Portal Vein Thrombosis after C-Section in a Patient with Polycythemia Vera (PV) Due to Pregnancy and Iron Deficiency Anemia (IDA)
Abstract
1. Introduction
2. Case Report
Upper Abdominal U/S Results
3. Discussion
4. Major Criteria
- Red cell mass above average expected value by more than 25%, hemoglobin above 16.5 g/dL in males and above 16 g/dL in women or hematocrit above 49% in men and above 48% in women.
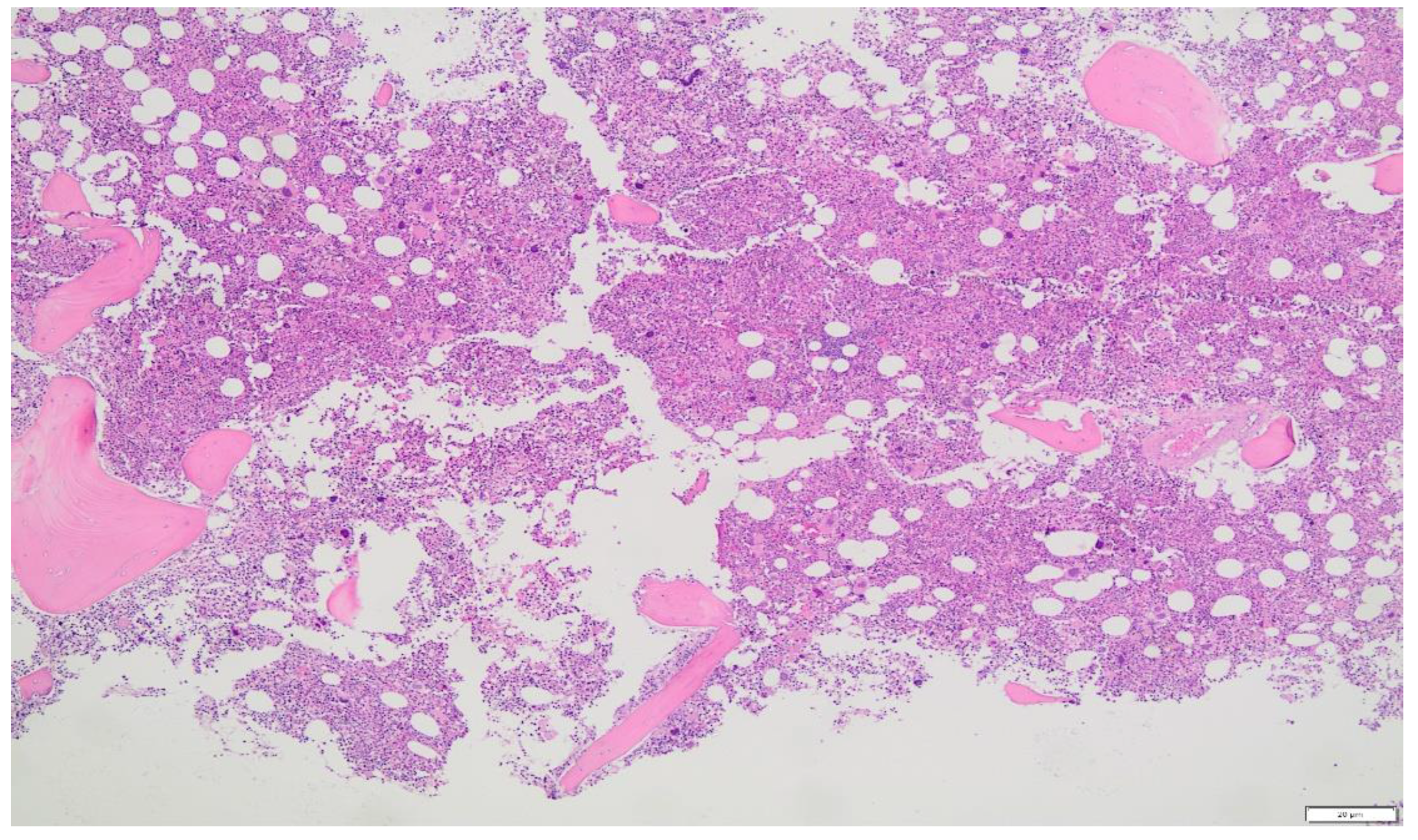
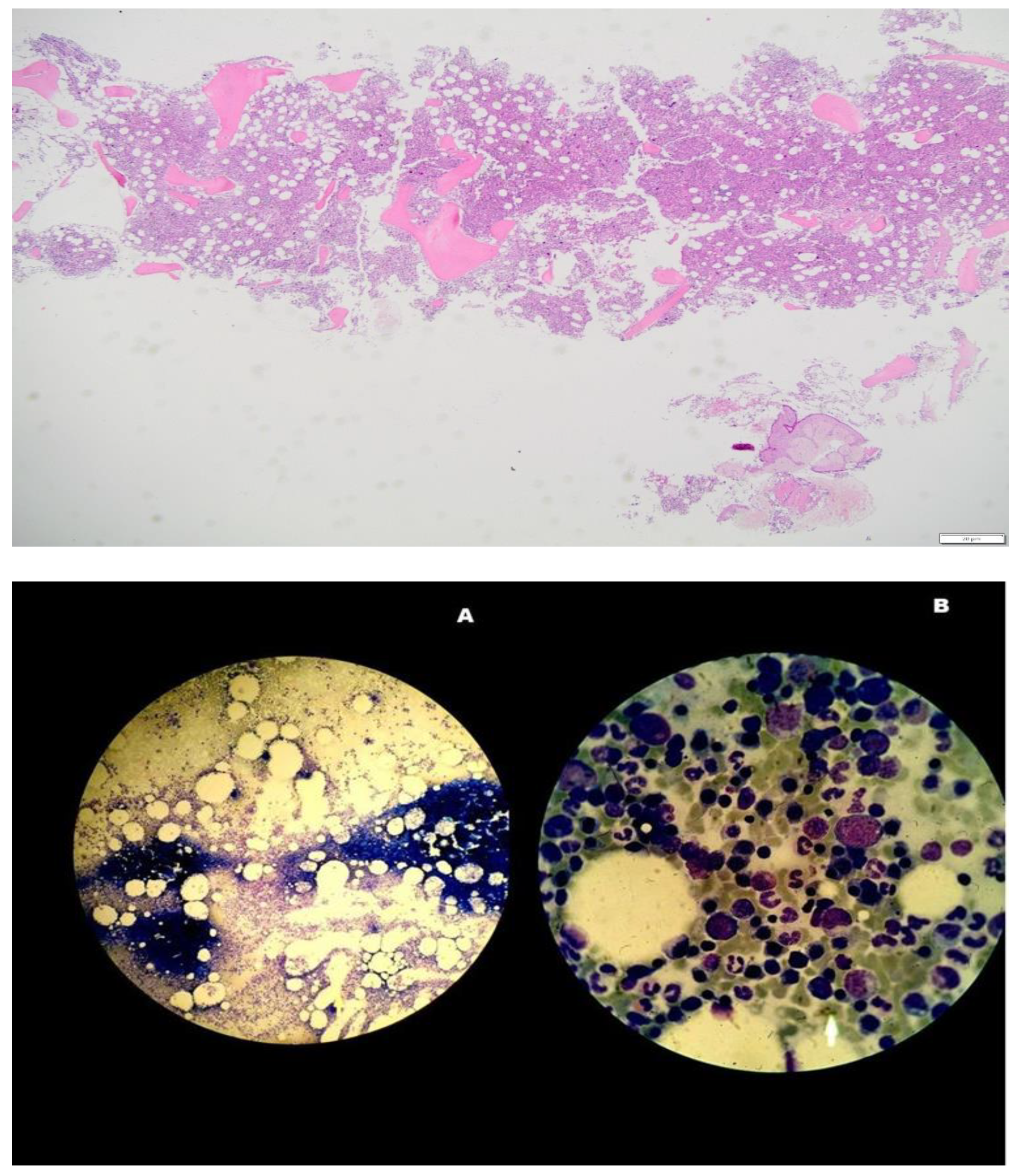
- A BM biopsy demonstrating trilineage growth (panmyelosis) and hypercellularity, with strong erythroid, granulocytic and megakaryocytic proliferation and pleomorphic adult megakaryocytes (differences in size).
- The existence of the JAK2V617F or exon 12 mutation.
5. Minor Criterion
- A low level of erythropoietin (EPO) in the serum.
5.1. Some Recommendations about Management of PV in Pregnancy
5.2. Important Issues Concerning Management of MPN and Pregnancy
5.2.1. Planning Pregnancy and Preconception Phase
- Avoid using teratogenic medications before getting pregnant.
- Planning joint care with an MPN-experienced hematologist and consultant obstetrician.
5.2.2. Pregnancy
- At-risk pregnancy low-aspirin use (PV, CVRF, JAK2 positive MPN, recurrent abortions or stillbirths).
- Strict venesection management of hematocrit (45%).
- In high-risk circumstances, add IFN and aspirin.
- Switch aspirin to LMWH two weeks before the anticipated delivery.
- Until 24 weeks, FBC every 4 weeks. Τhen, two FBCs every week.
- Blood pressure and a urine test at each appointment.
- US scans performed at 12, 20, 26, 30, 34 and 38 weeks.
- Uterine artery Doppler at 20 (+24 weeks if abnormal), i.e., bilateral high RI or notches:
- a.
- Increase the level of surveillance.
- b.
- Consider raising the LMWH dose.
- c.
- Include 1000 mg of vitamin C daily and 400 iu of vitamin E each day.
- d.
- An early birth before 38 weeks.
5.2.3. Delivery
- Once the patient enters labor, stop LMWH.
- Do not administer LMWH within 12 h of delivery if you are having an elective cesarean section.
5.2.4. Postpartum
- LMWH at a preventative dose for six weeks after delivery.
- Maintenance of the maternal hematocrit and platelet count at normal levels.
5.2.5. Breastfeeding
- Is not recommended for patients receiving cytoreductive treatment.
- Mother’s history of arterial or vein thrombosis (whether pregnant or not).
- Previous hemorrhages attributed to MPD (whether pregnant or not).
- A previous pregnancy condition that MDP may have contributed to, e.g.,:
- a.
- Pregnancy losses in either the second or third trimester or three first trimesters.
- b.
- Birth weight below the gestational fifth centile.
- c.
- Stillbirth or intrauterine mortality (with no obvious other cause or evidence of placental dysfunction and growth restricted fetus).
- d.
- Considerable antepartum bleeding.
- e.
- Postpartum bleeding (requiring red cell transfusion).
- f.
- Severe pre-eclampsia (necessitating preterm delivery < 37 weeks).
- g.
- Any such condition developing during the index pregnancy.
- 2.
- A spike in platelet count to about 1500 × 109/L.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alimam, S.; Bewley, S.; Chappell, L.C.; Knight, M.; Seed, P.; Gray, G.; Harrison, C.; Robinson, S. Pregnancy outcomes in myeloproliferative neoplasms: UK prospective cohort study. Br. J. Haemotol. 2016, 175, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.A.; Babiker, H.M. Polycythemia; Statpearls: Tampa, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526081/ (accessed on 19 September 2022).
- Mc Mullin, M.F.F.; Mead, A.J.; Ali, S.; Cargo, C.; Chen, F.; Ewing, J.; Garg, M.; Godfrey, A.; Knapper, S.; McLornan, D.P.; et al. A guideline for the management of specific situations in polycythaemia vera and secondary erythrocytosis. Br. J. Haematol. 2019, 184, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Bohiltea, R.E.; Niculescu-Mizil, E.; Mihai, B.M.; Furtunescu, F.; Ducu, I.; Munteanu, O.; Georgescu, T.A.; Grigoriu, C. Polycythemia vera in pregnancy represents a challenge for a multidisciplinary collaboration: A case report and literature review. Exp. Ther. Med. 2022, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Aaron, T.; Gerds, M.D. Myeloproliferative Neoplasms. Cleveland Clinic, Center for Continuing Education. 2016. Available online: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hematology-oncology/chronic-myeloproliferative-disorders/ (accessed on 7 October 2022).
- Barbui, T.; Finazzi, G. Myeloproliferative disease in pregnancy and other management issues. Hematol. Am. Soc. Hematol. Educ. Program. 2006, 2006, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Liesveld, J.; Reagan, P.; Polycythemia Vera (Primary Polycythemia). Last Full Review/Revision February 2019. MSD Manuel Professional Version. Available online: www.msdmanuals.com/professional/hematology-and-oncology/myeloproliferativedisorders/polycythemia-vera (accessed on 10 October 2022).
- Harrison, C. Pregnancy and its management in the Philadelphia negative myeloproliferative diseases. Br. J. Haematol. 2005, 129, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.E., 2nd; Ueland, K.; Aronson, W.J. Polycythemia rubra vera and pregnancy. Obstet. Gynecol. 1983, 62 (Suppl. 3), 16s–20s. [Google Scholar] [PubMed]
- Hoffman, R.; Prchal, J.T.; Samuelson, S.; Ciurea, S.O.; Rondelli, D. Philadelphia chromosome-negative myeloproliferative disorders: Biology and treatment. Biol. Blood Marrow Transplant. 2007, 13 (Suppl. 1), 64–72. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Thiele, J.; Gisslinger, H.; Kvasnicka, H.M.; Vannucchi, A.M.; Guglielmelli, P.; Orazi, A.; Tefferi, A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer J. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Griesshammer, M.; Struve, S.; Harrison, C.M. Essential thrombocythemia/polycythemia vera and pregnancy: The need for an observational study in Europe. Semin. Thromb. Hemost. 2006, 32 Pt 2, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Griesshammer, M.; Sadjadian, P.; Wille, K. Contemporary management of patients with BCR-ABL1-negative myeloproliferative neoplasms during pregnancy. J. Expert Rev. Hematol. 2018, 11, 697–706. [Google Scholar] [CrossRef] [PubMed]
| WBC: 14,300 | κ./μL | CRP: 117.6 | mg/L |
| Hb: 11.5 | g/dL | UREA: 15 | mg/dL |
| Hct: 38 | % | GLU: 92 | mg/dL |
| PLT: 219 | κ./μL | INR: 1.05 | |
| SGOT: 30 | U/L | CK-MB: 0.3 | IU/L |
| SGPT: 36 | U/L | ||
| γ-gt: 45 | IU/L | ||
| LDH: 516 | IU/L | ||
| ALP: 134 | U/L | ||
| CPK: 56 | IU/L | ||
| FBC | |
|---|---|
| WBC: 13.200 | κ./μL |
| RBC: 4.75 | κ./μL |
| Hb: 11.6 | g/dL |
| Hct: 40.3 | % |
| NEU: 76 | % |
| MCV: 84.8 | fl |
| MCH: 24.5 | pg |
| MCHC: 28.9 | g/dL |
| PLT: 260 | κ./μL |
| SGOT: 27 | U/L |
| SGPT: 41 | U/L |
| Serum amylase: 35 | IU/L |
| WBC: 7900 | κ./μL |
| RBC: 5160 | κ./μL |
| Hct: 44.6 | g/dL |
| Hb: 14 | % |
| MCV: 88.4 | fL |
| MCH: 26.8 | pg |
| MCHC: 31 | g/dL |
| PLT: 212 | κ./μL |
| Fe: 53 | ng/dL |
| Ferritin: 11 | mg/mL |
| SGOT: 66 | U/L |
| SGPT: 73 | U/L |
| ALP: 591 | U/L |
| LDH: 510 | IU/L |
| WBC: 7900 | κ./μL |
| RBC: 4490 | κ./μL |
| Hct: 38.7 | g/dL |
| Hb: 12 | % |
| MCV: 86.2 | fL |
| MCH: 26.8 | pg |
| MCHC: 31 | g/dL |
| PLT: 225 | κ./μL |
| Fe: 37 | mg/dL |
| Ferritin: 7.8 | ng/dL |
| SGOT: 48 | U/L |
| SGPT: 56 | U/L |
| γ-GT: 90 | IU/L |
| ALP: 239 | U/L |
| LDH: 417 | IU/L |
| 15 Years Ago | 15 Years Ago (3 Months Later) | ||||
|---|---|---|---|---|---|
| WBC: 5380 | κ./μL | WBC: 6100 | κ./μL | ||
| RBC: 4960 | κ./μL | RBC: 5200 | κ./μL | ||
| Hct: 44.1 | % | Hct: 45 | % | ||
| Hb: 13.6 | g/dL | Hb: 14.5 | g/dL | ||
| MCV: 88.9 | fL | MCV: 86.3 | fL | ||
| MCHC: 30.9 | pg | MCHC: 27.9 | pg | ||
| MCH: 27.9 | g/dL | MCH: 32 | g/dL | ||
| PLT: 250 | κ./μL | PLT: 261 | κ./μL | ||
| Fe: - | mg/dL | Fe: - | mg/dL | ||
| Fer: 4.7 | ng/mL | Fer: 6 | ng/mL | ||
| 12 Years Ago | 5 Years Ago | 4 Years Ago | |||
| WBC: 6000 | κ./μL | WBC: 6400 | κ./μL | WBC: 6400 | κ./μL |
| RBC: 5450 | κ./μL | RBC: 5160 | κ./μL | RBC: 5040 | κ./μL |
| Hct: 46.7 | % | Hct: 42.8 | % | Hct: 45.2 | % |
| Hb: 15.4 | g/dL | Hb: 13.7 | g/dL | Hb: 14.2 | g/dL |
| MCV: 85.7 | fL | MCV: 82.9 | fL | MCV: 89.7 | fL |
| MCHC: 28 | pg | MCHC: 26.6 | pg | MCHC: 28 | pg |
| MCH: 32 | g/dL | MCH: 32 | g/dL | MCH: 31.4 | g/dL |
| PLT: 300 | κ./μL | PLT: 349 | κ./μL | PLT: 340 | κ./μL |
| Fe: 49 | mg/dL | Fe: 46 | mg/dL | Fe: 45 | mg/dL |
| Fer: 6.7 | ng/mL | Fer: 8.9 | ng/mL | Fer: 7.3 | ng/mL |
| 3 Years Ago | |||||
| WBC: 6340 | κ./μL | ||||
| RBC: 5030 | κ./μL | ||||
| Hct: 43.5 | % | ||||
| Hb: 13.4 | g/dL | ||||
| MCV: 86.5 | fL | ||||
| MCHC: 26.6 | pg | ||||
| MCH: 30.8 | g/dL | ||||
| PLT: 400 | κ./μL | ||||
| Fe: 41 | mg/dL | ||||
| Fer: 4.5 | ng/mL | ||||
| 8th Week * | 26th Week ** | ||
|---|---|---|---|
| WBC: 8930 | κ./μL | WBC: 11,070 | κ./μL |
| RBC: 4870 | κ./μL | RBC: 4310 | κ./μL |
| Hb: 14.2 | g/dL | Hb: 12.2 | g/dL |
| Hct: 44 | % | Hct: 39.9 | % |
| MCV: 90.3 | fL | MCV: 92.6 | fL |
| MCH: 29.2 | pg | MCH: 28.3 | pg |
| MCHC: 32 | g/dL | MCHC: 30 | g/dL |
| PLT: 341 | κ./μL | PLT: 377 | κ./μL |
| FE: 81 | mg/dL | Fe: 75 | mg/dL |
| Fer: 11 | ng/mL | Fer: 3.9 | ng/mL |
| GLU: 83 | mg/dL | GLU: 84 | mg/dL |
| SGOT: 20 | U/L | ||
| SGPT: 20 | U/L | ||
| UA: 5.1 | mg/dL | ||
| Cr: 0.61 | mg/dL | ||
| U: 25 | mg/dL | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntounis, T.; Zioutos, K.A.; Koutras, A.; Prokopakis, I.; Fasoulakis, Z.; Sapantzoglou, I.; Perros, P.; Samara, A.A.; Spanoudakis, E.; Valsamaki, A.; et al. Portal Vein Thrombosis after C-Section in a Patient with Polycythemia Vera (PV) Due to Pregnancy and Iron Deficiency Anemia (IDA). Clin. Pract. 2022, 12, 1069-1077. https://doi.org/10.3390/clinpract12060109
Ntounis T, Zioutos KA, Koutras A, Prokopakis I, Fasoulakis Z, Sapantzoglou I, Perros P, Samara AA, Spanoudakis E, Valsamaki A, et al. Portal Vein Thrombosis after C-Section in a Patient with Polycythemia Vera (PV) Due to Pregnancy and Iron Deficiency Anemia (IDA). Clinics and Practice. 2022; 12(6):1069-1077. https://doi.org/10.3390/clinpract12060109
Chicago/Turabian StyleNtounis, Thomas, Konstantinos A. Zioutos, Antonios Koutras, Ioannis Prokopakis, Zacharias Fasoulakis, Ioakeim Sapantzoglou, Paraskevas Perros, Athina A. Samara, Emmanouil Spanoudakis, Asimina Valsamaki, and et al. 2022. "Portal Vein Thrombosis after C-Section in a Patient with Polycythemia Vera (PV) Due to Pregnancy and Iron Deficiency Anemia (IDA)" Clinics and Practice 12, no. 6: 1069-1077. https://doi.org/10.3390/clinpract12060109
APA StyleNtounis, T., Zioutos, K. A., Koutras, A., Prokopakis, I., Fasoulakis, Z., Sapantzoglou, I., Perros, P., Samara, A. A., Spanoudakis, E., Valsamaki, A., Krouskou, S.-E., Nikolettos, K., Palios, V.-C., Mousios, P., Goula, K., Konis, K., Chionis, A., & Kontomanolis, E. N. (2022). Portal Vein Thrombosis after C-Section in a Patient with Polycythemia Vera (PV) Due to Pregnancy and Iron Deficiency Anemia (IDA). Clinics and Practice, 12(6), 1069-1077. https://doi.org/10.3390/clinpract12060109







