A Scoping Review on the Reported Evidence and Gaps of the Risk of Diabetes in Dyslipidemic Patients under Statin Therapy
Abstract
:1. Introduction
2. Materials & Methods
2.1. Stage 1: Sources of Information
2.2. Stage 2: Search Strategy
2.3. Stage 3: Process of Selection
2.4. Inclusion and Exclusion Criteria
2.5. Data Charting
2.6. Data Items
3. Results
3.1. Selection of Source of Evidence
3.2. Characteristics and Results of Source of Evidence
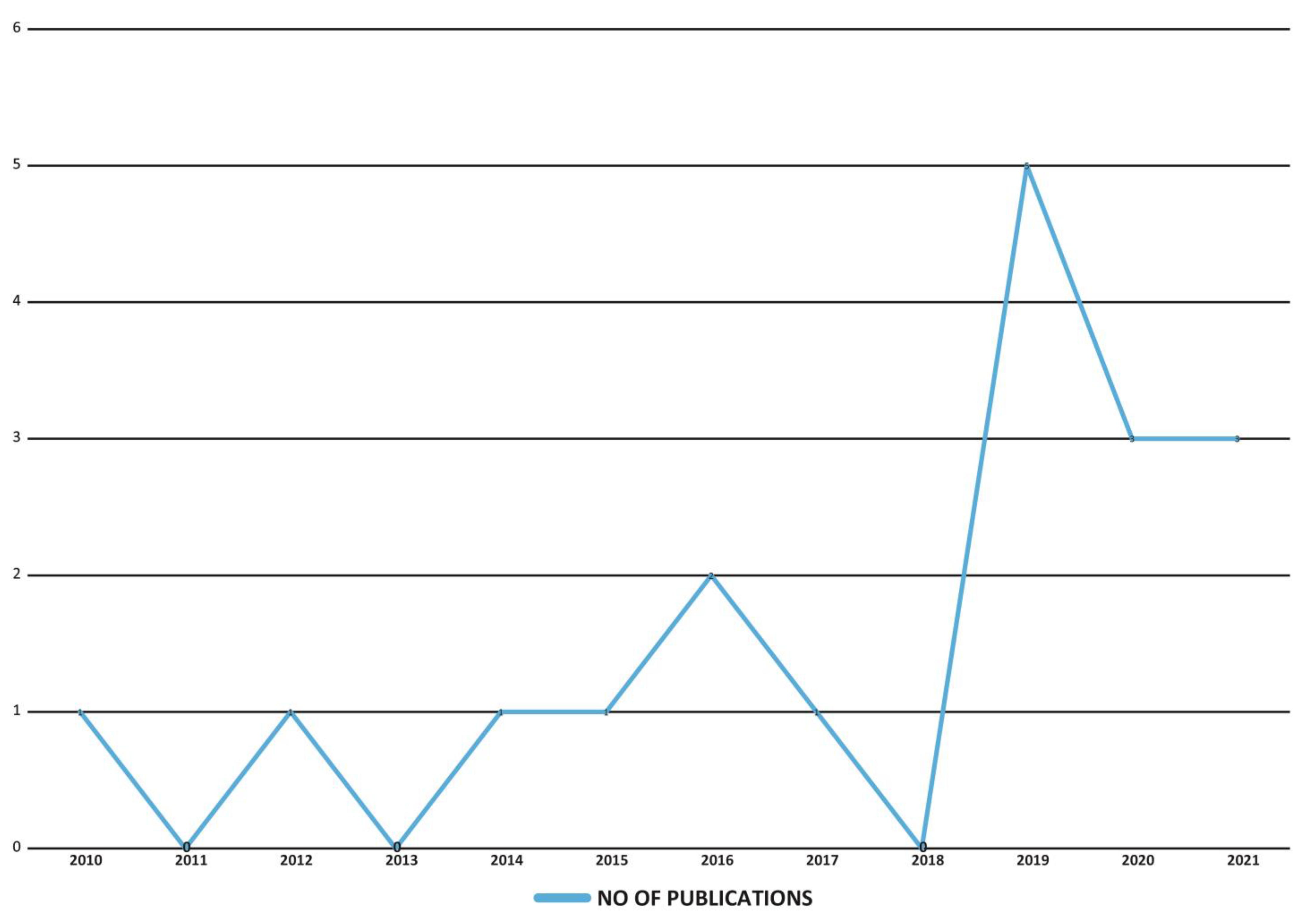
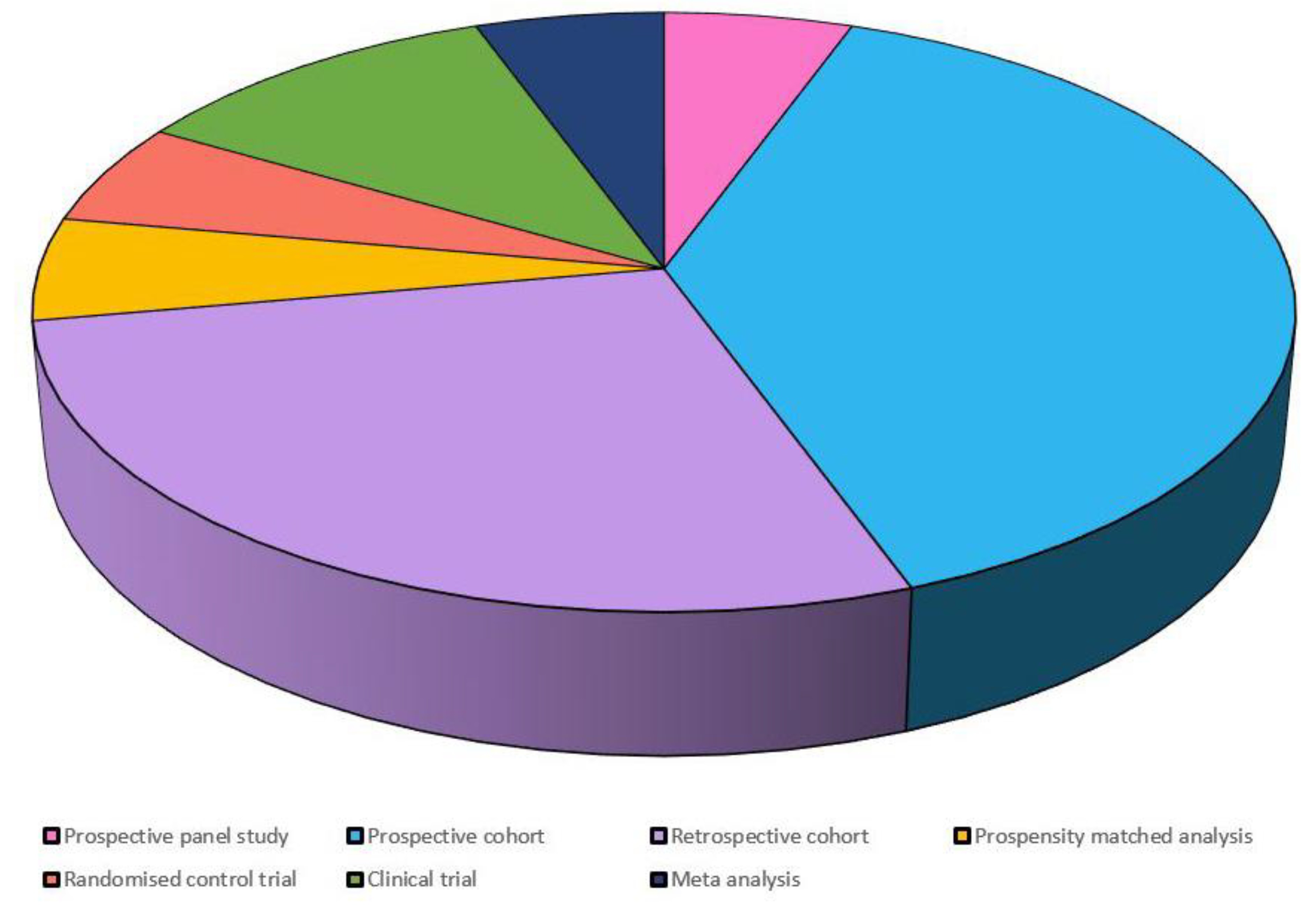
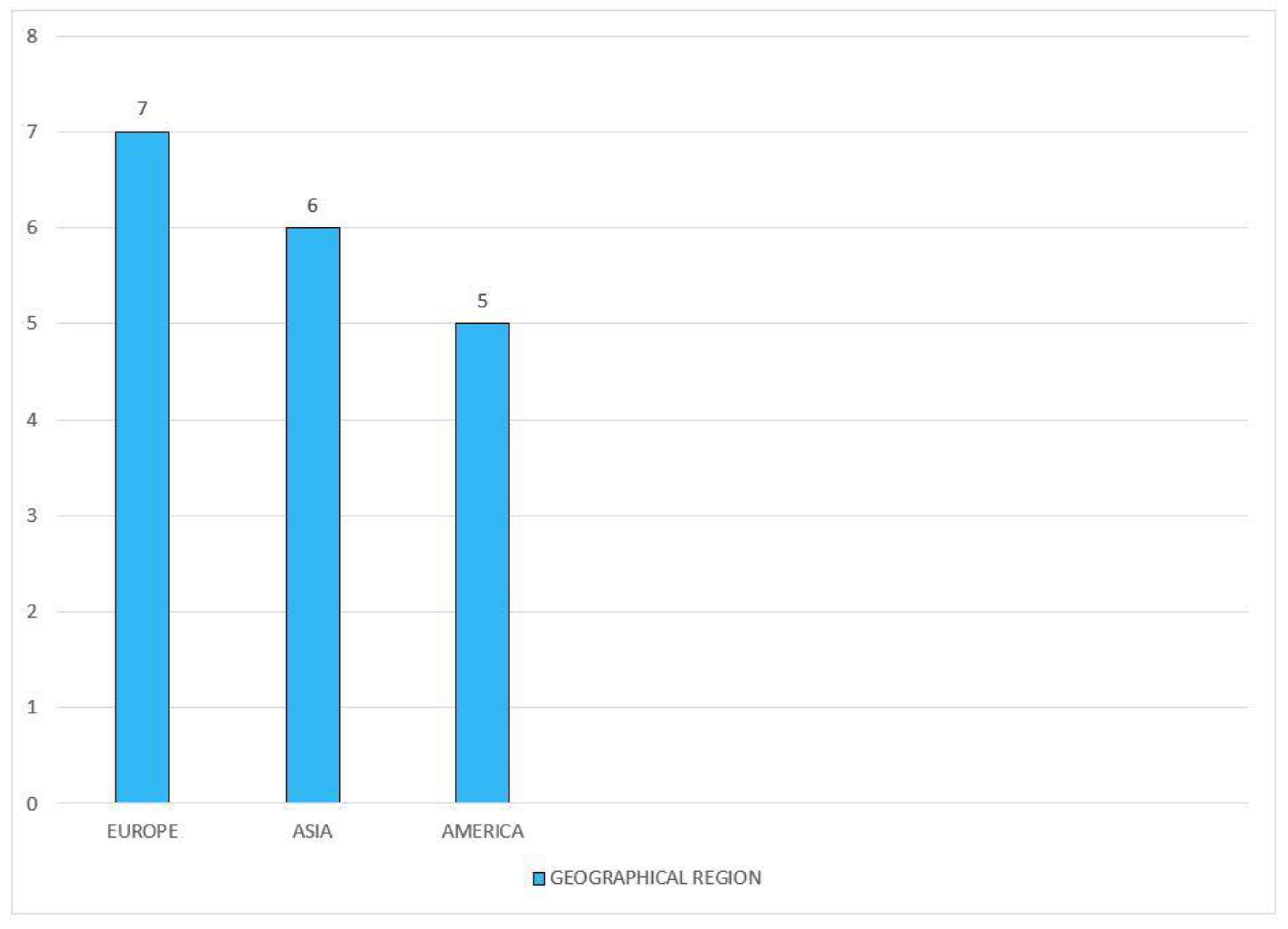
3.3. Summary of Charted Data
Characteristics of Charted Data
4. Discussion
5. Conclusions
6. Knowledge Gaps
7. Limitations
8. Directions for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Jain, V.; Patel, R.K.; Kapadia, Z.; Galiveeti, S.; Banerji, M.; Hope, L. Drugs and hyperglycemia: A practical guide. Maturitas 2017, 104, P80–P83. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Health Topics—Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 19 May 2022).
- Galicia-Garcia, U.; Jebari, S.; Larrea-Sebal, A.; Uribe, K.B.; Siddiqi, H.; Ostolaza, H.; Benito-Vicente, A.; Martín, C. Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 4725. [Google Scholar] [CrossRef] [PubMed]
- Carris, N.W.; Tipparaju, S.M.; Magness, D.J.; Chapalamadugu, K.C.; Magness, R.R. Pleiotropic effects of metformin to rescue statin-induced muscle injury and insulin resistance: A proposed mechanism and potential clinical implications. Med. Hypotheses 2017, 107, 39–44. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clin. 2018, 150, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Urina-Jassir, M.; Pacheco-Paez, T.; Paez-Canro, C.; Urina-Triana, M. Statin associated adverse reactions in Latin America: A scoping review. BMJ Open 2021, 11, e050675. [Google Scholar] [CrossRef]
- Liberale, L.; Carbone, F.; Camici, G.G.; Montecucco, F. IL-1β and Statin Treatment in Patients with Myocardial Infarction and Diabetic Cardiomyopathy. J. Clin. Med. 2019, 8, 1764. [Google Scholar] [CrossRef] [Green Version]
- Chogtu, B.; Magazine, R.; Bairy, K.L. Statin use and risk of diabetes mellitus. World J. Diabetes 2015, 6, 352–357. [Google Scholar] [CrossRef]
- Pappa, M.; Tsimihodimos, V.; Alaveras, A. Pathogenetic mechanisms of statin dysglycemia and risk of new-onset diabetes. J. Atheroscler. Prev. Treat. 2021, 12, 14–23. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Y.; Xu, C.B. Statins and New-Onset Diabetes Mellitus: LDL Receptor May Provide a Key Link. Front. Pharmacol. 2017, 8, 372. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Preiss, D. Genetic insights into statin-associated diabetes risk. Curr. Opin. Lipidol. 2016, 27, 125–130. [Google Scholar] [CrossRef]
- Abbasi, F.; Reaven, G.M. Statin-induced diabetes: How important is insulin resistance? J. Intern. Med. 2015, 277, 498–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahm, A.J.; Hegele, R.A. Incident Diabetes with Statins: Biology, Artifact, or Both? Can. J. Cardiol. 2015, 31, 963–965. [Google Scholar] [CrossRef]
- Carmena, R.; Betteridge, D.J. Diabetogenic Action of Statins: Mechanisms. Curr. Atheroscler. Rep. 2019, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Keni, R.; Sekhar, A.; Gourishetti, K.; Nayak, P.G.; Kinra, M.; Kumar, N.; Shenoy, R.R.; Kishore, A.; Nandakumar, K. Role of Statins in New-onset Diabetes Mellitus: The Underlying Cause, Mechanisms Involved, and Strategies to Combat. Curr. Drug Targets 2021, 22, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Henriksbo, B.D.; Tamrakar, A.K.; Phulka, J.S.; Barra, N.G.; Schertzer, J.D. Statins activate the NLRP3 inflammasome and impair insulin signaling via p38 and mTOR. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E110–E116. [Google Scholar] [CrossRef] [PubMed]
- Paseban, M.; Butler, A.E.; Sahebkar, A. Mechanisms of statin-induced new-onset diabetes. J. Cell. Physiol. 2018, 234, 12551–12561. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Ahmadizar, F.; Ochoa-Rosales, C.; Glisic, M.; Franco, O.H.; Muka, T.; Stricker, B.H. Associations of statin use with glycaemic traits and incident type 2 diabetes. Br. J. Clin. Pharmacol. 2019, 85, 993–1002. [Google Scholar] [CrossRef]
- Ko, M.J.; Jo, A.J.; Kim, Y.J.; Kang, S.H.; Cho, S.; Jo, S.H.; Park, C.Y.; Yun, S.C.; Lee, W.J.; Park, D.W. Time- and Dose-Dependent Association of Statin Use with Risk of Clinically Relevant New-Onset Diabetes Mellitus in Primary Prevention: A Nationwide Observational Cohort Study. J. Am. Heart Assoc. 2019, 8, e011320. [Google Scholar] [CrossRef]
- Min, H.K.; Kim, S.H.; Choi, J.H.; Choi, K.; Kim, H.R.; Lee, S.H. Impacts of statin and metformin on neuropathy in patients with type 2 diabetes mellitus: Korean Health Insurance data. World J. Clin. Cases 2021, 9, 10198–10207. [Google Scholar] [CrossRef]
- Hyun, M.H.; Jang, J.W.; Choi, B.G.; Na, J.O.; Choi, C.U.; Kim, J.W.; Kim, E.J.; Rha, S.; Park, C.G.; Lee, E.; et al. Risk of insulin resistance with statin therapy in individuals without dyslipidemia: A propensity-matched analysis in a registry population. Clin. Exp. Pharmacol. Physiol. 2020, 47, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Mansi, I.A.; Chansard, M.; Lingvay, I.; Zhang, S.; Halm, E.A.; Alvarez, C.A. Association of Statin Therapy Initiation with Diabetes Progression: A Retrospective Matched-Cohort Study. JAMA Intern. Med. 2021, 181, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Monzio Compagnoni, M.; Rea, F.; Merlino, L.; Catapano, A.L.; Mancia, G. Clinical significance of diabetes likely induced by statins: Evidence from a large population-based cohort. Diabetes Res. Clin. Pract. 2017, 133, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Barkas, F.; Elisaf, M.; Liberopoulos, E.; Liamis, G.; Ntzani, E.E.; Rizos, E.C. Atherogenic dyslipidemia increases the risk of incident diabetes in statin-treated patients with impaired fasting glucose or obesity. J. Cardiol. 2019, 74, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Hong, S.J.; Son, S.; An, H.; Kook, H.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.S. Incidence of new-onset diabetes with 1 mg versus 4 mg pitavastatin in patients at high risk of developing diabetes during a 3-year follow-up. Cardiovasc. Diabetol. 2019, 18, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederberg, H.; Stančáková, A.; Yaluri, N.; Modi, S.; Kuusisto, J.; Laakso, M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: A 6 year follow-up study of the METSIM cohort. Diabetologia 2015, 58, 1109–1117. [Google Scholar] [CrossRef] [Green Version]
- Parida, S.; Swain, T.R.; Routray, S.N.; Maiti, R. Effect of Atorvastatin on Glycaemic Parameters in Normoglycaemic and Prediabetic Subjects: A Prospective, Panel Study. J. Clin. Diagn. Res. 2017, 11, FC04–FC09. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Abbasi, F.; Lamendola, C.; Harris, C.S.; Harris, V.; Tsai, M.S.; Tripathi, P.; Abbas, F.; Reaven, G.M.; Reaven, P.D.; Snyder, M.P.; et al. Statins Are Associated with Increased Insulin Resistance and Secretion. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2786–2797. [Google Scholar] [CrossRef]
- Barkas, F.; Elisaf, M.; Liberopoulos, E.; Klouras, E.; Liamis, G.; Rizos, E.C. Statin therapy with or without ezetimibe and the progression to diabetes. J. Clin. Lipidol. 2016, 10, 306–313. [Google Scholar] [CrossRef]
- Ochoa-Rosales, C.; Portilla-Fernandez, E.; Nano, J.; Wilson, R.; Lehne, B.; Mishra, P.P.; Gao, X.; Ghanbari, M.; Rueda-Ochoa, O.L.; Juvinao-Quintero, D.; et al. Epigenetic Link Between Statin Therapy and Type 2 Diabetes. Diabetes Care 2020, 43, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Zigmont, V.A.; Shoben, A.B.; Lu, B.; Kaye, G.L.; Clinton, S.K.; Harris, R.E.; Olivo-Marston, S.E. Statin users have an elevated risk of dysglycemia and new-onset-diabetes. Diabetes/Metab. Res. Rev. 2019, 35, e3189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culver, A.L.; Ockene, I.S.; Balasubramanian, R.; Olendzki, B.C.; Sepavich, D.M.; Wactawski-Wende, J.; Manson, J.E.; Qiao, Y.; Liu, S.; Merriam, P.A.; et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch. Intern. Med. 2012, 172, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Macedo, A.F.; Douglas, I.; Smeeth, L.; Forbes, H.; Ebrahim, S. Statins and the risk of type 2 diabetes mellitus: Cohort study using the UK clinical practice pesearch datalink. BMC Cardiovasc. Disord. 2014, 14, 85. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Noh, Y.; Shin, S.; Lim, H.S.; Park, R.W.; Bae, S.K.; Oh, E.; Kim, G.J.; Kim, J.H.; Lee, S. Impact of statins on risk of new onset diabetes mellitus: A population-based cohort study using the Korean National Health Insurance claims database. Ther. Clin. Risk Manag. 2016, 12, 1533–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajpathak, S.N.; Kumbhani, D.J.; Crandall, J.; Barzilai, N.; Alderman, M.; Ridker, P.M. Statin Therapy and Risk of Developing Type 2 Diabetes: A Meta-Analysis. Diabetes Care 2009, 32, 1924–1929. [Google Scholar] [CrossRef] [Green Version]
- Cybulska, B.; Kłosiewicz-Latoszek, L. How do we know that statins are diabetogenic, and why? Is it an important issue in the clinical practice? Kardiol. Pol. 2018, 76, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- Millán Núñez-Cortés, J.; Cases Amenós, A.; Ascaso Gimilio, J.F.; Barrios Alonso, V.; Pascual Fuster, V.; Pedro-Botet Montoya, J.C.; Pintó Sala, X.; Serrano Cumplido, A. Consensus on the Statin of Choice in Patients with Impaired Glucose Metabolism: Results of the DIANA Study. Am. J. Cardiovasc. Drugs 2017, 17, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Banach, M.; Malodobra-Mazur, M.; Gluba, A.; Katsiki, N.; Rysz, J.; Dobrzyn, A. Statin therapy and new-onset diabetes: Molecular mechanisms and clinical relevance. Curr. Pharm. Des. 2013, 19, 4904–4912. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Kostapanos, M.; Elisaf, M.S. Statins and their increased risk of inducing diabetes. Expert Opin. Drug Saf. 2015, 14, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Kuusisto, J. Diabetes Secondary to Treatment with Statins. Curr. Diab. Rep. 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G. New onset diabetes mellitus induced by statins: Current evidence. Postgrad. Med. 2019, 129, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Crandall, J.P.; Mather, K.; Rajpathak, S.N.; Goldberg, R.B.; Watson, K.; Foo, S.; Ratner, R.; Barrett-Connor, E.; Temprosa, M. Statin use and risk of developing diabetes: Results from the Diabetes Prevention Program. BMJ Open Diabetes Res. Care 2017, 5, e000438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engeda, J.C.; Stackhouse, A.; White, M.; Rosamond, W.D.; Lhachimi, S.K.; Lund, J.L.; Keyserling, T.C.; Avery, C.L. Evidence of heterogeneity in statin-associated type 2 diabetes mellitus risk: A meta-analysis of randomized controlled trials and observational studies. Diabetes Res. Clin. Pract. 2019, 151, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, R.; Trompet, S.; Leong, A.; Goodarzi, M.O.; Postmus, I.; Warren, H.; Theusch, E.; Barnes, M.R.; Arsenault, B.J.; Li, X.; et al. Statin-induced LDL cholesterol response and type 2 diabetes: A bidirectional two-sample Mendelian randomization study. Pharm. J. 2020, 20, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Ganda, O.P. Statin-induced diabetes: Incidence, mechanisms, and implications. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Vavlukis, M.; Kedev, S. Effects of High Intensity Statin Therapy in the Treatment of Diabetic Dyslipidemia in Patients with Coronary Artery Disease. Curr. Pharm. Des. 2018, 24, 427–441. [Google Scholar] [CrossRef]
- Agarwala, A.; Kulkarni, S.; Maddox, T. The Association of Statin Therapy with Incident Diabetes: Evidence, Mechanisms, and Recommendations. Curr. Cardiol. Rep. 2018, 20, 50. [Google Scholar] [CrossRef]
- Maki, K.C.; Diwadkar-Navsariwala, V.; Kramer, M.W. Statin use and risk for type 2 diabetes: What clinicians should know. Postgrad. Med. 2018, 130, 166–172. [Google Scholar] [CrossRef]
- United Nations. The 2030 Agenda and the Sustainable Development Goals: An Opportunity for Latin America and the Caribbean; LC/G.2681-P/Rev.3; United Nations Publication: Santiago, Chile, 2018. [Google Scholar]
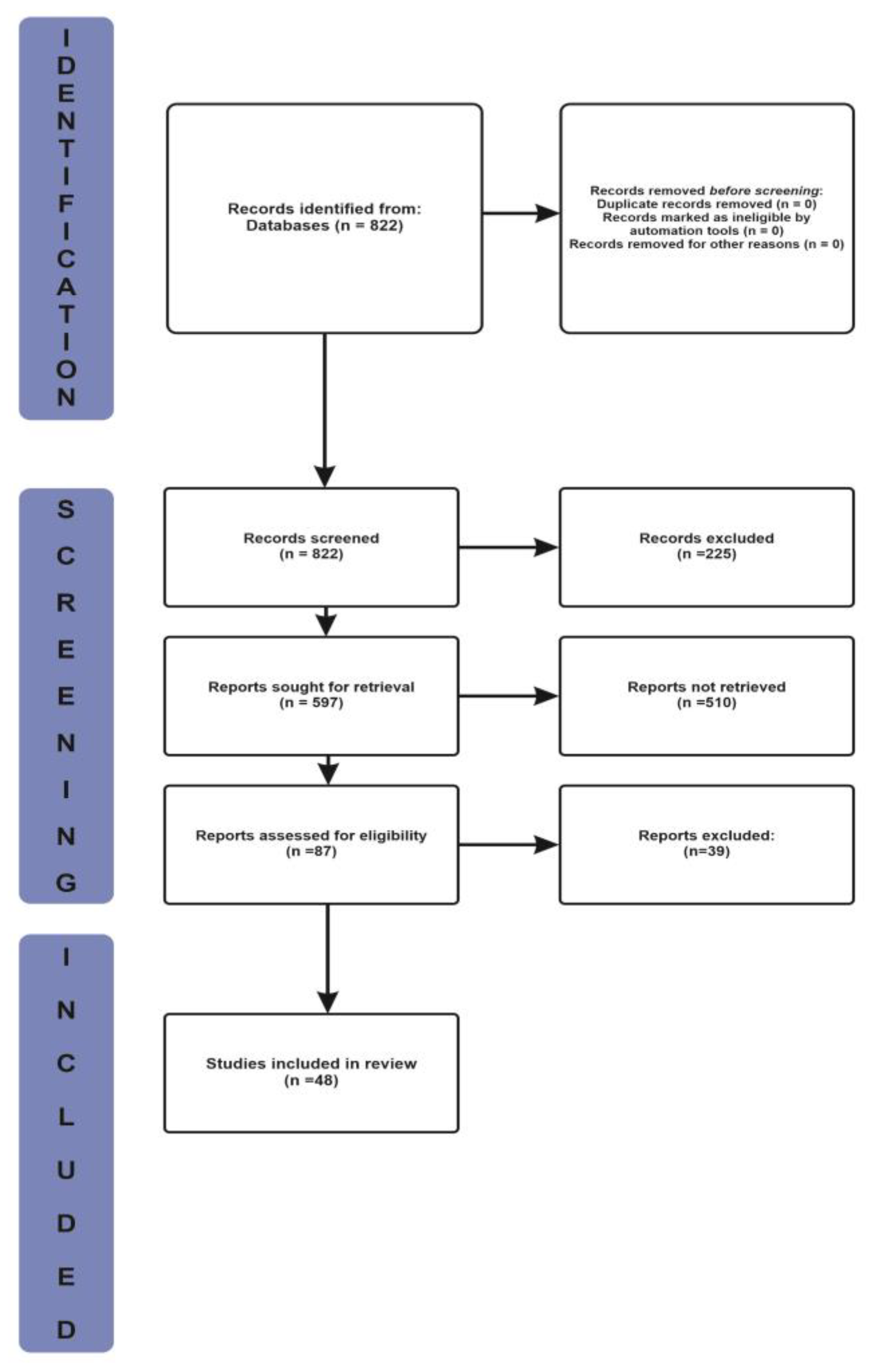
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Study design | Quantitative, qualitative and mixed-method designs. | Animal studies |
| Location | Low-, middle- and high-income countries | None |
| Year | 2009–2022 | Before 2009 |
| Language | English | Any other language |
| Comorbidities | Dyslipidaemia | Pre-existing diabetes mellitus, pancreatitis and any others |
| Reference | Year | Country | Main Objective | Study Design | Study Population | Population Characteristics | Statin Included | Comparison | Follow up Time | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
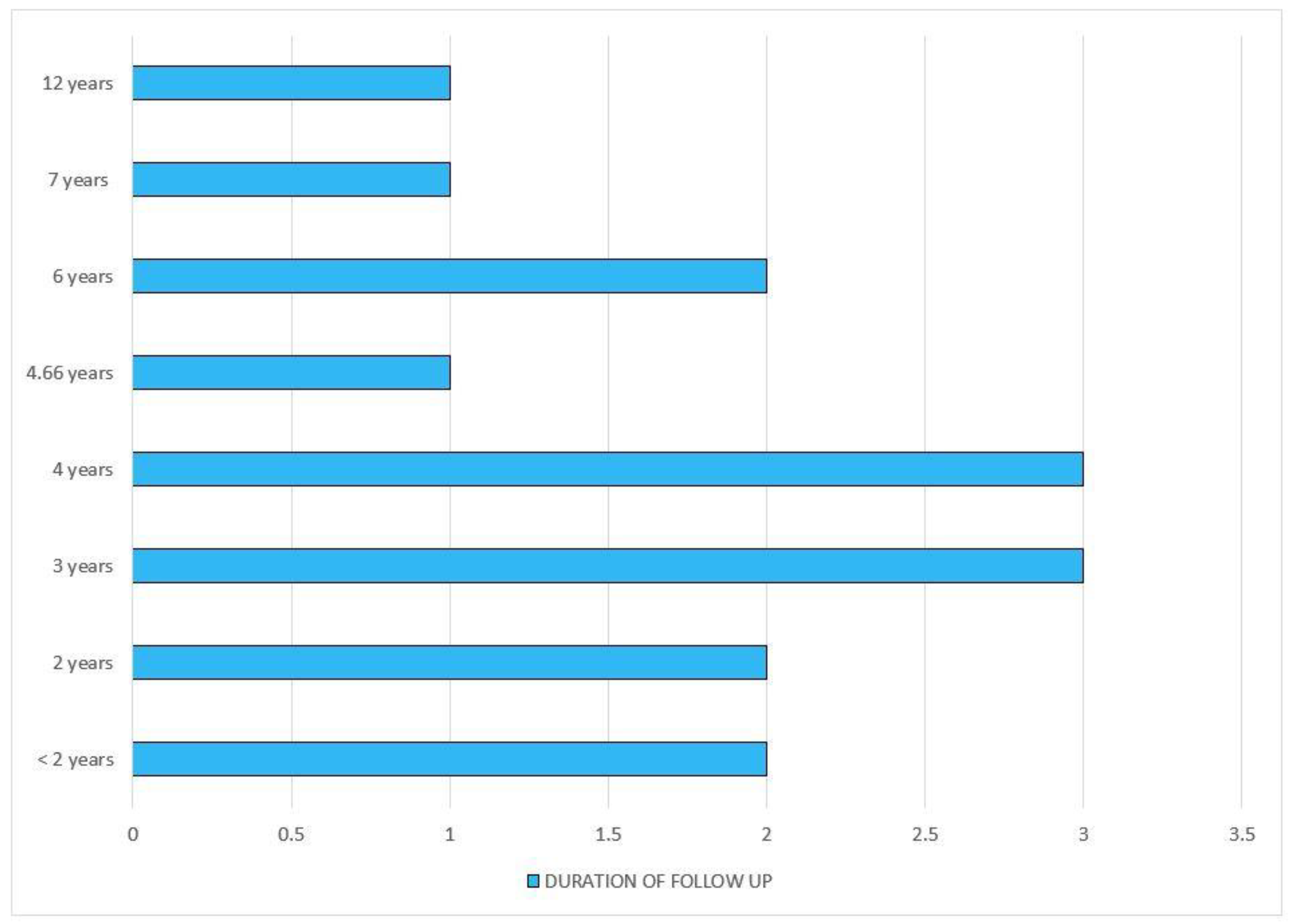
| [20] | 2019 | The Netherlands | To investigate the association of statin use with glycemic traits with incident type 2 diabetes | Prospective cohort study | 9535 individuals | Age—64.3 (SD-10.1) years; 41% were men | Not specified | Non statin users | 4 years | Statin users have increased serum fasting insulin and insulin resistance |
| [21] | 2019 | Korea | To find risk of diabetes mellitus associated with statin therapy | Prospective cohort study | 1,034,982 individuals | Never statin users—Age 51.9 years, ever statin users—Age 55.5 years | Not specified | Never statin users | 4 years | There are time- and dose-dependent associations of statin use with an increased risk of new-onset diabetes mellitus |
| [22] | 2021 | Korea | To evaluate the incidence of neuropathy among patients with T2DM associated with statin and metformin therapies | Prospective cohort study | 2016—34,964 individuals; 2017—35,887 individuals | Age—30 to 70 years divided into 5 groups. | Not specified | Combined statin and metformin users | 2 years | Statin users have increased risk of hyperglycemia, insulin resistance and type 2 diabetes mellitus |
| [23] | 2020 | Korea | To explore the relationship between baseline LDL—c and the risk of statin-induced insulin resistance | Propensity-matched analysis | 2660 patients | Not mentioned | Not specified | None | 2 years | Insulin resistance was higher in statin users without baseline dyslipidemia than inthose with dyslipidemia |
| [24] | 2021 | USA | To evaluate the progression of diabetes in diabetic patients after statin use | Retrospective cohort study | 83,022 individuals | Mean age of 60.1, 94.9% were men | Not specified | Non-statin users with diabetes | 12 years | The progression of diabetes was higher in statin users as compared to active comparators |
| [25] | 2020 | Italy | To find out the risk of macrovascular complications associated with type 2 diabetes induced by statins | Prospective cohort study | 84,828 individuals | Age group—40 to 80 years | Not specified | Non-diabetic patients | 6 years | The prognosis of statin-dependent type 2 diabetes is less adverse than that not induced by statins |
| [26] | 2019 | Greece | To explore the metabolic factors that influence the increased risk of diabetes mellitus in patients under statin therapy | Retrospective cohort study | 1241 patients with dyslipidemia | Age group 46–64 years | Not specified | None | 3 years | Atherogenic dyslipidemia is independently associated with NODM |
| [27] | 2019 | Korea | To identify the risk of developing diabetes in patients taking lowest and highest doses of pitavastatin | Single blinded randomized study | 1044 patients | Age—30 to 79 years | Pitavastatin | Highest and lowest dose of Pitavastatin | 3 years | There was no difference in the incidence of development of NODM between two groups |
| [28] | 2015 | Finland | To decipher the mechanism associated with statin induced diabetes mellitus | Prospective cohort study | 8749 individuals | Age—45 to 73 years | Not specified | Never statin users | 6 years | The factors contributing to increased diabetes risk are decreased insulin sensitivity and insulin secretion |
| [29] | 2017 | India | To analyze the effect of atorvastatin on the glycemic indexes of normoglycemic and prediabetic individuals | Prospective panel-based study | 75 patients | Group A (N = 25), female (36%), age 53.95 years (SD = 6.9); Group B (N = 25), female 24%, age 51.05 years (7.1) | Atorvastatin | None | Up to 1.5 years | In normoglycemic patients, atorvastatin therapy induced glucose intolerance, and in prediabetic individuals, it led to diabetic progression |
| [30] | 2010 | UK | To establish the relationship between statinuse and onset of diabetes | Meta-analysis | 91,140 participants | Not mentioned | Not specified | Not specified | 4 years | There is a marginal increase in the risk of developing diabetes with the use of statins |
| [31] | 2021 | USA | To elucidate the physiological mechanism underlying the increased risk of diabetes mellitus in statin treatment | Clinical trial | 75 participants | Median age 61 years, 37% women | Atorvastatin | None | 10 weeks | High-intensity atorvastatin elevates blood glucose levels by increasing insulin resistance and decreasing insulin secretion |
| [32] | 2016 | Greece | To evaluate the risk of progression from prediabetes to diabetes in individuals under statin therapy | Retrospective cohort study | 877 participants | Not mentioned | Not specified | Normoglycemic and prediabetic individuals | 7 years | High-intensity statin treatment posed a higher risk of the development of diabetes in prediabetic individuals |
| [33] | 2020 | The Netherlands | To determine the role of epigenetics in statin-induced diabetes mellitus | Prospective cohort study | 8270 participants | Age group; 35 to 75 years; 56% South Asian, 44% Caucasians | Not specified | None | Not specified | DNA methylation is associated with the diabetogenic potential of statins |
| [34] | 2019 | USA | To determine the risk of dysglycemia in patients under statin therapy | Retrospective cohort study | 7064 participants | Not mentioned | Not specified | None | Not specified | Elevated HbA1c was observed in non-diabetic statin users. |
| [35] | 2012 | USA | To investigate the association between the use of statins and the onset of diabetes in postmenopausal women | Clinical trial | 161,808 post-menopausal women | Age group—50 to 79 years | Not specified | None | 3 years | Statin use is associated with increased risk of diabetes in postmenopausal women. |
| [36] | 2014 | UK | To evaluate the effects of statins on NODM | Prospective cohort study | 2,016,094 individuals | Age group: 30 to 85 years | Not specified | Non statin users | Mean 4.66 years | Type 2 diabetes mellitus is proven to be associated with statin use. |
| [37] | 2016 | Korea | To investigate associations between statins and NODM in patients with ischemic heart disease | Retrospective cohort study | 156,360 patients | Age group: ≥18 years | Variety of statins | None | Not mentioned | All statins are associated with increased risk of NODM in ischemic heart disease patients, with pravastatin having the lowest risk. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Needamangalam Balaji, J.; Prakash, S.; Joshi, A.; Surapaneni, K.M. A Scoping Review on the Reported Evidence and Gaps of the Risk of Diabetes in Dyslipidemic Patients under Statin Therapy. Clin. Pract. 2022, 12, 565-578. https://doi.org/10.3390/clinpract12040060
Needamangalam Balaji J, Prakash S, Joshi A, Surapaneni KM. A Scoping Review on the Reported Evidence and Gaps of the Risk of Diabetes in Dyslipidemic Patients under Statin Therapy. Clinics and Practice. 2022; 12(4):565-578. https://doi.org/10.3390/clinpract12040060
Chicago/Turabian StyleNeedamangalam Balaji, Jyotsna, Sreenidhi Prakash, Ashish Joshi, and Krishna Mohan Surapaneni. 2022. "A Scoping Review on the Reported Evidence and Gaps of the Risk of Diabetes in Dyslipidemic Patients under Statin Therapy" Clinics and Practice 12, no. 4: 565-578. https://doi.org/10.3390/clinpract12040060
APA StyleNeedamangalam Balaji, J., Prakash, S., Joshi, A., & Surapaneni, K. M. (2022). A Scoping Review on the Reported Evidence and Gaps of the Risk of Diabetes in Dyslipidemic Patients under Statin Therapy. Clinics and Practice, 12(4), 565-578. https://doi.org/10.3390/clinpract12040060








