Implications of Cellular Immaturity in Necrosis and Microvascularization in Glioblastomas IDH-Wild-Type
Abstract
1. Introduction
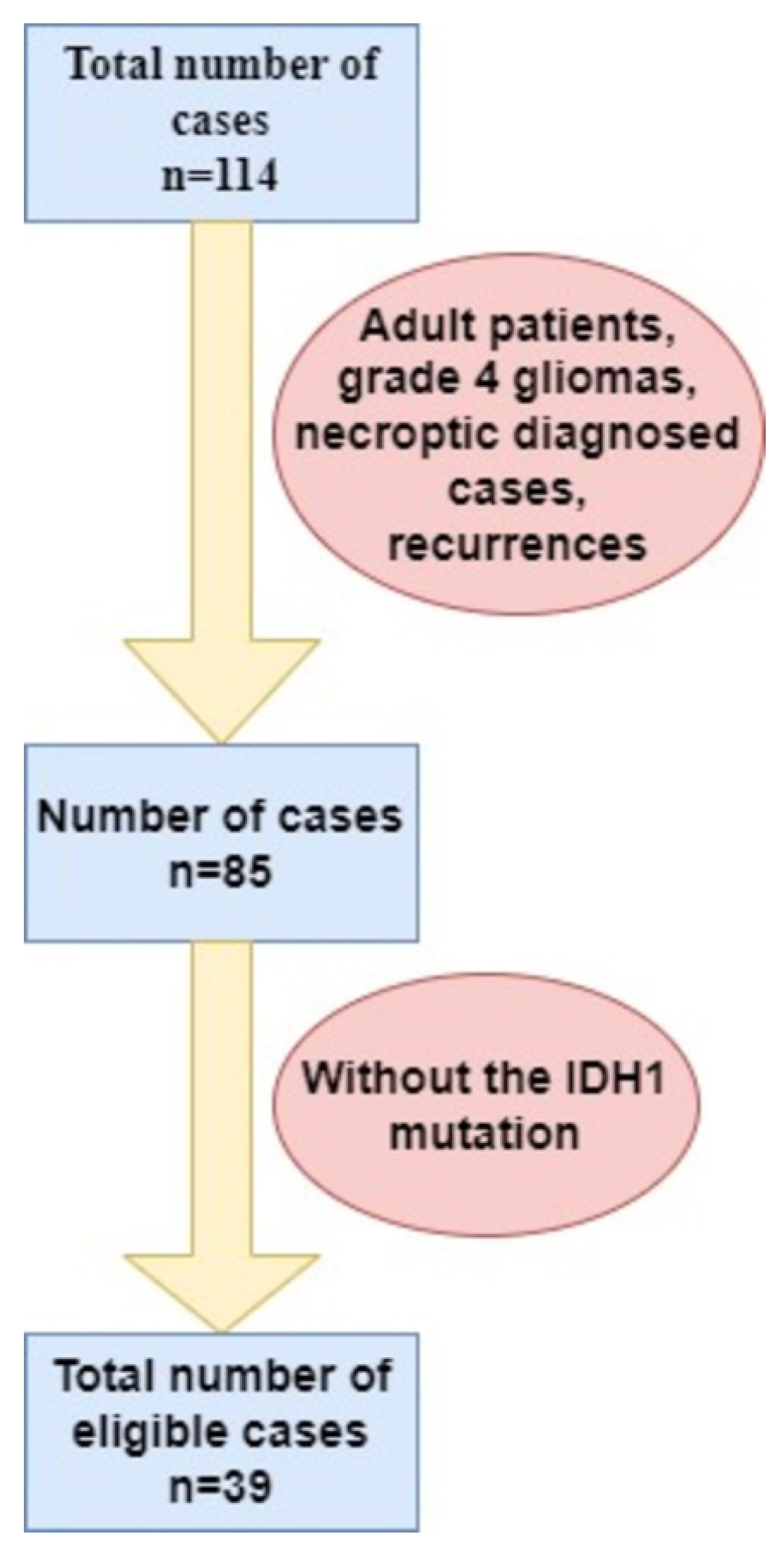
2. Materials and Methods
3. Results
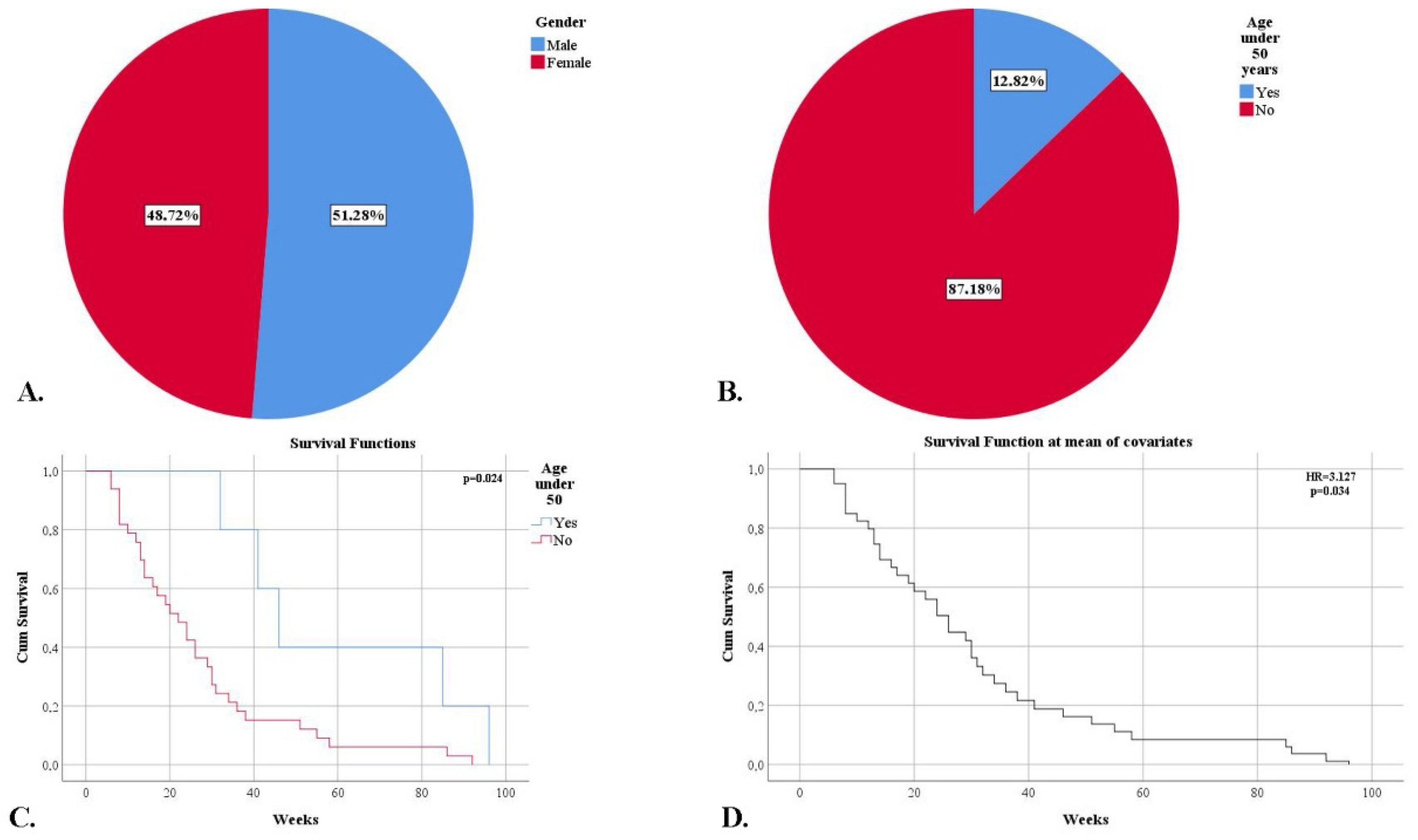
3.1. Demographic and Clinical Characteristics of the Patient Group
3.2. Imaging Characteristics of the Studied Cases
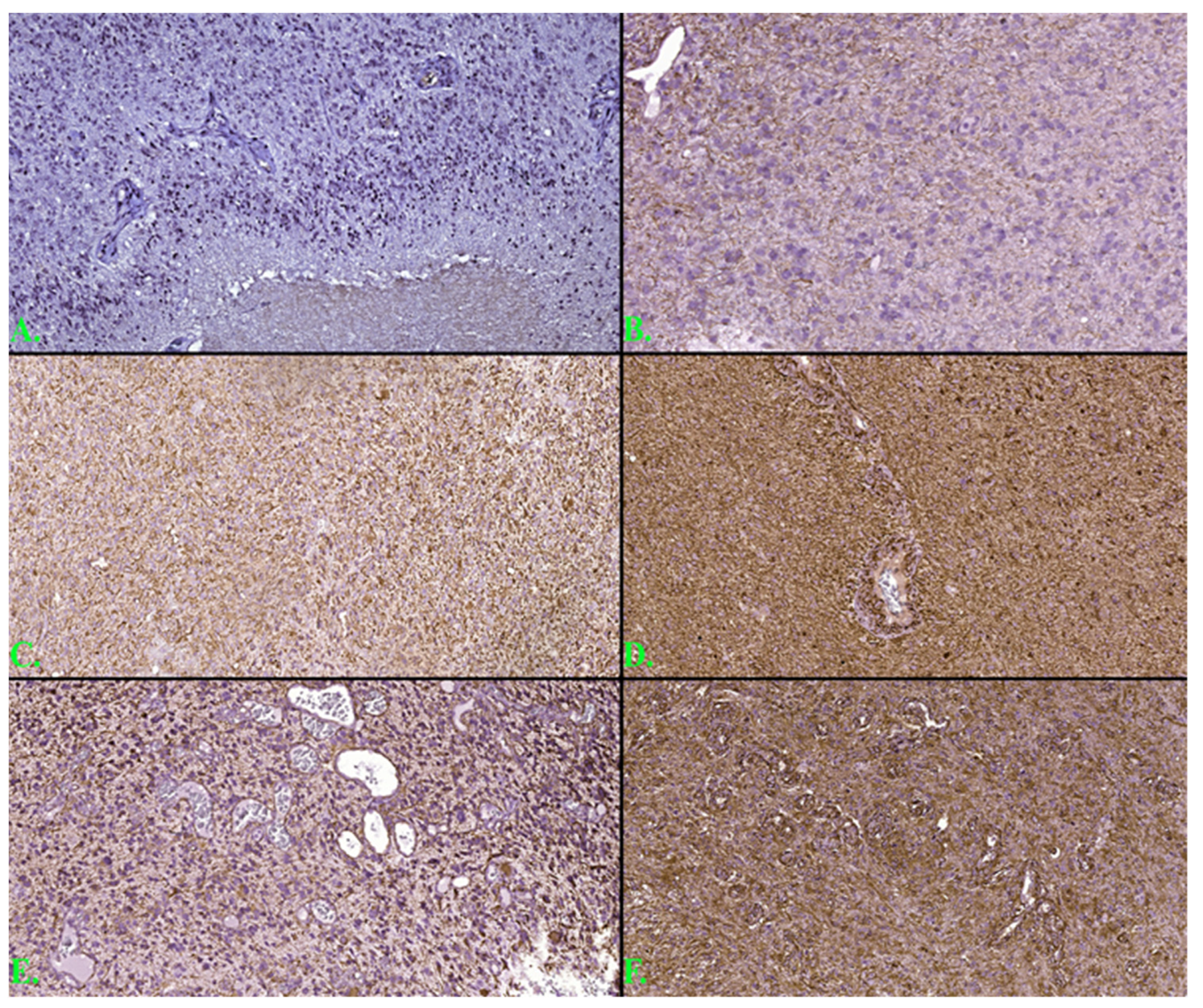
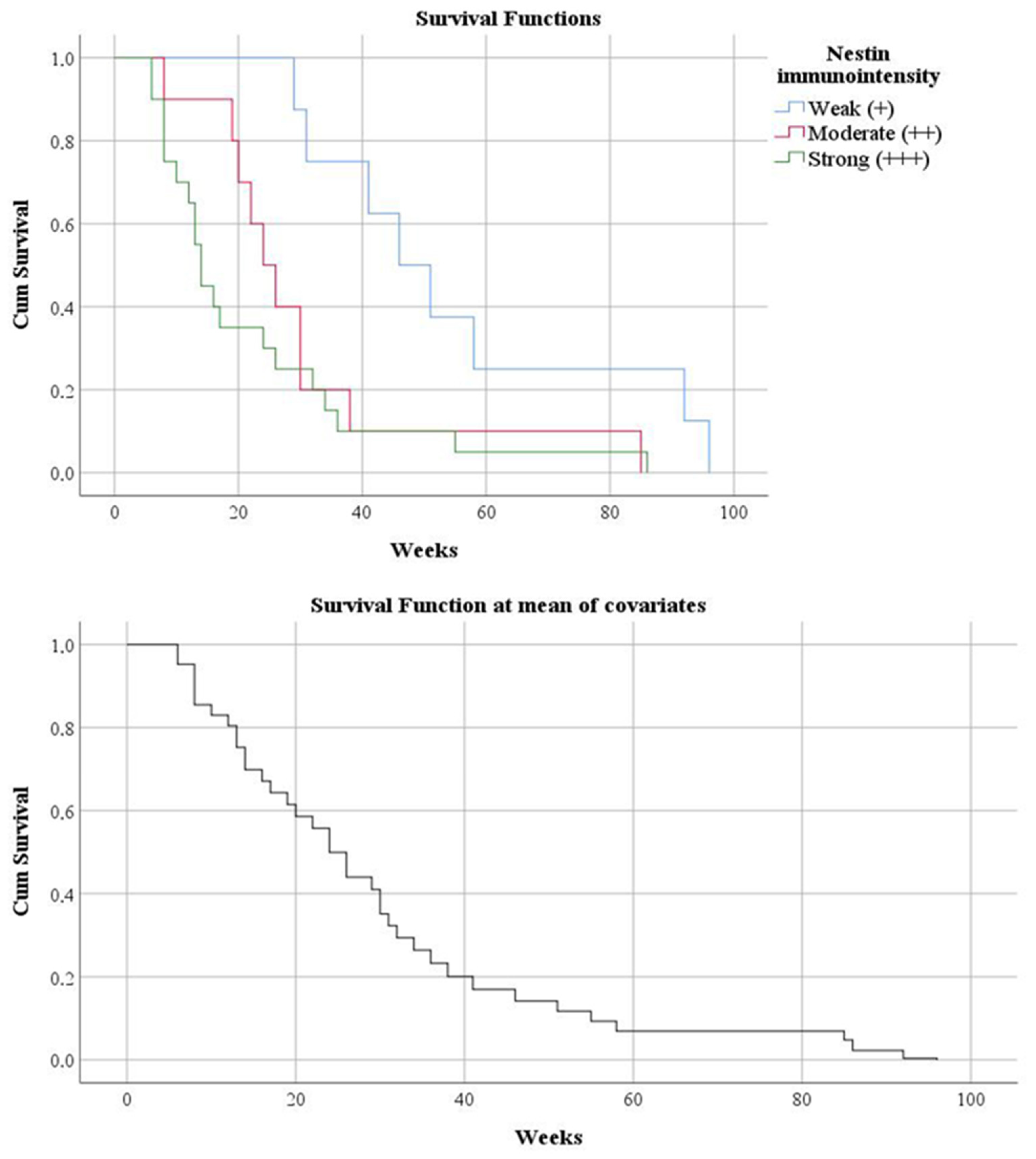
3.3. Histogenetic Aspects of the Analyzed Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- WHO Classification of Tomours Editorial Board. Central Nervous System Tymours. WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; pp. 39, 54–55. [Google Scholar]
- Schiffer, D.; Annovazzi, L.; Mazzucco, M.; Mellai, M. The origin of circumscribed necroses and perinecrotic niches in glioblastoma multiforme: An additional hypothesis. Integr. Cancer Sci. Therap. 2015, 2, 75–78. [Google Scholar] [CrossRef]
- Schiffer, D.; Annovazzi, L.; Casalone, C.; Corona, C.; Mellai, M. Glioblastoma: Microenvironment and Niche Concept. Cancers 2019, 11, 5. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Xu, K.; Wang, Z.; Fan, X.; Zhang, C.; Li, S.; Qiu, X.; Jiang, T. Relationship between necrotic patterns in glioblastoma and patient survival: Fractal dimension and lacunarity analyses using magnetic resonance imaging. Sci. Rep. 2017, 7, 8302. [Google Scholar] [CrossRef]
- Noch, E.; Khalili, K. Molecular mechanisms of necrosis in glioblastoma: The role of glutamate excitotoxicity. Cancer Biol. Ther. 2009, 8, 1791–1797. [Google Scholar] [CrossRef]
- Schiffer, D.; Mellai, M.; Annovazzi, L.; Caldera, V.; Piazzi, A.; Denysenko, T.; Melcarne, A. Stem cell niches in glioblastoma: A neuropathological view. Biomed. Res. Int. 2014, 2014, 725921. [Google Scholar] [CrossRef]
- Kargiotis, O.; Rao, J.S.; Kyritsis, A.P. Mechanisms of angiogenesis in gliomas. J. Neurooncol. 2006, 78, 281–293. [Google Scholar] [CrossRef]
- Mosteiro, A.; Pedrosa, L.; Ferrés, A.; Diao, D.; Sierra, À.; González, J.J. The Vascular Microenvironment in Glioblastoma: A Comprehensive Review. Biomedicines 2022, 10, 1285. [Google Scholar] [CrossRef]
- Birner, P.; Piribauer, M.; Fischer, I.; Gatterbauer, B.; Marosi, C.; Ambros, P.F.; Ambros, I.M.; Bredel, M.; Oberhuber, G.; Rössler, K.; et al. Vascular patterns in glioblastoma influence clinical outcome and associate with variable expression of angiogenic proteins: Evidence for distinct angiogenic subtypes. Brain Pathol. 2003, 13, 133–143. [Google Scholar] [CrossRef]
- Chen, J.; Mao, S.; Li, H.; Zheng, M.; Yi, L.; Lin, J.M.; Lin, Z.X. The pathological structure of the perivascular niche in different microvascular patterns of glioblastoma. PLoS ONE 2017, 12, e0182183. [Google Scholar] [CrossRef]
- Brown, N.F.; Ottaviani, D.; Tazare, J.; Gregson, J.; Kitchen, N.; Brandner, S.; Fersht, N.; Mulholland, P. Survival Outcomes and Prognostic Factors in Glioblastoma. Cancers 2022, 14, 3161. [Google Scholar] [CrossRef] [PubMed]
- Nobel, J.M.; van Geel, K.; Robben, S.G.F. Structured reporting in radiology: A systematic review to explore its potential. Eur. Radiol. 2022, 32, 2837–2854. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ladomersky, E.; Mozny, A.; Kocherginsky, M.; O’Shea, K.; Reinstein, Z.Z.; Zhai, L.; Bell, A.; Lauing, K.L.; Bollu, L.; et al. Glioblastoma as an age-related neurological disorder in adults. Neurooncol. Adv. 2021, 3, vdab125. [Google Scholar] [CrossRef]
- Barz, M.; Gerhardt, J.; Bette, S.; Aftahy, A.K.; Huber, T.; Combs, S.E.; Ryang, Y.M.; Wiestler, B.; Skardelly, M.; Gepfner-Tuma, I.; et al. Prognostic value of tumour volume in patients with a poor Karnofsky performance status scale–a bicentric retrospective study. BMC Neurol. 2021, 21, 446. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 2007, 170, 1445–1453. [Google Scholar] [CrossRef]
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Sandhu, N.; Knox, S.J. A Review of Newly Diagnosed Glioblastoma. Front. Oncol. 2021, 10, 574012. [Google Scholar] [CrossRef]
- Pantoja Cavalcante, S.; De Almeida, J.R.; Clara, C.A.; Scapulatempo Neto, C.; Verzinhase Peres, S.; Moriguchi, S.M.; Dos Santos, M.J.; Da Rocha, E.T. Evaluation of the microvascular density in astrocytomas in adults correlated using SPECT-MIBI. Exp. Ther. Med. 2010, 1, 293–299. [Google Scholar] [CrossRef][Green Version]
- Bette, S.; Barz, M.; Wiestler, B.; Huber, T.; Gerhardt, J.; Buchmann, N.; Combs, S.E.; Schmidt-Graf, F.; Delbridge, C.; Zimmer, C.; et al. Prognostic Value of Tumor Volume in Glioblastoma Patients: Size Also Matters for Patients with Incomplete Resection. Ann Surg. Oncol. 2018, 25, 558–564. [Google Scholar] [CrossRef]
- Iliadis, G.; Selviaridis, P.; Kalogera-Fountzila, A.; Fragkoulidi, A.; Baltas, D.; Tselis, N.; Chatzisotiriou, A.; Misailidou, D.; Zamboglou, N.; Fountzilas, G. The importance of tumor volume in the prognosis of patients with glioblastoma: Comparison of computerized volumetry and geometric models. Strahlenther. Onkol. 2009, 185, 743–750. [Google Scholar] [CrossRef]
- Sales, A.H.A.; Beck, J.; Schnell, O.; Fung, C.; Meyer, B.; Gempt, J. Surgical Treatment of Glioblastoma: State-of-the-Art and Future Trends. J. Clin. Med. 2022, 11, 5354. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Oppenlander, M.E.; Wolf, A.B.; Snyder, L.A.; Bina, R.; Wilson, J.R.; Coons, S.W.; Ashby, L.S.; Brachman, D.; Nakaji, P.; Porter, R.W.; et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J. Neurosurg. 2014, 120, 846–853. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, X. Which Parameter Is More Important for the Prognosis of New-Onset Adult Glioblastoma: Residual Tumor Volume or Extent of Resection? World Neurosurg. 2018, 116, e444–e451. [Google Scholar] [CrossRef]
- Pessina, F.; Navarria, P.; Cozzi, L.; Tomatis, S.; Riva, M.; Ascolese, A.M.; Santoro, A.; Simonelli, M.; Bello, L.; Scorsetti, M. Role of surgical resection in recurrent glioblastoma: Prognostic factors and outcome evaluation in an observational study. J. Neurooncol. 2017, 131, 377–384. [Google Scholar] [CrossRef]
- Sales, A.H.A.; Bette, S.; Barz, M.; Huber, T.; Wiestler, B.; Ryang, Y.M.; Schmidt-Graf, F.; Liesche, F.; Combs, S.E.; Meyer, B.; et al. Role of postoperative tumor volume in patients with MGMT-unmethylated glioblastoma. J. Neurooncol. 2019, 142, 529–536. [Google Scholar] [CrossRef]
- Nguyen, H.-M.; Guz-Montgomery, K.; Lowe, D.B.; Saha, D. Pathogenetic Features and Current Management of Glioblastoma. Cancers 2021, 13, 856. [Google Scholar] [CrossRef]
- Magrowski, Ł.; Nowicka, E.; Masri, O.; Tukiendorf, A.; Tarnawski, R.; Miszczyk, M. The survival impact of significant delays between surgery and radiochemotherapy in glioblastoma patients: A retrospective analysis from a large tertiary center. J. Clin. Neurosci. 2021, 90, 39–47. [Google Scholar] [CrossRef]
- Whitfield, B.T.; Huse, J.T. Classification of adult-type diffuse gliomas: Impact of the World Health Organization 2021 update. Brain Pathol. 2022, 32, e13062. [Google Scholar] [CrossRef]
- Armocida, D.; Frati, A.; Salvati, M.; Santoro, A.; Pesce, A. Is Ki-67 index overexpression in IDH wild type glioblastoma a predictor of shorter Progression Free survival? A clinical and Molecular analytic investigation. Clin. Neurol. Neurosurg. 2020, 198, 106126. [Google Scholar] [CrossRef]
- Dumke, R.; Dumke, C.; Eberle, F.; Nimsky, C.; Keber, U.; Engenhart-Cabillic, R.; Lautenschläger, S. Monocentric evaluation of Ki-67 labeling index in combination with a modified RPA score as a prognostic factor for survival in IDH-wildtype glioblastoma patients treated with radiochemotherapy. Strahlenther Onkol. 2022, 198, 892–906. [Google Scholar] [CrossRef]
- Senhaji, N.; Squalli Houssaini, A.; Lamrabet, S.; Louati, S.; Bennis, S. Molecular and Circulating Biomarkers in Patients with Glioblastoma. Int. J. Mol. Sci. 2022, 23, 7474. [Google Scholar] [CrossRef]
- Bouvier-Labit, C.; Chinot, O.; Ochi, C.; Gambarelli, D.; Dufour, H.; Figarella-Branger, D. Prognostic significance of Ki67, p53 and epidermal growth factor receptor immunostaining in human glioblastomas. Neuropathol. Appl. Neurobiol. 1998, 24, 381–388. [Google Scholar] [CrossRef]
- Appay, R.; Dehais, C.; Maurage, C.A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Escande, F.; Idbaih, A.; Kamoun, A.; et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol. 2019, 21, 1519–1528. [Google Scholar] [CrossRef]
- Huang, L.E. Impact of CDKN2A/B Homozygous Deletion on the Prognosis and Biology of IDH-Mutant Glioma. Biomedicines 2022, 10, 246. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Hata, N.; Takigawa, K.; Arita, H.; Kuga, D.; Hatae, R.; Sangatsuda, Y.; Fujioka, Y.; Sako, A.; Umehara, T.; et al. Clinical significance of CDKN2A homozygous deletion in combination with methylated MGMT status for IDH-wildtype glioblastoma. Cancer Med. 2021, 10, 3177–3187. [Google Scholar] [CrossRef]
- Chang, Y.Z.; Li, G.Z.; Pang, B.; Zhang, K.N.; Zhang, X.H.; Wang, Y.Z.; Jiang, Z.L.; Chai, R.C. Transcriptional Characteristics of IDH-Wild Type Glioma Subgroups Highlight the Biological Processes Underlying Heterogeneity of IDH-Wild Type WHO Grade IV Gliomas. Front. Cell Dev. Biol. 2020, 8, 580464. [Google Scholar] [CrossRef]
- Esemen, Y.; Awan, M.; Parwez, R.; Baig, A.; Rahman, S.; Masala, I.; Franchini, S.; Giakoumettis, D. Molecular Pathogenesis of Glioblastoma in Adults and Future Perspectives: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 2607. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.K. Current Understanding of Hypoxia in Glioblastoma Multiforme and Its Response to Immunotherapy. Cancers 2022, 14, 1176. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.; Arranz, L. Nestin-expressing progenitor cells: Function, identity and therapeutic implications. Cell Mol Life Sci. 2018, 75, 2177–2195. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.G. Clinicopathological Significance of Nestin Expression as a Diagnostic and Prognostic Marker in Brain Gliomas, Independent of IDH Mutation. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Szymańska-Chabowska, A.; Świątkowski, F.; Jankowska-Polańska, B.; Mazur, G.; Chabowski, M. Nestin Expression as a Diagnostic and Prognostic Marker in Colorectal Cancer and Other Tumors. Clin. Med. Insights Oncol. 2021, 15, 11795549211038256. [Google Scholar] [CrossRef]
- Neradil, J.; Veselska, R. Nestin as a marker of cancer stem cells. Cancer Sci. 2015, 106, 803–811. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hagio, M.; Ishiwata, T. Nestin: A novel angiogenesis marker and possible target for tumor angiogenesis. World J. Gastroenterol. 2013, 19, 42–48. [Google Scholar] [CrossRef]
- Suzuki, S.; Namiki, J.; Shibata, S.; Mastuzaki, Y.; Okano, H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J. Histochem. Cytochem. 2010, 58, 721–730. [Google Scholar] [CrossRef]
- Schiffer, D.; Annovazzi, L.; Caldera, V.; Mellai, M. On the origin and growth of gliomas. Anticancer. Res. 2010, 30, 1977–1998. [Google Scholar]
- Dong, J.; Zhao, Y.; Huang, Q.; Fei, X.; Diao, Y.; Shen, Y.; Xiao, H.; Zhang, T.; Lan, Q.; Gu, X. Glioma stem/progenitor cells contribute to neovascularization via transdifferentiation. Stem. Cell Rev. Rep. 2011, 7, 141–152. [Google Scholar] [CrossRef]
- Sica, G.; Lama, G.; Anile, C.; Geloso, M.C.; La Torre, G.; De Bonis, P.; Maira, G.; Lauriola, L.; Jhanwar-Uniyal, M.; Mangiola, A. Assessment of angiogenesis by CD105 and nestin expression in peritumor tissue of glioblastoma. Int. J. Oncol. 2011, 38, 41–49. [Google Scholar] [CrossRef]
- Nowak, A.; Grzegrzolka, J.; Paprocka, M.; Piotrowska, A.; Rys, J.; Matkowski, R.; Dziegiel, P. Nestin-positive microvessel density is an independent prognostic factor in breast cancer. Int. J. Oncol. 2017, 51, 668–676. [Google Scholar] [CrossRef][Green Version]
- Chabowski, M.; Nowak, A.; Grzegrzolka, J.; Piotrowska, A.; Janczak, D.; Dziegiel, P. Comparison of Microvessel Density Using Nestin and CD34 in Colorectal Cancer. Anticancer. Res. 2018, 38, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Matini, A.H.; Mofidi Naeini, M.; Haddad Kashani, H.; Vakili, Z. Evaluation of Nestin and EGFR in Patients with Glioblastoma Multiforme in a Public Hospital in Iran. Asian Pac. J. Cancer Prev. 2020, 21, 2889–2894. [Google Scholar] [CrossRef]
- Guadagno, E.; Borrelli, G.; Califano, M.; Calì, G.; Solari, D.; Del Basso De Caro, M. Immunohistochemical expression of stem cell markers CD44 and nestin in glioblastomas: Evaluation of their prognostic significance. Pathol. Res. Pract. 2016, 212, 825–832. [Google Scholar] [CrossRef]
- Saffar, H.; Mirzaii, M.; Mirzaian, E.; Kosari, F. Assessment of Micro-vessel Density in Brain Gliomaby CD105 Expression. Iran J. Pathol. 2018, 13, 205–211. [Google Scholar]
- Birlik, B.; Canda, S.; Ozer, E. Tumour vascularity is of prognostic significance in adult, but not paediatric astrocytomas. Neuropathol Appl. Neurobiol. 2006, 32, 532–538. [Google Scholar] [CrossRef]
- Mihić, J.; Rotim, K.; Vučić, M.; Hude Dragičević, I.; Borić, M.; Lugović-Mihić, L. Prognostic role of CD44 expression and neovascularization determined by endoglin (CD105) in glioblastoma patients. Acta Clin. Croat. 2019, 58, 455–462. [Google Scholar] [CrossRef]
- Tena-Suck, M.L.; Celis-Lopez, M.A.; Collado-Ortiz, M.A.; Castillejos-Lopez, M.; Tenorio-Serralta, M. Glioblastoma Multiforme and Angiogenesis: A Clinicopathological and Immunohistochemistry Approach. J. Neurol. Res. 2015, 5, 199–206. [Google Scholar] [CrossRef][Green Version]
- Fan, C.; Zhang, J.; Liu, Z.; He, M.; Kang, T.; Du, T.; Song, Y.; Fan, Y.; Xu, J. Prognostic role of microvessel density in patients with glioma. Medicine 2019, 98, e14695. [Google Scholar] [CrossRef]
- Shirahata, M.; Ono, T.; Stichel, D.; Schrimpf, D.; Reuss, D.E.; Sahm, F.; Koelsche, C.; Wefers, A.; Reinhardt, A.; Huang, K.; et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018, 136, 153–166. [Google Scholar] [CrossRef]
- Palpan Flores, A.; Vivancos Sanchez, C.; Roda, J.M.; Cerdán, S.; Barrios, A.J.; Utrilla, C.; Royo, A.; Gandía González, M.L. Assessment of Pre-operative Measurements of Tumor Size by MRI Methods as Survival Predictors in Wild Type IDH Glioblastoma. Front. Oncol. 2020, 10, 1662. [Google Scholar] [CrossRef]

| Means for Survival Time | ||||||
|---|---|---|---|---|---|---|
| After the surgical intervention | Radiotherapy | Chemotherapy | Mean | p-value | ||
| Weeks | C.I. 95% | |||||
| No | No | 7.71 | 6.692 | 8.737 | p = 0.004 | |
| Yes | 26.00 | 26.000 | 26.000 | |||
| Yes | No | 12.00 | 12.000 | 12.000 | ||
| Yes | 37.65 | 28.730 | 46.581 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orasanu, C.I.; Aschie, M.; Deacu, M.; Bosoteanu, M.; Vamesu, S.; Enciu, M.; Bălţătescu, G.I.; Cozaru, G.C.; Mitroi, A.F.; Voda, R.I. Implications of Cellular Immaturity in Necrosis and Microvascularization in Glioblastomas IDH-Wild-Type. Clin. Pract. 2022, 12, 1054-1068. https://doi.org/10.3390/clinpract12060108
Orasanu CI, Aschie M, Deacu M, Bosoteanu M, Vamesu S, Enciu M, Bălţătescu GI, Cozaru GC, Mitroi AF, Voda RI. Implications of Cellular Immaturity in Necrosis and Microvascularization in Glioblastomas IDH-Wild-Type. Clinics and Practice. 2022; 12(6):1054-1068. https://doi.org/10.3390/clinpract12060108
Chicago/Turabian StyleOrasanu, Cristian Ionut, Mariana Aschie, Mariana Deacu, Madalina Bosoteanu, Sorin Vamesu, Manuela Enciu, Gabriela Izabela Bălţătescu, Georgeta Camelia Cozaru, Anca Florentina Mitroi, and Raluca Ioana Voda. 2022. "Implications of Cellular Immaturity in Necrosis and Microvascularization in Glioblastomas IDH-Wild-Type" Clinics and Practice 12, no. 6: 1054-1068. https://doi.org/10.3390/clinpract12060108
APA StyleOrasanu, C. I., Aschie, M., Deacu, M., Bosoteanu, M., Vamesu, S., Enciu, M., Bălţătescu, G. I., Cozaru, G. C., Mitroi, A. F., & Voda, R. I. (2022). Implications of Cellular Immaturity in Necrosis and Microvascularization in Glioblastomas IDH-Wild-Type. Clinics and Practice, 12(6), 1054-1068. https://doi.org/10.3390/clinpract12060108






