A Case of Severe Hypoxia Caused by Phenazopyridine-Induced Methemoglobinemia: A near Fatal Event from Over-the-Counter Medication Use
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cortazzo, J.A.; Lichtman, A.D. Methemoglobinemia: A review and recommendations for management. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Darling, R.C.; Roughton, F.J. The effect of methemoglobin on the equilibrium between oxygen and hemoglobin. Am. J. Physiol. 1942, 137, 56–68. [Google Scholar] [CrossRef]
- Rehman, H.U. Evidence-based case review: Methemoglobinemia. West J. Med. 2001, 175, 193. [Google Scholar] [CrossRef] [PubMed]
- Ash-Bernal, R.; Wise, R.; Wright, S.M. Acquired methemoglobinemia: A retrospective series of 138 cases at 2 teaching hospitals. Medicine 2004, 83, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Shahani, L.; Sattovia, S. Acquired methaemoglobinaemia related to phenazopyridine ingestion. BMJ Case Rep. 2012, 2012, bcr2012006756. [Google Scholar] [CrossRef] [PubMed]
- Gibson, Q. Introduction: Congenital methemoglobinemia revisited. Blood 2002, 100, 3445–3446. [Google Scholar] [CrossRef] [PubMed]
- Prchal, J.T. Methemoglobinemia and other dyshemoglobinemias. In Williams Hematology, 10th ed.; McGraw Hill: New York, NY, USA, 2021; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2962§ionid=252529476 (accessed on 29 August 2022).
- Singh, N.K.; Mirza, N. Elderly woman with orange urine and purple hands. Mayo. Clin. Proc. 2008, 83, 744. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Davis, N.; Hsieh, R.; Stallard, S. Methemoglobinaemia with chronic phenazopyridine ingestion. BMJ Case Rep. 2019, 12, 744. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Wang, C.H.; Chang, C.C. Chocolate-colored blood with normal artery oxygen: Methemoglobinemia related to phenazopyridine. Am. J. Med. Sci. 2011, 341, 337. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Fernandez, M. Acquired methemoglobinemia from phenazopyridine use. Int. J. Emerg. Med. 2018, 11, 45. [Google Scholar] [CrossRef]
- Hamza, A.; Nasrullah, A.; Singh, R.; DiSilvio, B. Phenazopyridine-Induced Methaemoglobinaemia The Aftermath of Dysuria Treatment. Eur. J. Case Rep. Intern. Med. 2022, 9, 003191. [Google Scholar] [CrossRef] [PubMed]
- Banimahd, F.; Loo, T.; Amin, M.; Ahadiat, O.R.; Chakravarthy, B.; Lotfipour, S. A Rare but Important Clinical Presentation of Induced Methemoglobinemia. West J. Emerg. Med. 2016, 17, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.K.; Kraft, K.H. Perioperative hypoxia secondary to phenazopyridine-induced methemoglobinemia in an adolescent patient without renal insufficiency or overdose: An unusual case. Urology 2019, 130, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Shatila, W.; Clark, A. Unexplained hypoxia in a woman presenting with acute on chronic abdominal pain. Am. J. Med. 2013, 126, e1–e2. [Google Scholar] [CrossRef]
- Iolascon, A.; Bianchi, P.; Andolfo, I.; Russo, R.; Barcellini, W.; Fermo, E.; Toldi, G.; Ghirardello, S.; Rees, D.; Van Wijk, R.; et al. Recommendations for diagnosis and treatment of methemoglobinemia. Am. J. Hemat. 2021, 96, 1666–1678. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Shailesh, F.; Tiwari, U.; Sharma, S.G.; Malik, B. Phenazopyridine associated acute interstitial nephritis and review of literature. Ren. Fail. 2014, 36, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.J.; Tremper, K.K.; Hyatt, J. Effects of methemoglobinemia on pulse oximetry and mixed venous oximetry. Anesthesiology 1989, 70, 112–117. [Google Scholar] [CrossRef]
- Tejesh, C.A.; Shivanna, S.; Manjunath, A.C.; Prathima, P.T. ICU management of methemoglobinemia due to unknown compound poisoning. J. Anaesthesiol. Clin. Pharmacol. 2013, 29, 139. [Google Scholar] [CrossRef]
- Singh, P.; Rakesh, K.; Agarwal, R.; Tripathi, P.P.; Dhooria, S.; Sehgal, I.S.; Prasad, K.T.; Hans, R.; Sharma, R.; Sharma, N.; et al. Therapeutic whole blood exchange in the management of methaemoglobinemia: Case series and systematic review of literature. Transfus. Med. 2020, 30, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Park, S.W.; Han, S.K.; bin Kim, H.; Yeom, S.R. A case of methemoglobinemia successfully treated with hyperbaric oxygenation monotherapy. J. Emerg. Med. 2017, 53, 685–687. [Google Scholar] [CrossRef] [PubMed]
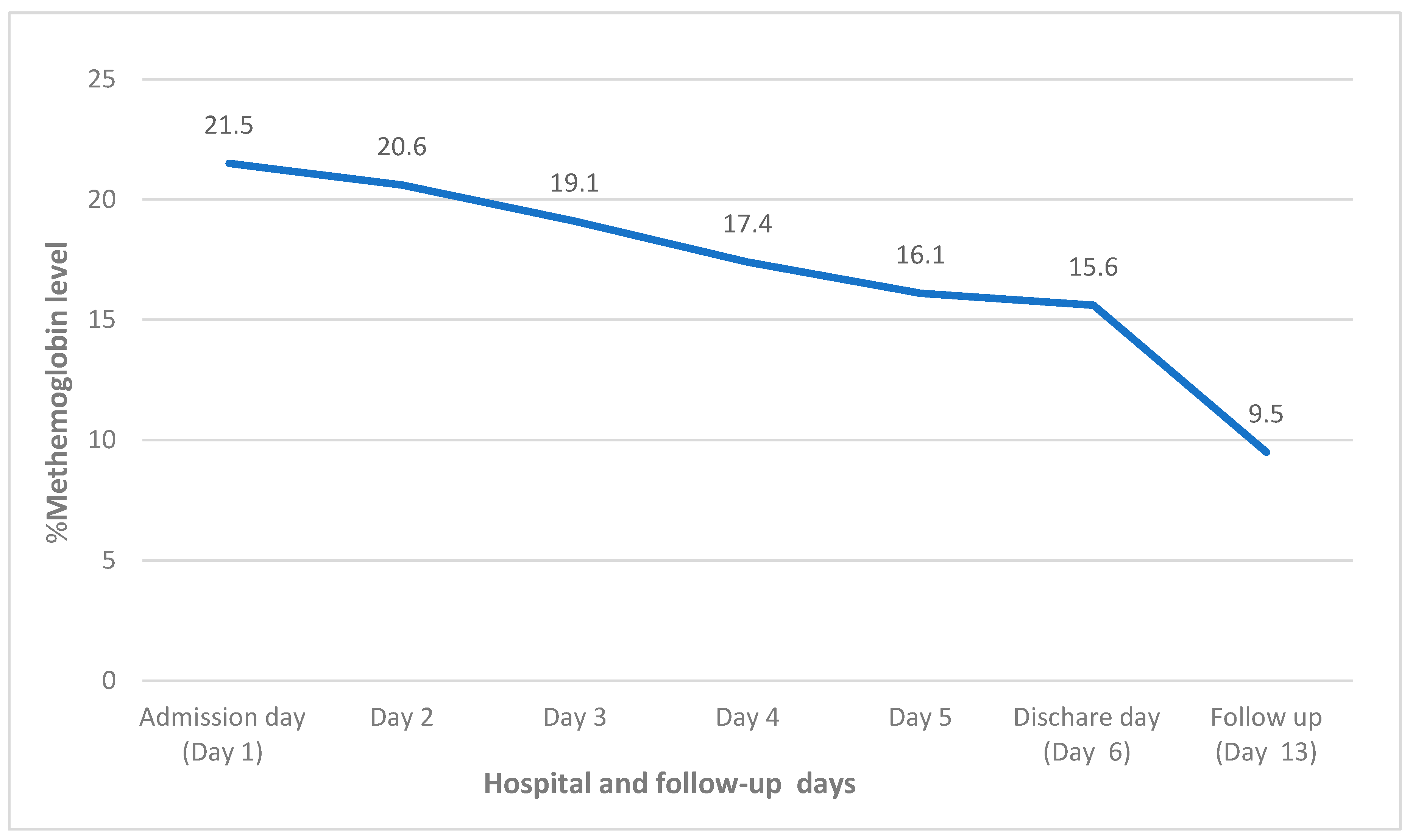
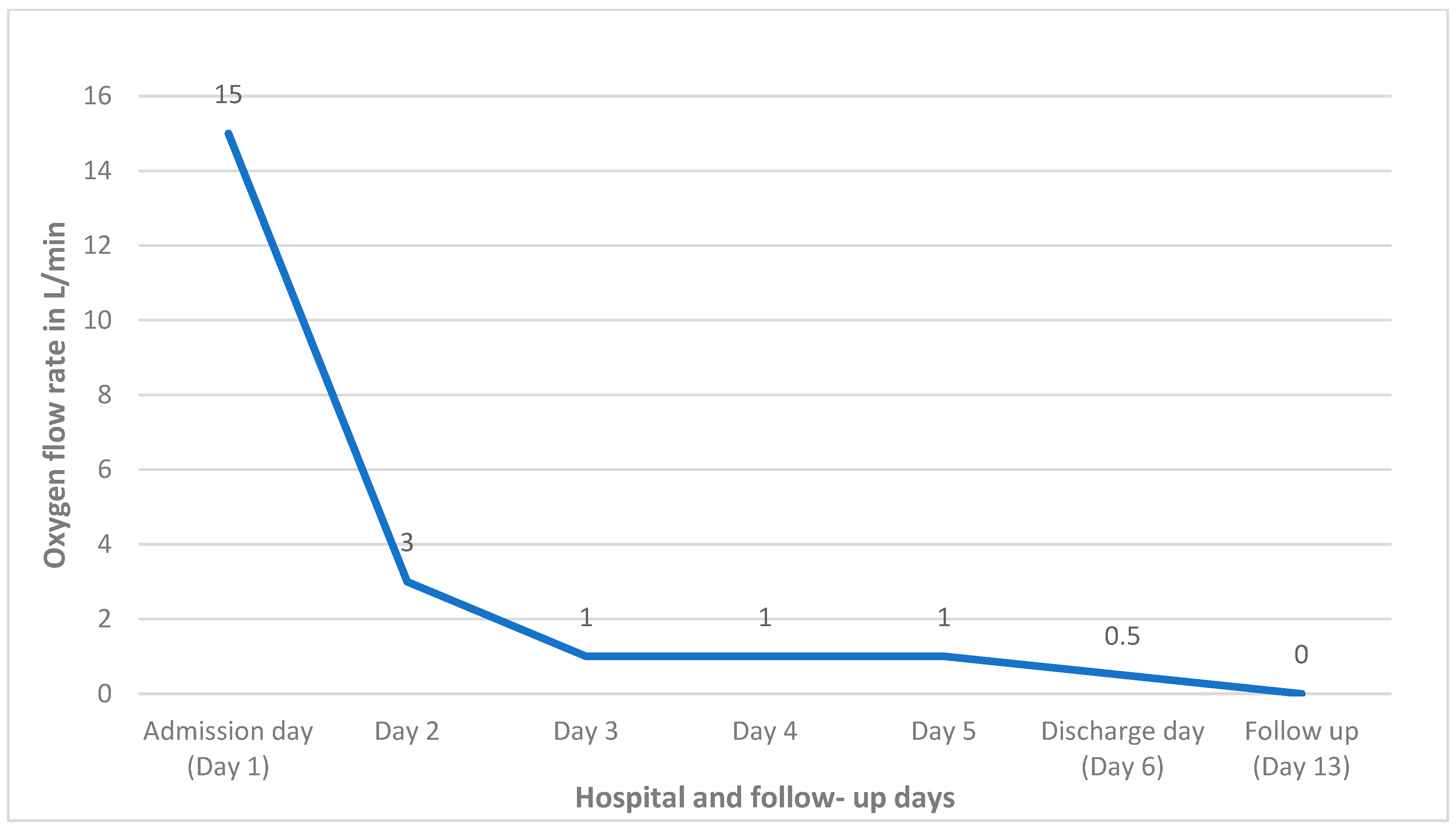
| Variables | References | Observed Value |
|---|---|---|
| pH, arterial blood | 7.35–7.45 units | 7.39 units |
| pCO2, arterial | 32–45 mmHg | 43 mmHg |
| pO2, arterial | 70–100 mmHg | 336 mmHg |
| HCO3, arterial | 21–28 mM/L | 26 mM/L |
| Hemoglobin | 12.0–15.5 gm/dL | 10.3 gm/dL |
| White blood cell count | 4.0–10.0 × 103/µL | 7.8 × 103/µL |
| Platelets | 150–450 × 103/µL | 232 × 103/µL |
| Sodium | 135–146 mEq/L | 138 mEq/L |
| Potassium | 3.5–5.3 mEq/L | 4.9 mEq/L |
| Chloride | 98–110 mEq/L | 107 mEq/L |
| Bicarbonate | 21–30 mM/L | 24 mM/L |
| Blood urea nitrogen | 7.0–30 mg/dL | 54 mg/dL |
| Creatinine | 0.55–1.02 mg/dL | 1.35 mg/dL |
| Glucose | 65–99 mg/dL | 99 mg/dL |
| Glomerular filtration rate (GFR) | CKD if < 60 mL/min/1.73 sq m | 35 mL/min/1.73 sq |
| D-dimer | <230 ng/mL | <150 ng/mL |
| Chronic heart failure peptide (BNP) | 0–100.0 pg/mL | 30.0 pg/mL |
| Procalcitonin | <0.10 ng/mL | 0.14 ng/mL |
| Methemoglobin | 0–1.5% | 21.5% |
| Carboxyhemoglobin | <1.5% non-smoker <5.0% smoker | 0.0 |
| Methemoglobin Level | Symptoms |
|---|---|
| <0–3% (mean: 1%) | Normal range for adults |
| 3–12% | Minimal level, cyanosis may present at level above 5–10% |
| 13–20% | Usually asymptomatic unless preexisting condition such as anemia, heart and lung disease are present that may exacerbate toxicity |
| 20–50% | Mild to moderate symptoms such as lightheadedness, fatigue, tachycardia, dyspnea, and lethargy |
| 50–70% | Severe symptoms such as altered sensorium, respiratory depression, shock, seizures and coma |
| >70% | Usually fatal |
| Author | Age/Sex | Comorbidities | Symptoms | Dose of Phenzopyridine | Methemoglobin Levels | Treatment |
|---|---|---|---|---|---|---|
| Shahani L. et al. [5] | 74/F | Chronic obstructive pulmonary disease, hypertension | Shortness of breath | Phenazopyridine for a month | 18.9% | Methylene blue |
| Singh N.K. et al. [8] | 79/F | Recurrent urinary tract infection | Purple hands, hypoxia | 200 mg 3 times daily for 10 days | 11.8% | Methylene blue |
| Agrawal A. et al. [9] | 55/F | Multiple sclerosis, neurogenic bladder | Lethargy, suprapubic pain and hypoxia | 200 mg TIDS for 5 months | 9.3% | Pyridium discontinued |
| Yu C.H. et al. [10] | 78/F | CKD stage III | Purple lips and hands, hypoxia | Phenazopyridine for 5 days | 37.4% | Methylene blue |
| Murphy T. et al. [11] | 57/F | Breast cancer, seizures, recent dysuria | Lethargy, light-headedness, headache, hypoxia. | 24 tablets in 48 h | 24.2% | Methylene blue |
| Hamza A. et al. [12] | 55/F | Urinary retention with chronic foley catheter, pulmonary embolism | Dusky skin and hypoxia | 400 mg TID for 2 months | 22% | Vitamin C used as methylene blue was contraindicated due to use of mirtazapine |
| Banimah F. et al. [13] | 32/F | No comorbidities | Lower abdominal pain, dysuria | 1 tablet 3 times daily for 4 days | 11.9% | Methylene blue |
| Shah P.K. et al. [14] | 17/M | Obesity, cerebral palsy, muscular dystrophy | Desaturation with higher oxygen requirement | 200 mg three times daily for 2 days | 15.8% | Phenazopyridine discontinued |
| Shatia W. et al. [15] | 28/F | Chronic interstitial cystitis | Acute on chronic suprapubic pain, hypoxia | 200 mg 3–4 tablets daily | 22.2% | Methylene blue, riboflavin and ascorbic acid. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
KC, O.; Subedi, A.; Sharma, R.; Dahal, P.H.; Koirala, M. A Case of Severe Hypoxia Caused by Phenazopyridine-Induced Methemoglobinemia: A near Fatal Event from Over-the-Counter Medication Use. Clin. Pract. 2022, 12, 845-851. https://doi.org/10.3390/clinpract12060089
KC O, Subedi A, Sharma R, Dahal PH, Koirala M. A Case of Severe Hypoxia Caused by Phenazopyridine-Induced Methemoglobinemia: A near Fatal Event from Over-the-Counter Medication Use. Clinics and Practice. 2022; 12(6):845-851. https://doi.org/10.3390/clinpract12060089
Chicago/Turabian StyleKC, Ojbindra, Ananta Subedi, Rakshya Sharma, Punya Hari Dahal, and Manisha Koirala. 2022. "A Case of Severe Hypoxia Caused by Phenazopyridine-Induced Methemoglobinemia: A near Fatal Event from Over-the-Counter Medication Use" Clinics and Practice 12, no. 6: 845-851. https://doi.org/10.3390/clinpract12060089
APA StyleKC, O., Subedi, A., Sharma, R., Dahal, P. H., & Koirala, M. (2022). A Case of Severe Hypoxia Caused by Phenazopyridine-Induced Methemoglobinemia: A near Fatal Event from Over-the-Counter Medication Use. Clinics and Practice, 12(6), 845-851. https://doi.org/10.3390/clinpract12060089





