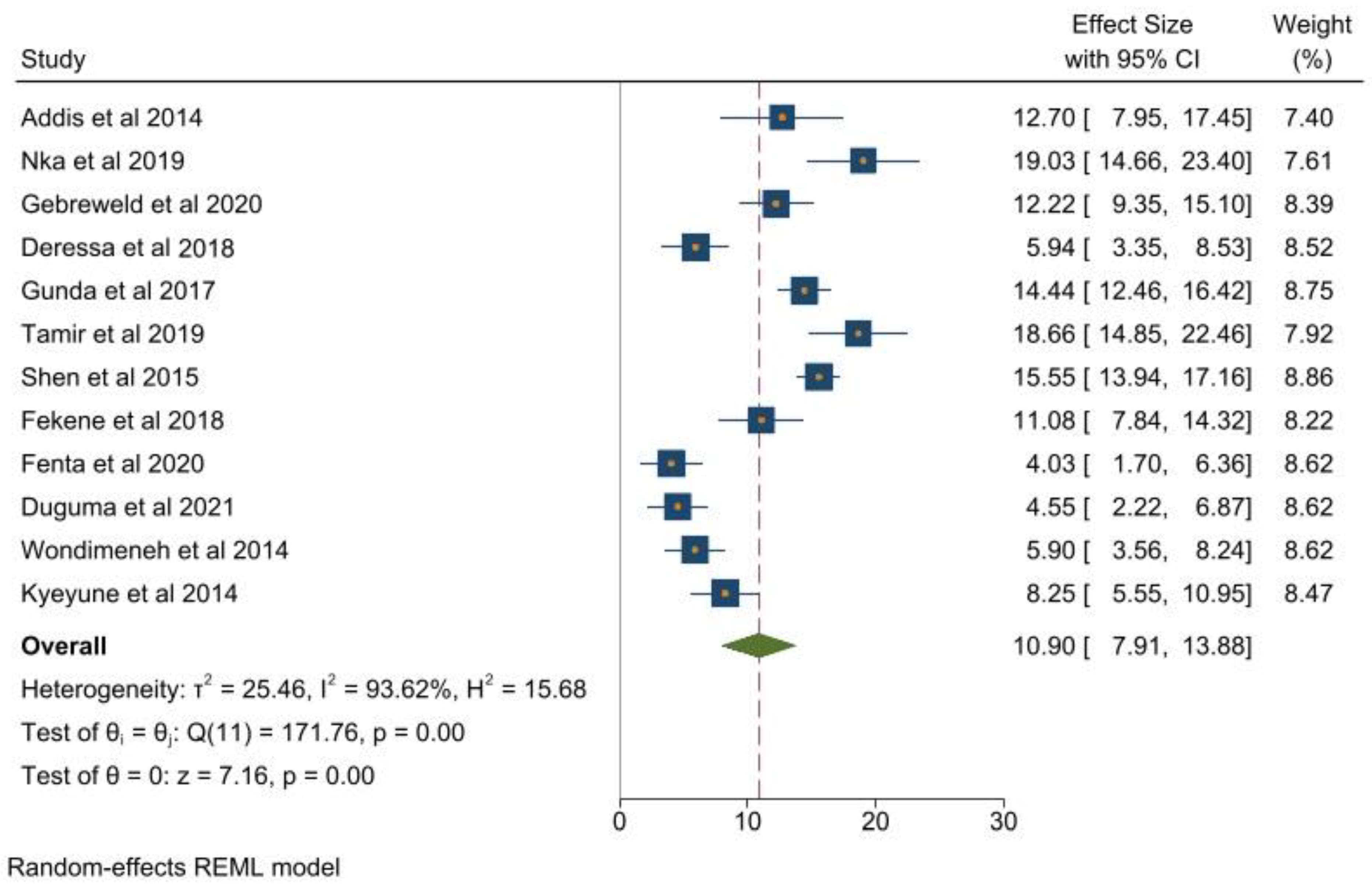
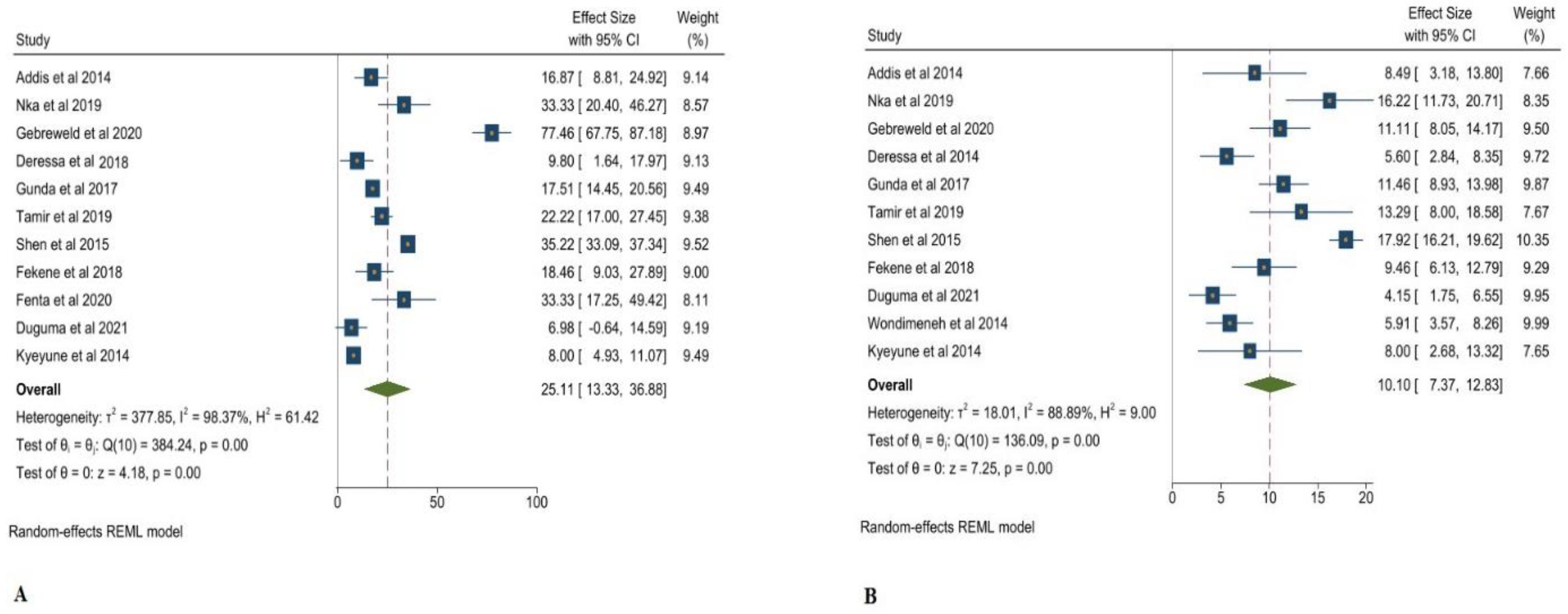
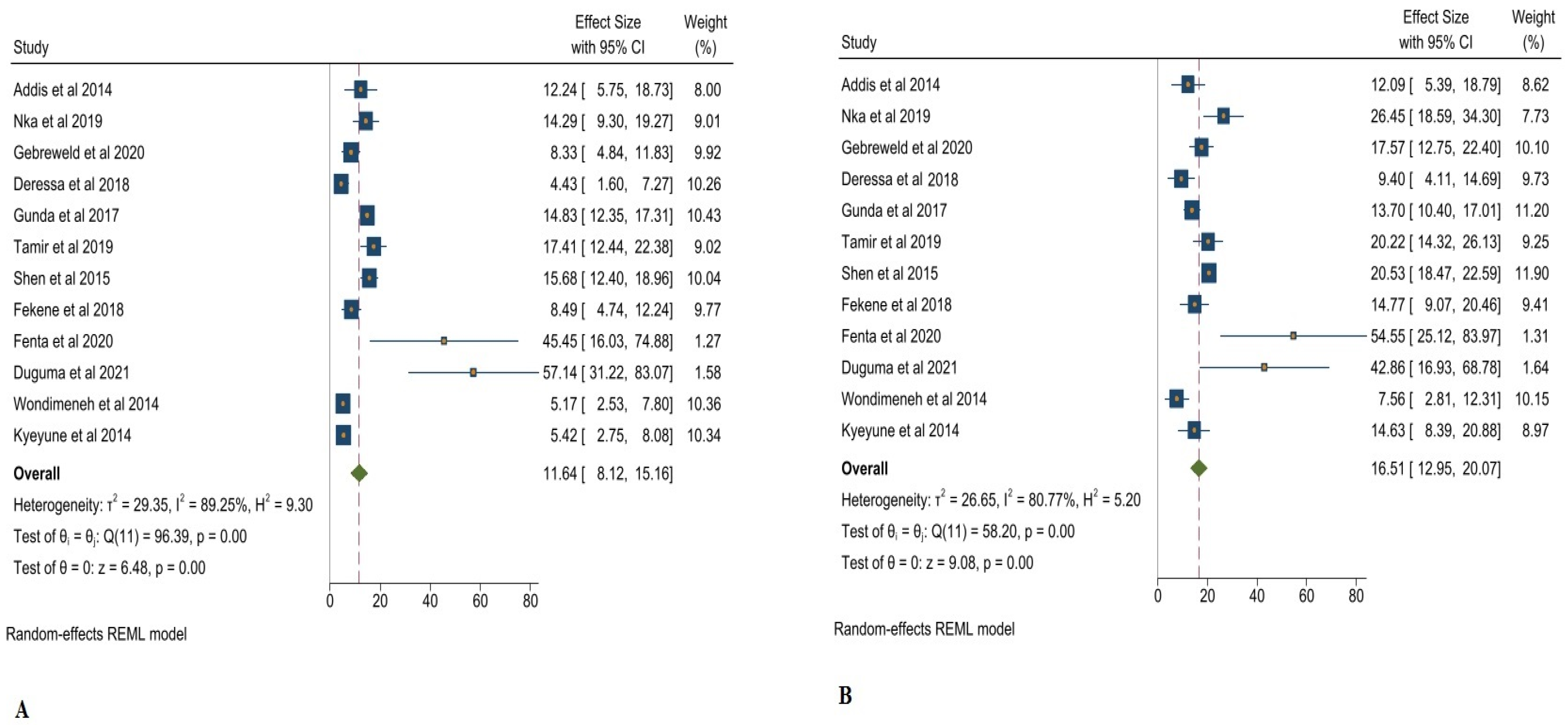
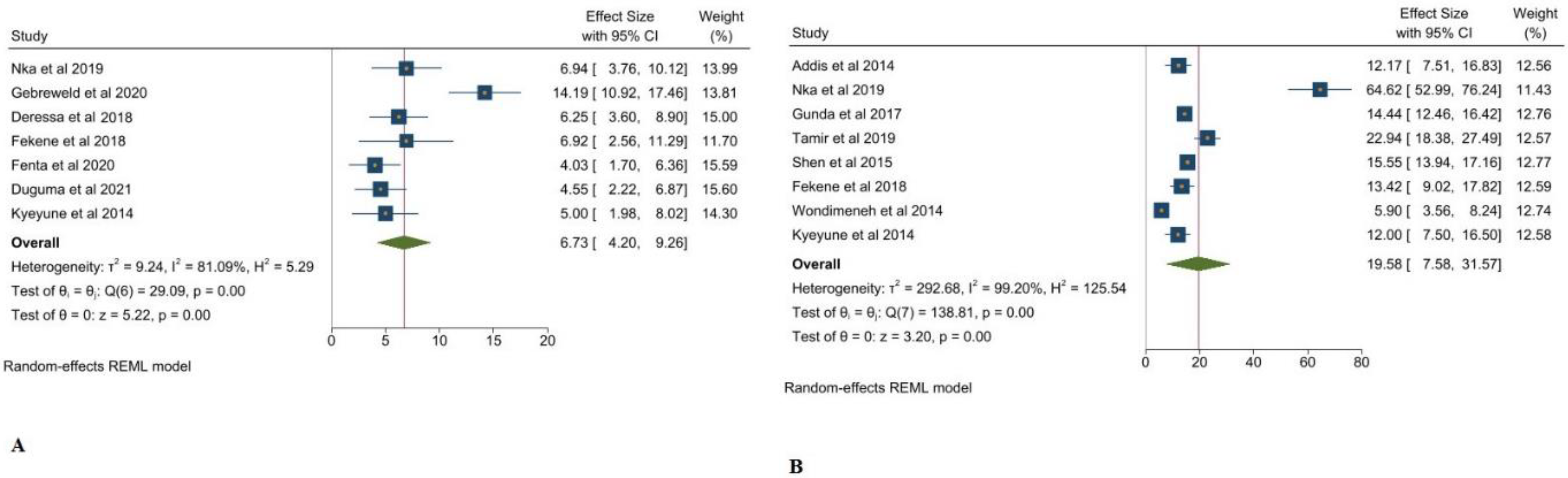
3. Results
3.1. Thrombocytopenia and Its Associated Clinical Markers in Adults Living with HIV
3.2. Publication Bias and Sensitivity Analysis
4. Discussion
4.1. Main Findings
4.2. Interpretation of the Findings
5. Implication
6. Conclusions
7. Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Addis, Z. Prevalence of Some Hematological Abnormalities among HIV Positive Patients on Their First Visit to a Tertiary Health Institution in Ethiopia; A Cross Sectional Study. Int. Blood Res. Rev. 2014, 2, 270–278. [Google Scholar] [CrossRef]
- Woldeamanuel, G.G.; Wondimu, D.H. Prevalence of Thrombocytopenia before and after Initiation of HAART among HIV Infected Patients at Black Lion Specialized Hospital, Addis Ababa, Ethiopia: A Cross Sectional Study. BMC Hematol. 2018, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.B.; Thippeswamy, T.; Shankar, R.; Prathima, C. Hematological Abnormalities in Early and Advanced HIV Infection Patients. Int. J. Sci. Study 2016, 3, 10–15. [Google Scholar]
- Fan, H.-W.; Guo, F.-P.; Li, Y.-J.; Li, N.; Li, T.-S. Prevalence of Thrombocytopenia among Chinese Adult Antiretroviral-Naïve HIV-Positive Patients. Chin. Med. J. 2015, 128, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Sahle, T.; Yemane, T.; Gedefaw, L. Effect of Malaria Infection on Hematological Profiles of People Living with Human Immunodeficiency Virus in Gambella, Southwest Ethiopia. BMC Hematol. 2017, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Almaeen, A.; Wani, F.A.; Thirunavukkarasu, A. Hematologic Derangements in HIV/AIDS Patients and Their Relationship with the CD4 Counts: A Cross-Sectional Study. Int. J. Clin. Exp. Pathol. 2020, 13, 756–763. [Google Scholar] [PubMed]
- Nascimento, F.G.; Tanaka, P.Y. Thrombocytopenia in HIV-Infected Patients. Indian J. Hematol. Blood Transfus. 2012, 28, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Trickey, A.; May, M.T.; Vehreschild, J.-J.; Obel, N.; Gill, M.J.; Crane, H.M.; Boesecke, C.; Patterson, S.; Grabar, S.; Cazanave, C.; et al. Survival of HIV-Positive Patients Starting Antiretroviral Therapy between 1996 and 2013: A Collaborative Analysis of Cohort Studies. Lancet HIV 2017, 4, e349–e356. [Google Scholar] [CrossRef]
- Pereira, B.; Mazzitelli, M.; Milinkovic, A.; Casley, C.; Rubio, J.; Channa, R.; Girometti, N.; Asboe, D.; Pozniak, A.; Boffito, M. Evaluation of a Clinic Dedicated to People Aging with HIV at Chelsea and Westminster Hospital: Results of a 10-Year Experience. AIDS Res. Hum. Retrovir. 2022, 38, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Reibling, N. The International Performance of Healthcare Systems in Population Health: Capabilities of Pooled Cross-Sectional Time Series Methods. Health Policy 2013, 112, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Vrabel, M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Oncol. Nurs. Forum 2015, 42, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata Command to Perform Meta-Analysis of Binomial Data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Riitano, D.; Lisy, K. The Development of a Critical Appraisal Tool for Use in Systematic Reviews Addressing Questions of Prevalence. Int. J. Health Policy Manag. 2014, 3, 123–128. [Google Scholar] [CrossRef]
- Gebreweld, A.; Fiseha, T.; Girma, N.; Haileslasie, H.; Gebretsadik, D. Prevalence of Cytopenia and Its Associated Factors among HIV Infected Adults on Highly Active Antiretroviral Therapy at Mehal Meda Hospital, North Shewa Zone, Ethiopia. PLoS ONE 2020, 15, e0239215. [Google Scholar] [CrossRef]
- Deressa, T.; Damtie, D.; Workineh, M.; Genetu, M.; Melku, M. Anemia and Thrombocytopenia in the Cohort of HIV-Infected Adults in Northwest Ethiopia: A Facility-Based Cross-Sectional Study. EJIFCC 2018, 29, 36–47. [Google Scholar]
- Tamir, Z.; Seid, A.; Haileslassie, H. Magnitude and Associated Factors of Cytopenias among Antiretroviral Therapy Naïve Human Immunodeficiency Virus Infected Adults in Dessie, Northeast Ethiopia. PLoS ONE 2019, 14, e0211708. [Google Scholar] [CrossRef]
- Fekene, T.E.; Juhar, L.H.; Mengesha, C.H.; Worku, D.K. Prevalence of Cytopenias in Both HAART and HAART Naïve HIV Infected Adult Patients in Ethiopia: A Cross Sectional Study. BMC Hematol. 2018, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Fenta, D.A.; Wube, T.B.; Nuru, M.M. Hematological and Immunological Abnormalities among Children Receiving Highly Active Antiretroviral Therapy at Hawassa University College of Medicine and Health Sciences, Southern Ethiopia. Res. Sq. 2020, 1, 1–10. [Google Scholar]
- Wondimeneh, Y.; Muluye, D.; Ferede, G. Prevalence and Associated Factors of Thrombocytopenia among HAART Naive HIV Positive Patients at Gondar University Hospital, Northwest Ethiopia. BMC Res. Notes 2014, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Duguma, N.; Tesfaye Kiya, G.; Adissu Maleko, W.; Bimerew, L.G. Hematological Parameters Abnormalities and Associated Factors in HIV-Positive Adults before and after Highly Active Antiretroviral Treatment in Goba Referral Hospital, Southeast Ethiopia: A Cross-Sectional Study. SAGE Open Med. 2021, 9, 205031212110201. [Google Scholar] [CrossRef] [PubMed]
- Nka, A.D.; Sosso, S.M.; Fokam, J.; Bouba, Y.; Teto, G.; Simo Rachel, R.; Tiga, A.; Yimga, J.; Nukenine, E.N.; Nanfack, A.J.; et al. Thrombocytopenia According to Antiretroviral Drug Combinations, Viremia and CD4 Lymphocytes among HIV-Infected Patients in Cameroon: A Snapshot from the City of Yaoundé. BMC Res. Notes 2019, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, J.; Wang, Z.; Shen, J.; Qi, T.; Song, W.; Tang, Y.; Liu, L.; Zhang, R.; Zeng, Y.; et al. A Cross-Sectional Study of Leukopenia and Thrombocytopenia among Chinese Adults with Newly Diagnosed HIV/AIDS. Biosci. Trends 2015, 9, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Gunda, D.W.; Godfrey, K.G.; Kilonzo, S.B.; Mpondo, B.C. Cytopenias among ART-Naive Patients with Advanced HIV Disease on Enrolment to Care and Treatment Services at a Tertiary Hospital in Tanzania: A Crosssectional Study. Malawi Med. J. 2017, 29, 43. [Google Scholar] [CrossRef] [PubMed]
- Kyeyune, R.; Saathoff, E.; Ezeamama, A.E.; Löscher, T.; Fawzi, W.; Guwatudde, D. Prevalence and Correlates of Cytopenias in HIV-Infected Adults Initiating Highly Active Antiretroviral Therapy in Uganda. BMC Infect. Dis. 2014, 14, 496. [Google Scholar] [CrossRef]
- Miguez-Burbano, M.; Jackson, J., Jr.; Hadrigan, S. Thrombocytopenia in HIV Disease: Clinical Relevance, Physiopathology and Management. Curr. Med. Chem. Hematol. Agents 2005, 3, 365–376. [Google Scholar] [CrossRef]
- O’Bryan, T.A.; Okulicz, J.F.; Bradley, W.P.; Ganesan, A.; Wang, X.; Agan, B.K. Impact of the Highly Active Antiretroviral Therapy Era on the Epidemiology of Primary HIV-Associated Thrombocytopenia. BMC Res. Notes 2015, 8, 595. [Google Scholar] [CrossRef]
- Moh, R.; Danel, C.; Sorho, S.; Sauvageot, D.; Anzian, A.; Minga, A.; Gomis, O.B.; Kanga, C.; Inwoley, A.; Gabillard, D.; et al. Haematological Changes in Adults Receiving a Zidovudine-Containing Haart Regimen in Combination with Cotrimoxazole in Côte D’ivoire. Antivir. Ther. 2005, 10, 615–624. [Google Scholar] [CrossRef] [PubMed]
- The Swiss Group for Clinical Studies on the Acquired Immunodeficiency Syndrome (AIDS). Hirsche Zidovudine for the Treatment of Thrombocytopenia Associated with Human Immunodeficiency Virus (HIV). Ann. Intern. Med. 1988, 109, 718. [Google Scholar] [CrossRef] [PubMed]
- Talargia, F.; Getacher, L. Thrombocytopenia and Associated Factors Among HIV Infected Patients in Pre- and Post-Anti-Retroviral Therapy, North East Ethiopia. J. Blood Med. 2021, 12, 741–748. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Inclusion Criteria |
|---|---|
| Participants | HIV patients (aged 18 years or older) |
| Interventions |
|
| Comparators |
|
| Outcomes | Event of thrombocytopenia |
| Study designs | Cross sectional study |
| Study Omitted | Estimated (95% CI) | Heterogeneity | |
|---|---|---|---|
| I2 p-Value | |||
| Addis et al. 2014 [1] | 10.76 (7.54–13.98) | 99.67 | ≤0.001 |
| Nka et al. 2019 [25] | 10.22 (7.32–13.12) | 93.08 | ≤0.001 |
| Gebreweld et al. 2020 [18] | 10.22 (7.53–14.05) | 94.26 | ≤0.001 |
| Deressa et al. 2014 [19] | 11.36 (8.23–14.49) | 93.64 | ≤0.001 |
| Gunda et al. 2017 [27] | 10.56 (7.37–13.76) | 93.52 | ≤0.001 |
| Tamir et al. 2019 [20] | 10.22 (7.31–13.13) | 93.03 | ≤0.001 |
| Shen et al. 2015 [26] | 10.44 (7.31–13.57) | 92.79 | ≤0.001 |
| Fekene et al. 2018 [21] | 10.89 (7.63–14.16) | 94.38 | ≤0.001 |
| Fenta et al. 2020 [22] | 11.53 (8.56–14.51) | 92.81 | ≤0.001 |
| Duguma et al. 2021 [24] | 11.49 (8.47–14.51) | 93.03 | ≤0.001 |
| Wondimeneh et al. 2014 [23] | 11.37 (8.24–14.49) | 93.50 | ≤0.001 |
| Kyeyune et al. 2014 [28] | 11.15 (7.92–14.39) | 94.11 | ≤0.001 |
| Combined | 10.90 (7.91–13.88) | 93.62 | ≤0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkholifi, F.K.; Abdi, S.A.H.; Qadri, M. Global Prevalence and Associated Clinical Markers of Thrombocytopenia in People Living with HIV: Evidence from Meta-Analysis. Clin. Pract. 2022, 12, 867-875. https://doi.org/10.3390/clinpract12060091
Alkholifi FK, Abdi SAH, Qadri M. Global Prevalence and Associated Clinical Markers of Thrombocytopenia in People Living with HIV: Evidence from Meta-Analysis. Clinics and Practice. 2022; 12(6):867-875. https://doi.org/10.3390/clinpract12060091
Chicago/Turabian StyleAlkholifi, Faisal K., Sayed Aliul Hasan Abdi, and Marwa Qadri. 2022. "Global Prevalence and Associated Clinical Markers of Thrombocytopenia in People Living with HIV: Evidence from Meta-Analysis" Clinics and Practice 12, no. 6: 867-875. https://doi.org/10.3390/clinpract12060091
APA StyleAlkholifi, F. K., Abdi, S. A. H., & Qadri, M. (2022). Global Prevalence and Associated Clinical Markers of Thrombocytopenia in People Living with HIV: Evidence from Meta-Analysis. Clinics and Practice, 12(6), 867-875. https://doi.org/10.3390/clinpract12060091






