Vertical Transmission of SARS-CoV-2 Infection and Miscarriage in the Second Trimester: Report of an Immunohistochemically Proven Case
Abstract
:1. Introduction
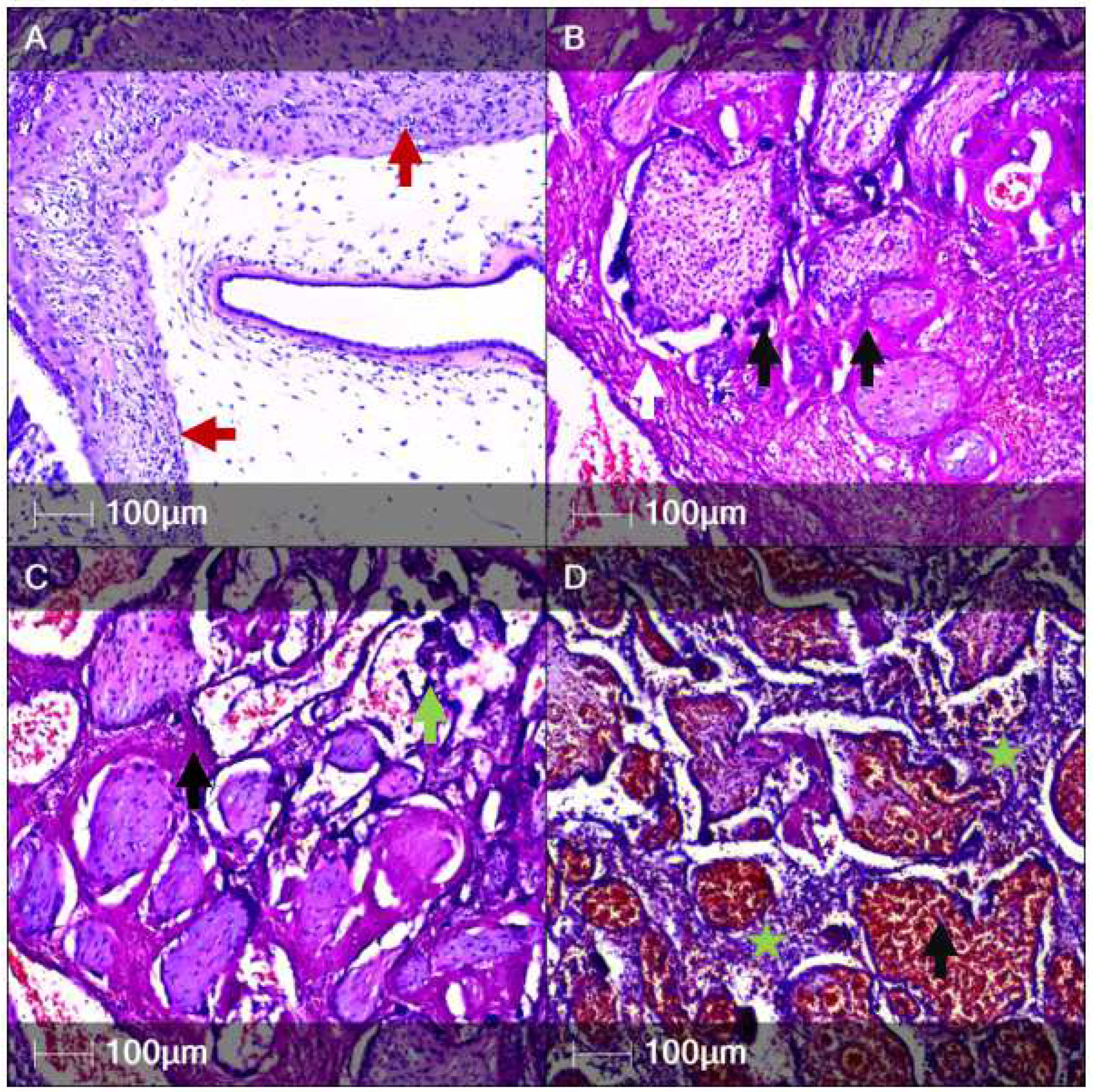
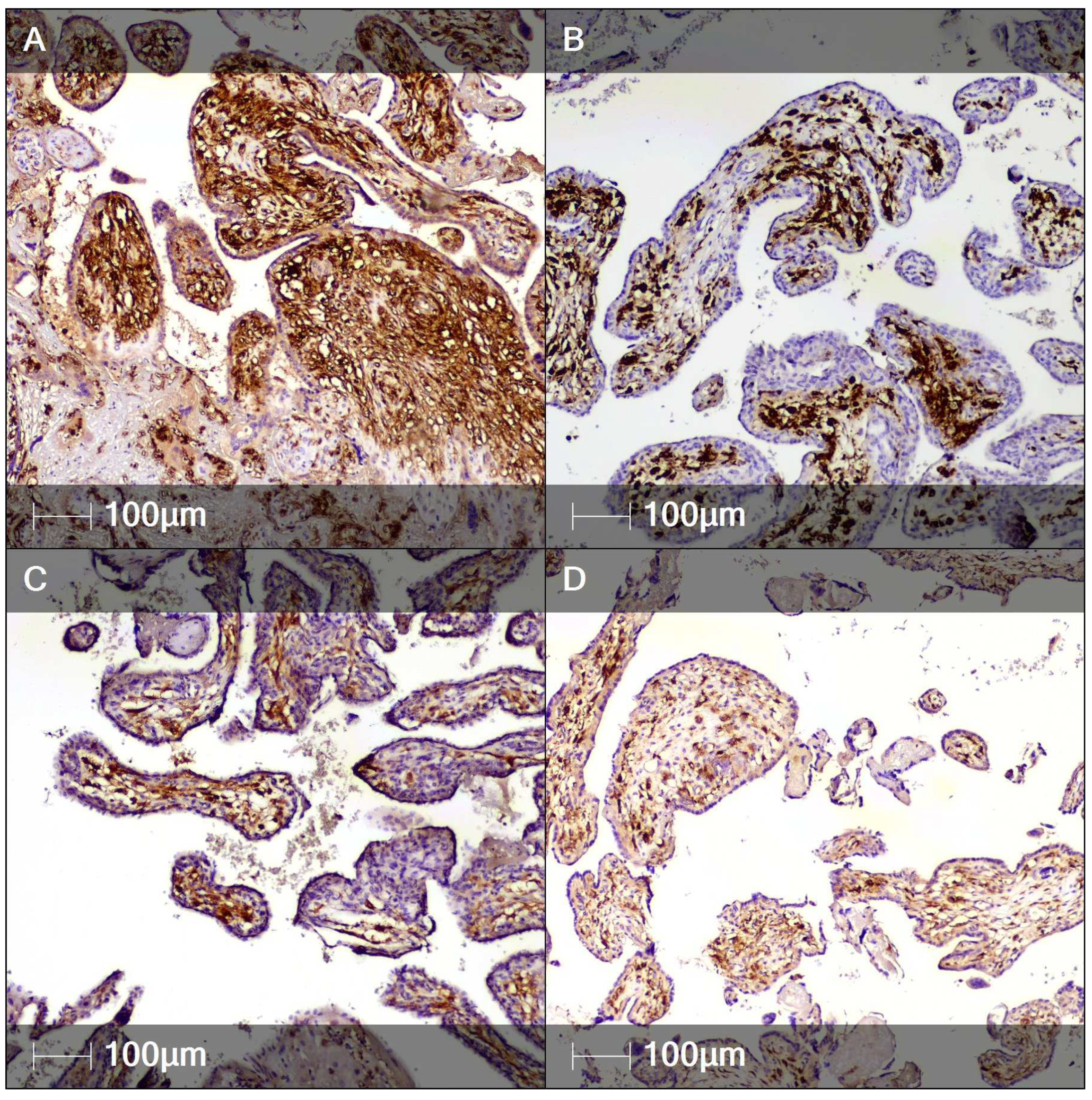
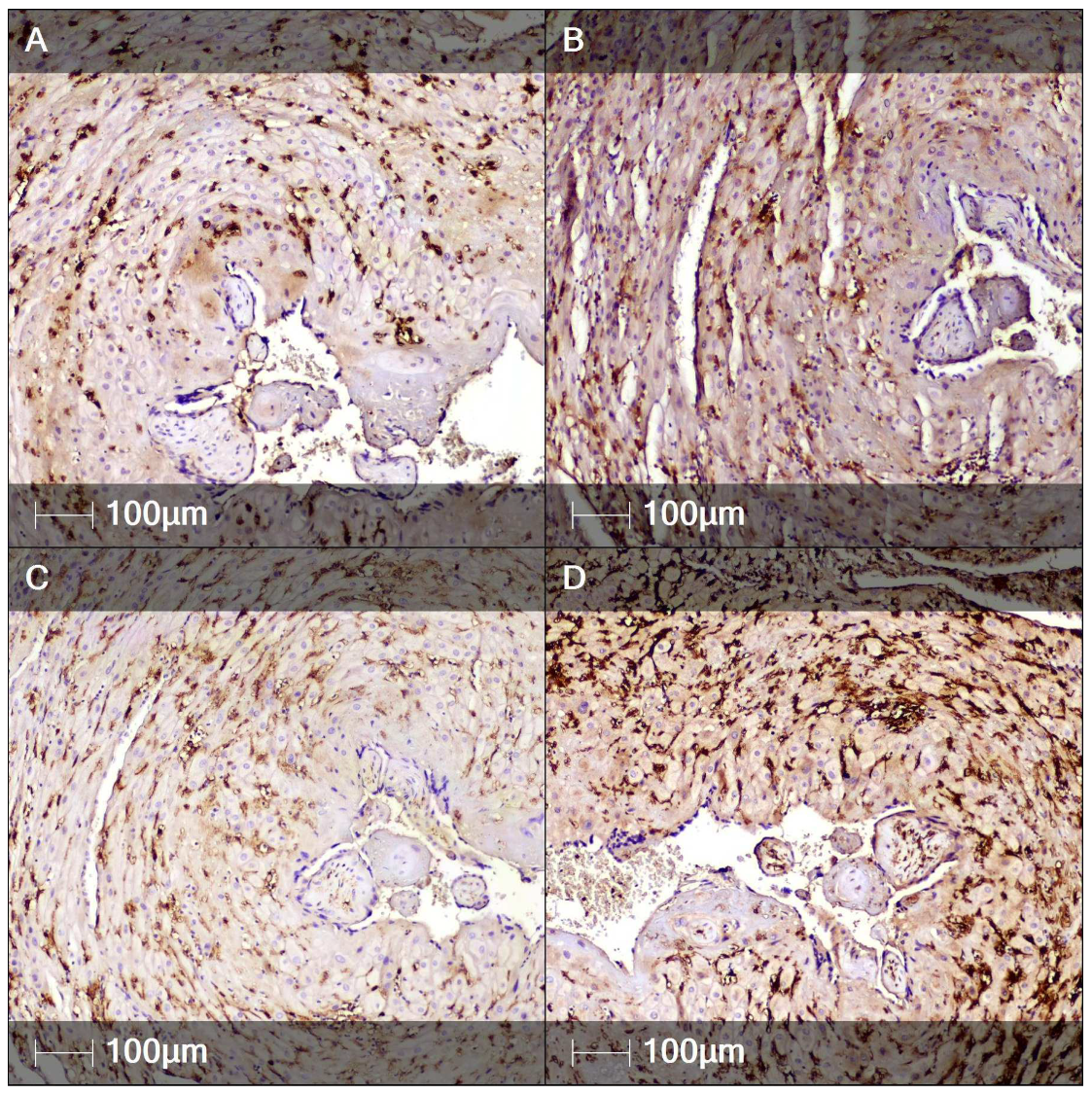
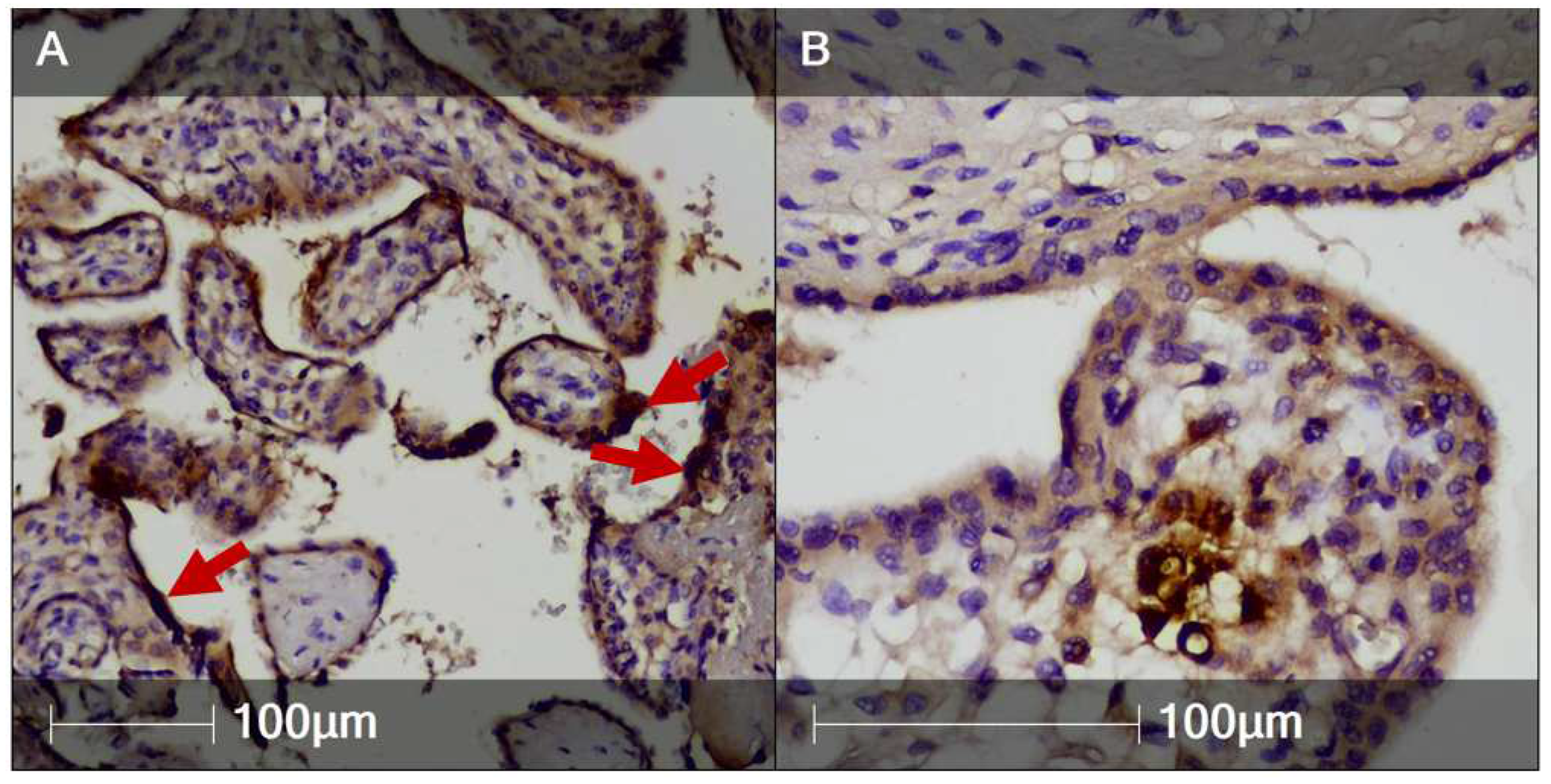
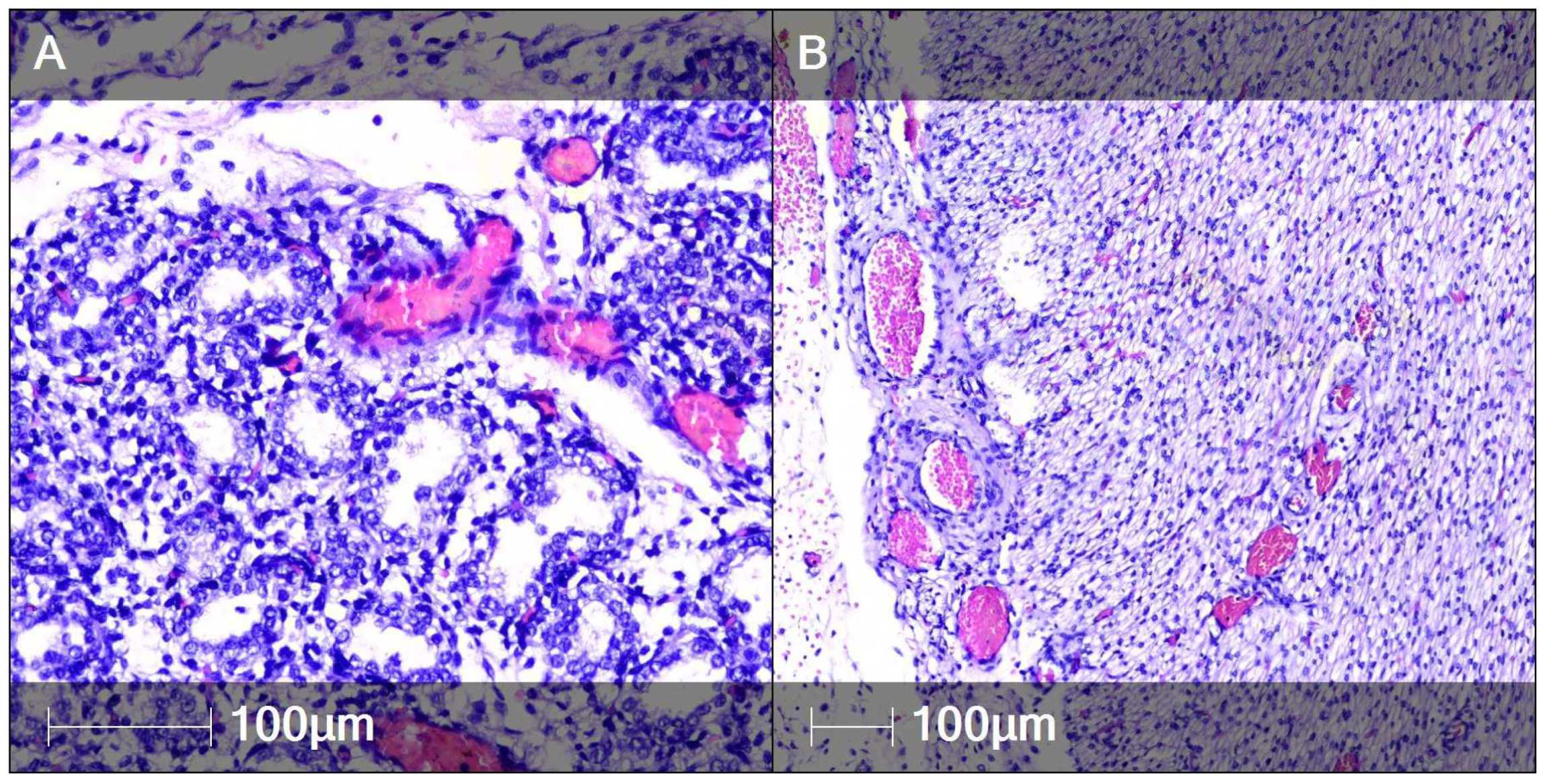
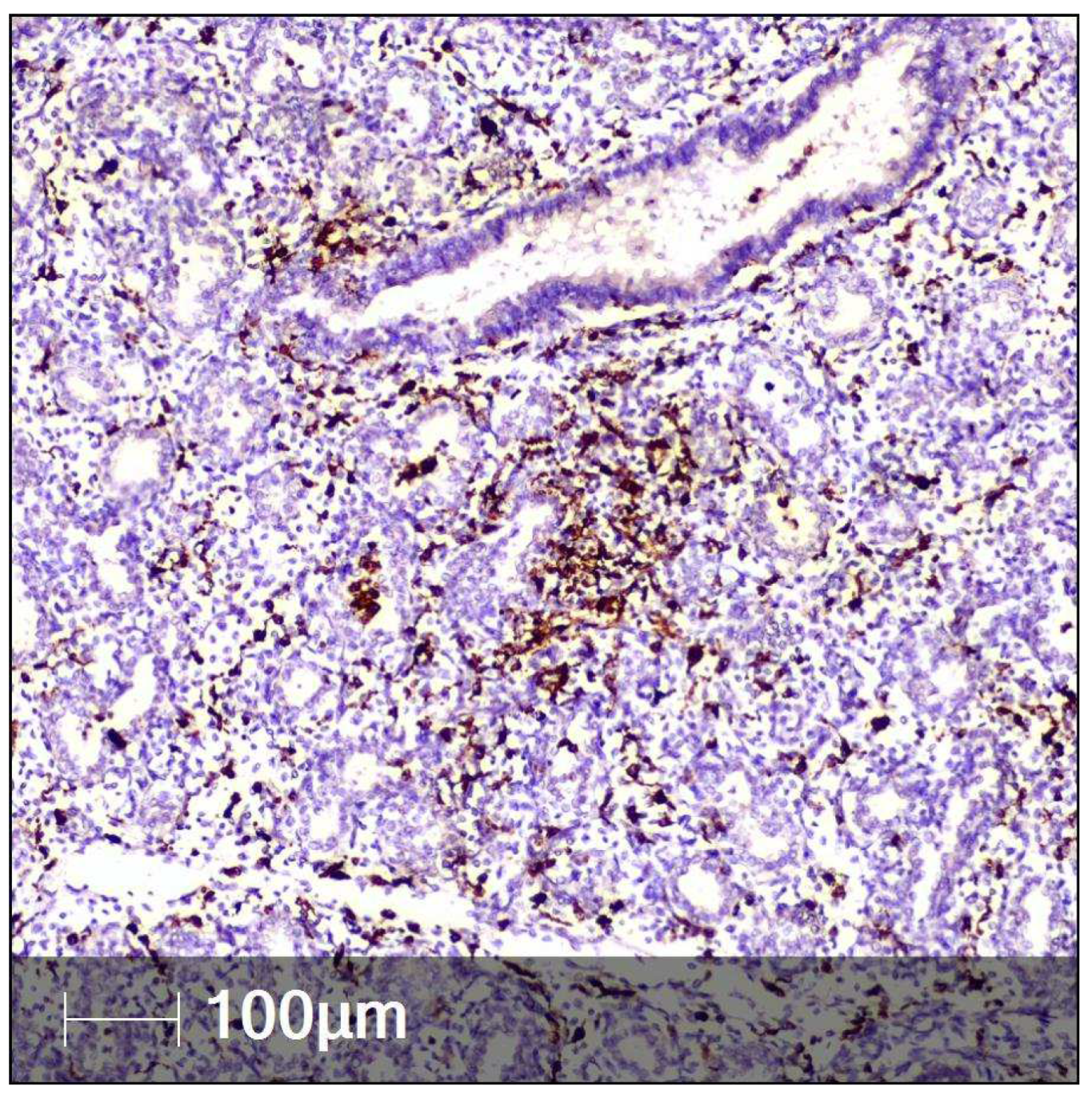
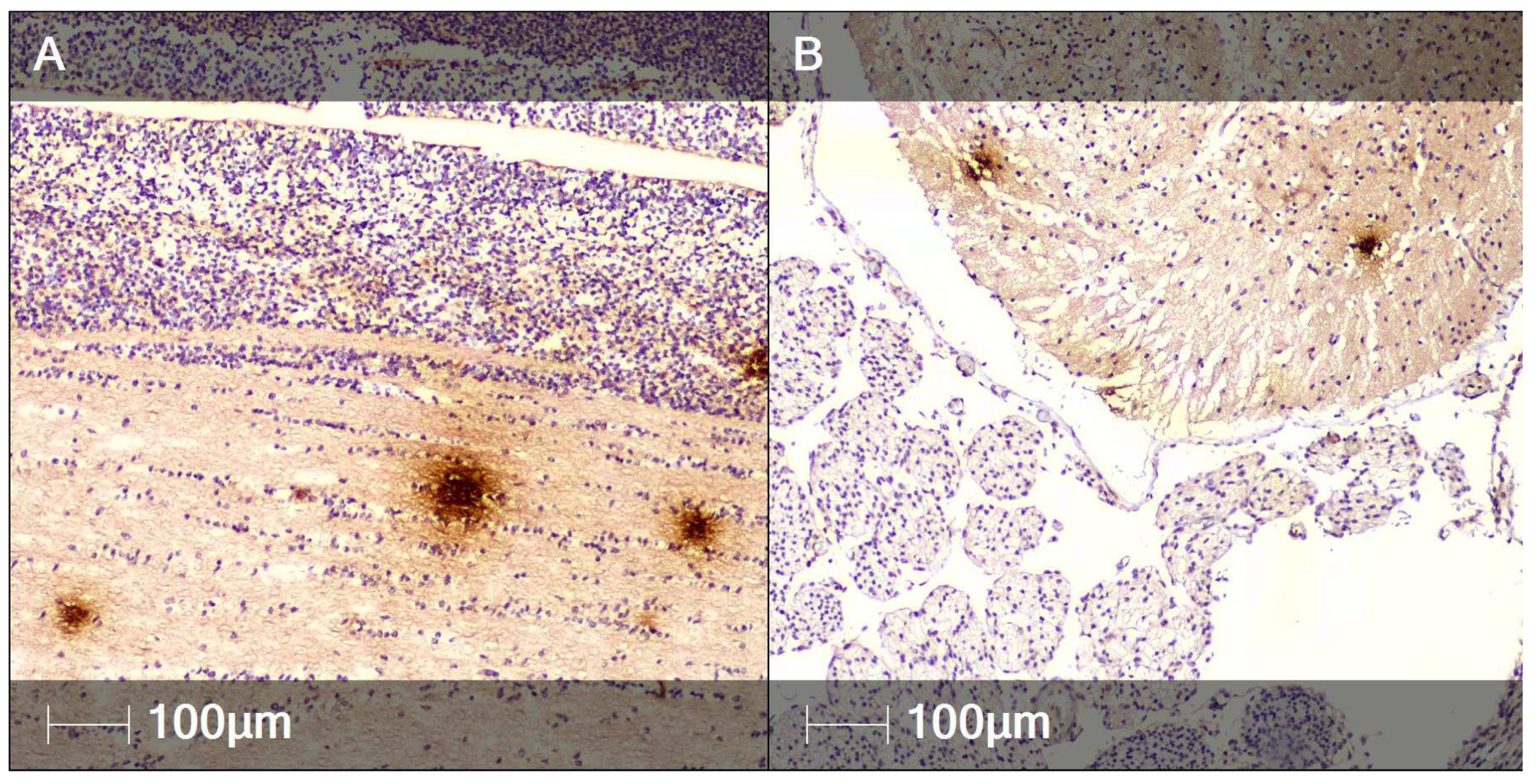
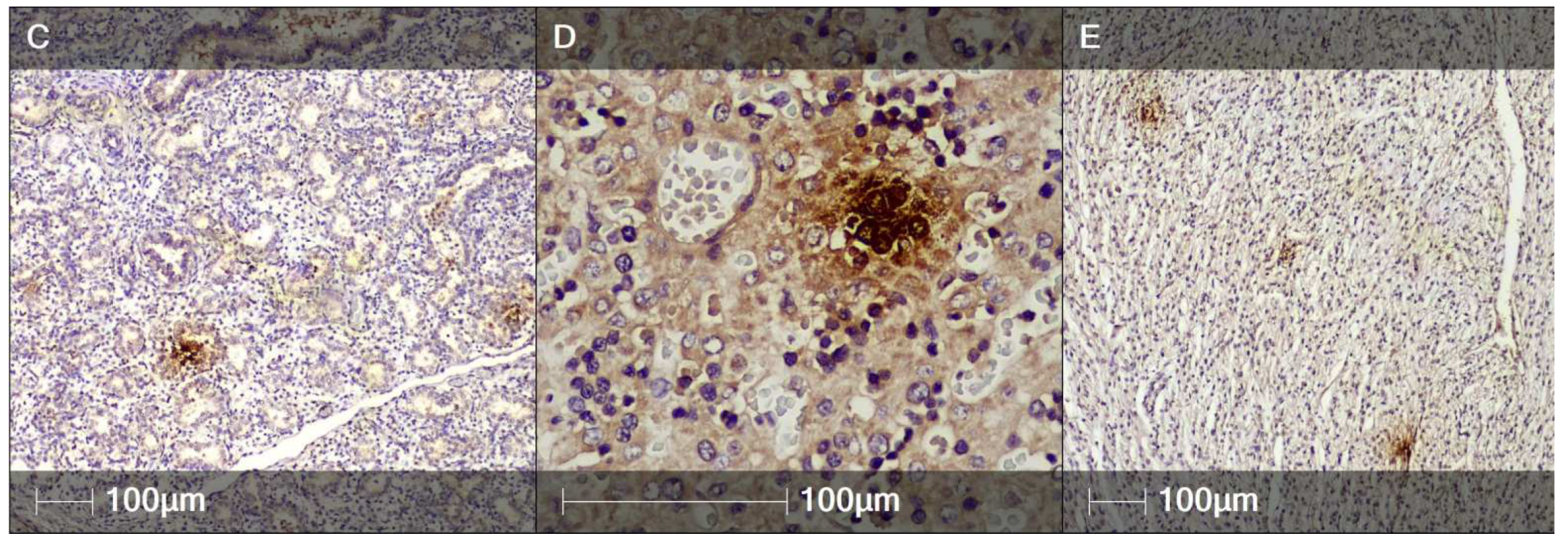
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogan, S.C.; Beigi, R.H. Treatment of viral infections during pregnancy. J. Perinatol. 2019, 46, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Mor, G. Risks associated with viral infections during pregnancy. J. Clin. Investig. 2017, 127, 1591–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Wang, L.; Fang, C.; Peng, S.; Zhang, L.; Chang, G.; Xia, S.; Zhou, W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Greub, G.; Favre, G.; Gengler, C.; Jaton, K.; Dubruc, E.; Pomar, L. Second-Trimester Miscarriage in a Pregnant Woman with SARS-CoV-2 Infection. JAMA 2020, 323, 2198–2200. [Google Scholar] [CrossRef]
- Pulinx, B.; Kieffer, D.; Michiels, I.; Petermans, S.; Strybol, D.; Delvaux, S.; Baldewijns, M.; Raymaekers, M.; Cartuyvels, R.; Maurissen, W. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2441–2445. [Google Scholar] [CrossRef]
- Wong, S.F.; Chow, K.M.; Leung, T.N.; Ng, W.F.; Ng, T.K.; Shek, C.C.; Ng, P.C.; Lam, P.W.; Ho, L.C.; To, W.W.; et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004, 191, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Alfaraj, S.H.; Al-Tawfiq, J.A.; Memish, Z.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: Report of two cases & review of the literature. J. Microbiol. Immunol. Infect. 2019, 52, 501–503. [Google Scholar] [CrossRef]
- León-Juárez, M.; Martínez–Castillo, M.; González-García, L.D.; Helguera-Repetto, A.C.; Zaga-Clavellina, V.; García-Cordero, J.; Flores-Pliego, A.; Herrera-Salazar, A.; Vázquez-Martínez, E.R.; Reyes-Muñoz, E. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog. Dis. 2017, 75, ftx093. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X. The SARS-CoV-2 receptor ACE2 expression of maternal–fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15, e0230295. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Maidji, E. Cytomegalovirus infection in the human placenta: Maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr. Top. Microbiol. Immunol. 2008, 325, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Chen, S.F.; Hsu, J.J.; Hsieh, C.C.; Hsueh, S.; Hsieh, T.T. Tumour necrosis factor-alpha converting enzyme in human gestational tissues from pregnancies complicated by chorioamnionitis. Placenta 2006, 27, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection. Arch. Gynecol. Obstet. 2017, 295, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boily-Larouche, G.; Milev, M.P.; Zijenah, L.S.; Labbe, A.C.; Zannou, D.M.; Humphrey, J.H.; Ward, B.J.; Poudrier, J.; Mouland, A.J.; Cohen, E.A.; et al. Naturally-occurring genetic variants in human DC-SIGN increase HIV-1 capture, cell-transfer and risk of mother-to-child transmission. PLoS ONE 2012, 7, e40706. [Google Scholar] [CrossRef] [PubMed]
- Valdespino-Vázquez, M.Y.; Helguera-Repetto, C.A.; León-Juárez, M.; Villavicencio-Carrisoza, O.; Flores-Pliego, A.; Moreno-Verduzco, E.R.; Díaz-Pérez, D.L.; Villegas-Mota, I.; Carrasco-Ramírez, E.; López-Martínez, I.E.; et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J. Med. Virol. 2021, 93, 4480–4487. [Google Scholar] [CrossRef]
- Schwartz, D.A. Autopsy and postmortem studies are concordant: Pathology of Zika virus infection in neonates and stillborn fetuses with microcephaly following transplacental transmission. Arch. Pathol. Lab. Med. 2017, 141, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Knight, M.; Bunch, K.; Vousden, N.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J. Characteristics and Outcomes of Pregnant Women Admitted to Hospital with Confirmed SARS-CoV-2 Infection in UK: National Population Based Cohort Study. BMJ 2020, 369, m2107. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.L.; Zhao, S.J.; Kwak-Kim, J.; Mor, G.; Liao, A.H. Why are Pregnant Women Susceptible to COVID-19? An Immunological Viewpoint. J. Reprod. Immunol. 2020, 139, 103122. [Google Scholar] [CrossRef]
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020, 323, 1846–1848. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Fang, Y.; Zuo, Q.; Huang, X.; Lei, Y.; Ren, X.; Liu, D. Vertical Transmission and Kidney Damage in Newborns Whose Mothers had Coronavirus Disease 2019 during Pregnancy. Int. J. Antimicrob. Agents 2021, 57, 106260. [Google Scholar] [CrossRef] [PubMed]
- Golden, T.N.; Simmons, R.A. Maternal and Neonatal Response to COVID-19. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E315–E319. [Google Scholar] [CrossRef] [PubMed]
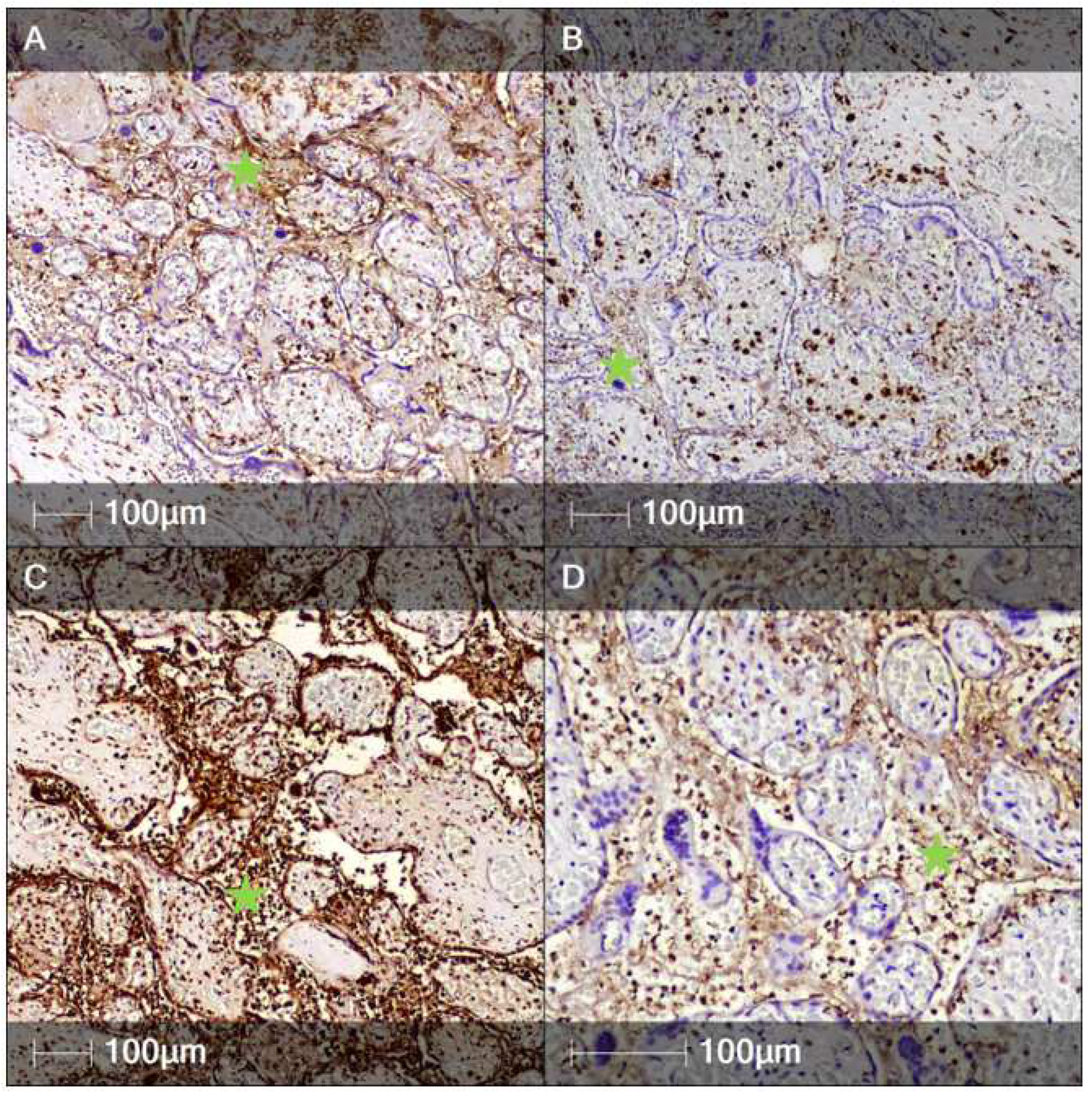
| Organ | Average Density of SARS-CoV-2-Positively Stained Regions (N/mm2) | Average Area of the SARS-CoV-2-Positive Regions (μm2) | Average SARS-CoV-2-Positive Cell Density (Cells/μm2) |
|---|---|---|---|
| Brain | 3 | 3257 | 2.38 × 10−5 |
| Myocardium | 1 | 4072 | 1.55 × 10−5 |
| Liver | 2 | 3448 | 1.58 × 10−4 |
| Lungs | 1 | 5792 | 2.20 × 10−4 |
| Average (all organs) | 1.75 | 4129.6 | 2.00 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metodiev, D.; Ruseva, M.; Parvanov, D.; Ganeva, R.; Handzhiyska, M.; Vidolova, N.; Stamenov, G. Vertical Transmission of SARS-CoV-2 Infection and Miscarriage in the Second Trimester: Report of an Immunohistochemically Proven Case. Clin. Pract. 2022, 12, 579-590. https://doi.org/10.3390/clinpract12040061
Metodiev D, Ruseva M, Parvanov D, Ganeva R, Handzhiyska M, Vidolova N, Stamenov G. Vertical Transmission of SARS-CoV-2 Infection and Miscarriage in the Second Trimester: Report of an Immunohistochemically Proven Case. Clinics and Practice. 2022; 12(4):579-590. https://doi.org/10.3390/clinpract12040061
Chicago/Turabian StyleMetodiev, Dimitar, Margarita Ruseva, Dimitar Parvanov, Rumiana Ganeva, Maria Handzhiyska, Nina Vidolova, and Georgi Stamenov. 2022. "Vertical Transmission of SARS-CoV-2 Infection and Miscarriage in the Second Trimester: Report of an Immunohistochemically Proven Case" Clinics and Practice 12, no. 4: 579-590. https://doi.org/10.3390/clinpract12040061
APA StyleMetodiev, D., Ruseva, M., Parvanov, D., Ganeva, R., Handzhiyska, M., Vidolova, N., & Stamenov, G. (2022). Vertical Transmission of SARS-CoV-2 Infection and Miscarriage in the Second Trimester: Report of an Immunohistochemically Proven Case. Clinics and Practice, 12(4), 579-590. https://doi.org/10.3390/clinpract12040061






