Association between Empagliflozin Use and Electrocardiographic Changes
Abstract
:1. Introduction
2. Materials and Methods
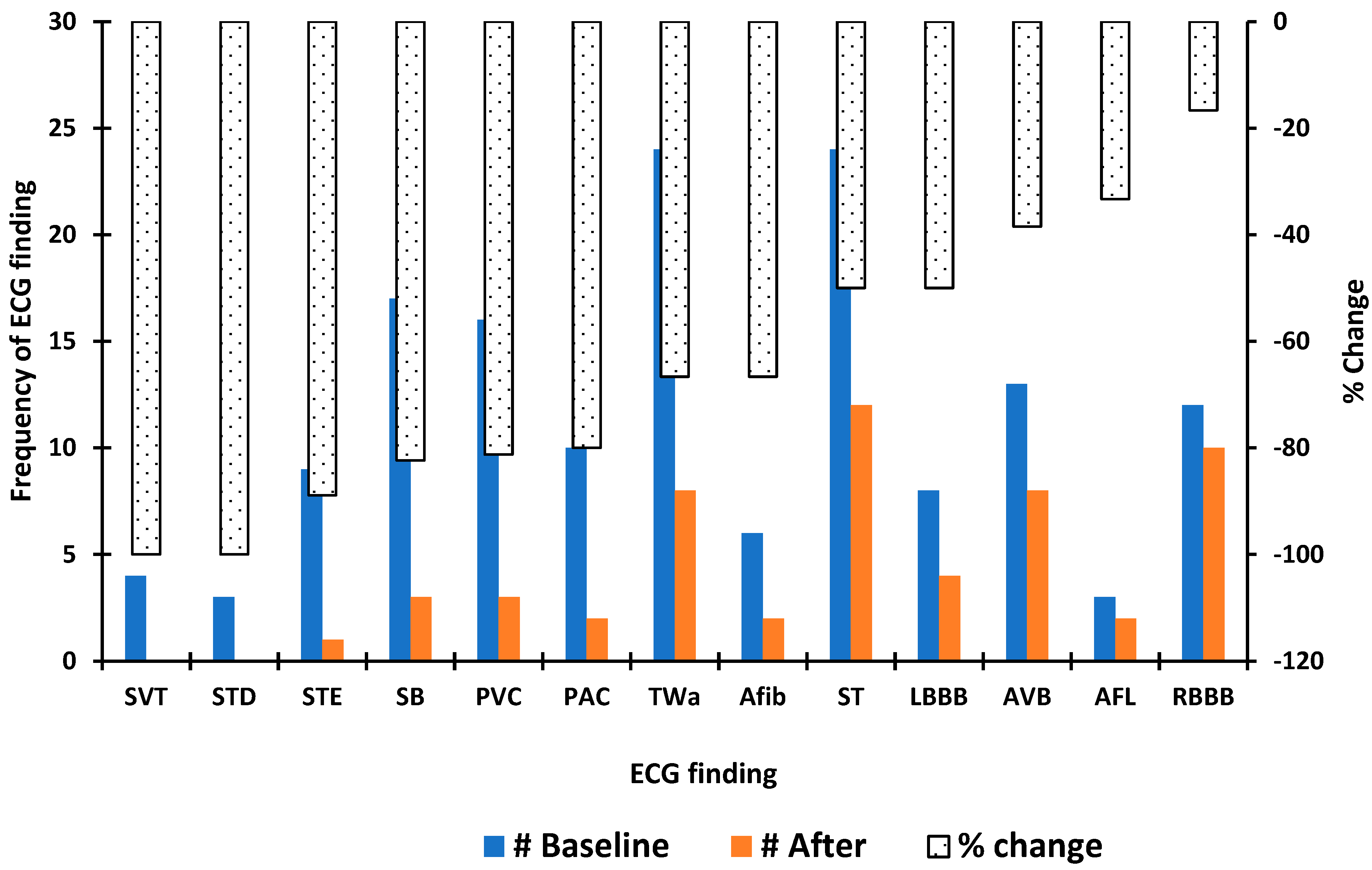
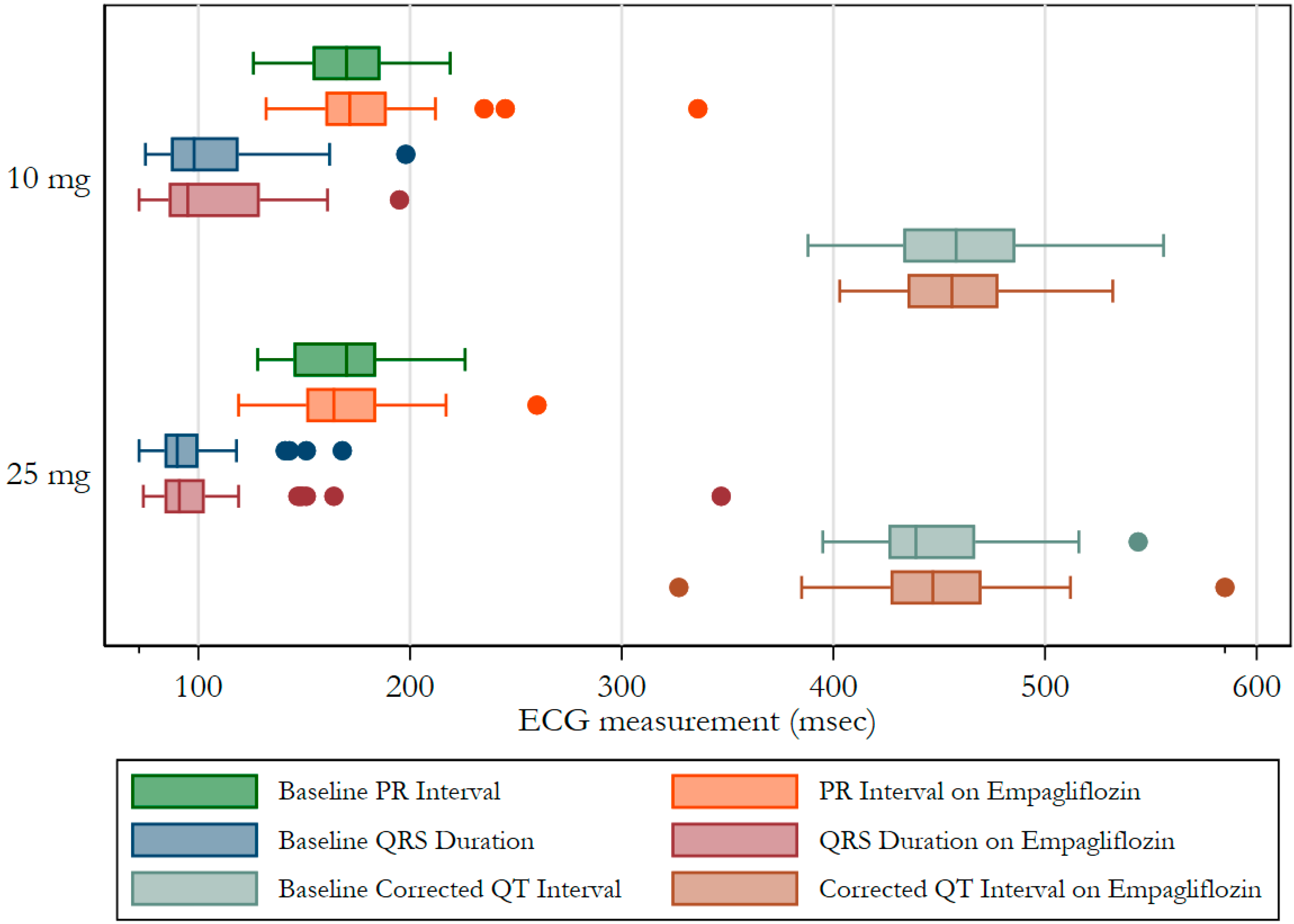
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maddox, T.M.; Januzzi, J.L.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; Masoudi, F.A.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Huang, J.-Y.; Siao, W.-Z.; Jong, G.-P. The association between SGLT2 inhibitors and new-onset arrhythmias: A nationwide population-based longitudinal cohort study. Cardiovasc. Diabetol. 2020, 19, 73. [Google Scholar] [CrossRef]
- Fernandes, G.C.; Fernandes, A.; Cardoso, R.; Penalver, J.; Knijnik, L.; Mitrani, R.D.; Myerburg, R.J.; Goldberger, J.J. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: A meta-analysis of 34 randomized controlled trials. Heart. Rhythm 2021, 18, 1098–1105. [Google Scholar] [CrossRef]
- Li, W.-J.; Chen, X.-Q.; Xu, L.-L.; Li, Y.-Q.; Luo, B.-H. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: A systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc. Diabetol. 2020, 19, 130. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Bonaca, M.P.; Furtado, R.H.; Mosenzon, O.; Kuder, J.F.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.; et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus. Circulation 2020, 141, 1227–1234. [Google Scholar] [CrossRef]
- Philippaert, K.; Kalyaanamoorthy, S.; Fatehi, M.; Long, W.; Soni, S.; Byrne, N.J.; Barr, A.; Singh, J.; Wong, J.; Palechuk, T.; et al. Cardiac Late Sodium Channel Current Is a Molecular Target for the Sodium/Glucose Cotransporter 2 Inhibitor Empagliflozin. Circulation 2021, 143, 2188–2204. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Hézső, T.; Kiss, D.; Kistamas, K.; Magyar, J.; Nánási, P.P.; Bányász, T. Late Sodium Current Inhibitors as Potential Antiarrhythmic Agents. Front. Pharmacol. 2020, 11, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Li, G.; Huang, C.L.-H.; Lei, M.; Wu, L. Late sodium current associated cardiac electrophysiological and mechanical dysfunction. Pflugers Arch. 2018, 470, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Huxley, R.R.; Filion, K.B.; Konety, S.; Alonso, A. Meta-Analysis of Cohort and Case–Control Studies of Type 2 Diabetes Mellitus and Risk of Atrial Fibrillation. Am. J. Cardiol. 2011, 108, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Feng, T.; Schlesinger, S.; Janszky, I.; Norat, T.; Riboli, E. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. J. Diabetes Its Complicat. 2018, 32, 501–511. [Google Scholar] [CrossRef]
- Movahed, M.-R.; Hashemzadeh, M.; Jamal, M.M. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int. J. Cardiol. 2005, 105, 315–318. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Salles, G.F.; Deccache, W. Prognostic value of QT interval parameters in type 2 diabetes mellitus: Results of a long-term follow-up prospective study. J. Diabetes Its Complicat. 2003, 17, 169–178. [Google Scholar] [CrossRef]
- Grisanti, L.A. Diabetes and Arrhythmias: Pathophysiology, Mechanisms and Therapeutic Outcomes. Front. Physiol. 2018, 9, 1669. [Google Scholar] [CrossRef]
- Ma¨kimattila, S.; Ma¨ntysaari, M.; Groop, P.-H.; Summanen, P.; Virkama¨ki, A.; Schlenzka, A.; Fagerudd, J.; Yki-Ja¨rvinen, H. Hyperreactivity to Nitrovasodilators in Forearm Vasculature Is Related to Autonomic Dysfunction in Insulin-Dependent Diabetes Mellitus. Circulation 1997, 95, 618–625. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, A.H.; Schildcrout, J.S.; Blakemore, D.L.; Masys, D.R.; Pulley, J.M.; Basford, M.A.; Roden, D.M.; Denny, J.C. Modulators of normal electrocardiographic intervals identified in a large electronic medical record. Heart Rhythm 2011, 8, 271–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.-H.; Wu, L.-S.; Chiou, M.-J.; Liu, J.-R.; Yu, K.-H.; Kuo, C.-F.; Wen, M.-S.; Chen, W.-J.; Yeh, Y.-H.; See, L.-C. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: A population-based dynamic cohort and in vitro studies. Cardiovasc. Diabetol. 2014, 13, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, T.-F.; Leu, H.-B.; Huang, C.-C.; Chen, J.-W.; Chan, W.-L.; Lin, S.-J.; Chen, S.-A. Thiazolidinediones can prevent new onset atrial fibrillation in patients with non-insulin dependent diabetes. Int. J. Cardiol. 2012, 156, 199–202. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Yeh, Y.-H.; Chan, Y.-H.; Liu, J.-R.; Chang, S.-H.; Lee, H.-F.; Wu, L.-S.; Yen, K.-C.; Kuo, C.-T.; See, L.-C. Dipeptidyl peptidase-4 inhibitor decreases the risk of atrial fibrillation in patients with type 2 diabetes: A nationwide cohort study in Taiwan. Cardiovasc. Diabetol. 2017, 16, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtain, J.P.; Docherty, K.F.; Jhund, P.S.; Petrie, M.C.; Inzucchi, E.S.; Køber, L.; Kosiborod, M.N.; Martinez, A.F.; Ponikowski, P.; Sabatine, M.S.; et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur. Heart J. 2021, 42, 3727–3738. [Google Scholar] [CrossRef]
- Jhuo, S.-J.; Liu, I.-H.; Tasi, W.-C.; Chou, T.-W.; Lin, Y.-H.; Wu, B.-N.; Lee, K.-T.; Lai, W.-T. Characteristics of Ventricular Electrophysiological Substrates in Metabolic Mice Treated with Empagliflozin. Int. J. Mol. Sci. 2021, 22, 6105. [Google Scholar] [CrossRef]
- Maier, L.S. A Novel Mechanism for the Treatment of Angina, Arrhythmias, and Diastolic Dysfunction: Inhibition of Late INa Using Ranolazine. J. Cardiovasc. Pharmacol. 2009, 54, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Frommeyer, G.; Milberg, P.; Maier, L.; Eckardt, L. Late sodium current inhibition: The most promising antiarrhythmic principle in the near future? Curr. Med. Chem. 2014, 21, 1271–1280. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Nesterenko, V.; Shryock, J.C.; Rajamani, S.; Song, Y.; Belardinelli, L. The Role of Late I Na in Development of Cardiac Arrhythmias. Handb. Exp. Pharmacol. 2014, 221, 137–168. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-I.; Chen, Y.-C.; Lin, Y.-K.; Chung, C.-C.; Lu, Y.-Y.; Kao, Y.-H.; Chen, Y.-J. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burashnikov, A.; Antzelevitch, C. Role of Late Sodium Channel Current Block in the Management of Atrial Fibrillation. Cardiovasc. Drugs Ther. 2012, 27, 79–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitbeck, M.G.; Charnigo, R.J.; Shah, J.; Morales, G.; Leung, S.W.; Fornwalt, B.; Bailey, A.L.; Ziada, K.; Sorrell, V.L.; Zegarra, M.M.; et al. QRS duration predicts death and hospitalization among patients with atrial fibrillation irrespective of heart failure: Evidence from the AFFIRM study. Europace 2013, 16, 803–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauchier, L.; Marie, O.; Casset-Senon, D.; Babuty, D.; Cosnay, P.; Fauchier, J.P. Reliability of QRS duration and morphology on surface electrocardiogram to identify ventricular dyssynchrony in patients with idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2003, 92, 341–344. [Google Scholar] [CrossRef]
- Iuliano, S.; Fisher, S.G.; Karasik, P.E.; Fletcher, R.D.; Singh, S.N. QRS duration and mortality in patients with congestive heart failure. Am. Heart J. 2002, 143, 1085–1091. [Google Scholar] [CrossRef]
- Stanaway, S.E.R.S.; Gill, G.V. Protein glycosylation in diabetes mellitus: Biochemical and clinical considerations. Pract. Diabetes Int. 2000, 17, 21–25. [Google Scholar] [CrossRef]
- Baycin-Hizal, D.; Gottschalk, A.; Jacobson, E.; Mai, S.; Wolozny, D.; Zhang, H.; Krag, S.S.; Betenbaugh, M.J. Physiologic and pathophysiologic consequences of altered sialylation and glycosylation on ion channel function. Biochem. Biophys. Res. Commun. 2014, 453, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Margraf-Schönfeld, S.; Böhm, C.; Watzl, C. Glycosylation Affects Ligand Binding and Function of the Activating Natural Killer Cell Receptor 2B4 (CD244) Protein. J. Biol. Chem. 2011, 286, 24142–24149. [Google Scholar] [CrossRef] [Green Version]
- Jordan, J.; Tank, J.; Heusser, K.; Heise, T.; Wanner, C.; Heer, M.; Macha, S.; Mattheus, M.; Lund, S.S.; Woerle, H.J.; et al. The effect of empagliflozin on muscle sympathetic nerve activity in patients with type II diabetes mellitus. J. Am. Soc. Hypertens. 2017, 11, 604–612. [Google Scholar] [CrossRef]
| Condition | n (%) |
|---|---|
| Alcohol use | 18 (17.8) |
| Tobacco use | 61 (60.4) |
| Illicit drugs use | 12 (11.9) |
| Cancer | 25 (24.8) |
| Coronary artery disease | 33 (32.7) |
| Chronic obstructive pulmonary disease | 16 (15.8) |
| Chronic kidney disease | 30 (29.7) |
| Heart failure | 17 (16.8) |
| Hypertension | 86 (85.2) |
| Hyperlipidemia | 89 (88.1) |
| Obesity | 57 (56.4) |
| Peripheral artery disease | 9 (8.9) |
| Sleep apnea | 25 (24.8) |
| Stroke | 13 (12.9) |
| Empagliflozin Initiation | |||
|---|---|---|---|
| Medication | before n (%) | after n (%) | p-Value |
| Aspirin | 26 (33.3) | 33 (42.3) | 0.48 |
| ACE inhibitors | 58 (74.4) | 44 (56.4) | 0.06 |
| Angiotensin receptor blockers | 26 (33.3) | 32 (41) | 0.53 |
| Beta blockers | 40 (51.3) | 44 (56.4) | 0.65 |
| Calcium channel blockers | 26 (33.3) | 25 (32.1) | 0.94 |
| Statin | 68 (87.2) | 66 (84.6) | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antwi-Amoabeng, D.; Sathappan, S.; Beutler, B.D.; Ulanja, M.B.; Awad, M.; Gullapalli, N.; Duncan, P.; Gbadebo, T.D. Association between Empagliflozin Use and Electrocardiographic Changes. Clin. Pract. 2022, 12, 557-564. https://doi.org/10.3390/clinpract12040059
Antwi-Amoabeng D, Sathappan S, Beutler BD, Ulanja MB, Awad M, Gullapalli N, Duncan P, Gbadebo TD. Association between Empagliflozin Use and Electrocardiographic Changes. Clinics and Practice. 2022; 12(4):557-564. https://doi.org/10.3390/clinpract12040059
Chicago/Turabian StyleAntwi-Amoabeng, Daniel, Sunil Sathappan, Bryce D. Beutler, Mark B. Ulanja, Munadel Awad, Nageshwara Gullapalli, Phillip Duncan, and T. David Gbadebo. 2022. "Association between Empagliflozin Use and Electrocardiographic Changes" Clinics and Practice 12, no. 4: 557-564. https://doi.org/10.3390/clinpract12040059
APA StyleAntwi-Amoabeng, D., Sathappan, S., Beutler, B. D., Ulanja, M. B., Awad, M., Gullapalli, N., Duncan, P., & Gbadebo, T. D. (2022). Association between Empagliflozin Use and Electrocardiographic Changes. Clinics and Practice, 12(4), 557-564. https://doi.org/10.3390/clinpract12040059






