Abstract
(1) Background: Quality of life (QOL) is used as a health indicator to assess the effectiveness and impact of therapies in certain groups of patients. This study aimed to analyze the QOL of patients with acute coronary syndrome (ACS) who received medical treatment by a public or private health care system. (2) Methods: This observational, prospective, longitudinal study was carried out in four referral hospitals providing cardiology services in Sergipe, Brazil. QoL was evaluated using the Medical Outcomes Study 36-Item Short-Form Health Survey. The volunteers were divided into two groups (public or private health care group) according to the type of health care provided. Multiple linear regression models were used to evaluate QoL at 180 days after ACS. (3) Results: A total of 581 patients were eligible, including 44.1% and 55.9% for public and private health care, respectively. At 180 days after ACS, the public health care group had lower QoL scores for all domains (functional capacity, physical aspects, pain, general health status, vitality, social condition, emotional profile, and health) (p < 0.05) than the private group. The highest QoL level was associated with male sex (p < 0.05) and adherence to physical activity (p ≤ 0.003) for all assessed domains. (4) Conclusions: This shows that social factors and health status disparities influence QoL after ACS in Sergipe.
1. Introduction
Acute coronary syndrome (ACS) is one of the most important causes of morbidity and mortality in Brazil and worldwide [1,2]. Despite the progress in the diagnosis and treatment of patients with ACS, which have contributed to a significant increase in the number of survivors after an acute event, it is still a challenge for health systems to provide effective, equitable secondary prevention measures [3,4,5,6] and addressing disparities in health care system for these patients.
Brazilian [1] and international [7,8] guidelines point to the importance of adequate secondary prevention guidance in patients with ACS. Prognosis and clinical evolution of patients after hospital discharge can be modified based on the therapy adopted and compliance to treatment, contributing to a reduction and control of risk factors (RF) and comorbidities, collaborating to an increase in survival [7,8,9] and improvement in the quality of life (QoL) of these patients [10].
QoL has become one of the most discussed topics in recent decades and is considered to be of great interdisciplinary interest nowadays [9,11,12], since the improvement in QoL has become an outcome of aftercare practices and public policies for health promotion and disease prevention [11,12]. Therefore, information about QoL has been used as an indicator to assess the effectiveness and impact of determined treatments on groups of patients [11,12,13,14].
In Brazil, the Brazilian Unified Health System (SUS in Portuguese), with universal coverage for 150 million Brazilians, coexists with the Supplementary Health Care, which is predominantly a private system, with 50 million beneficiaries. SUS was developed to meet the principles of universality, equality, and integrality [15,16]. However, there are reports of existing disparities between private and public health care with regard to the appropriate treatment of patients with ACS [4,17]. Moreover, evidence shows that distortions in the quality of health care may have a negative influence on treatment adherence, compromising the prognosis and QoL of patients [18]. However, information is scarce in the literature on the QOL of patients with ACS assisted in the SUS or private health care, and on the presence of disparity between health care systems.
2. Materials and Methods
2.1. Study Design and Locations
This observational, prospective, longitudinal study was carried out in four referral hospitals providing cardiology services in Aracaju City, Sergipe, Brazil. Among these hospitals, only one offers services through SUS and does not have an “open-door” service, which means that it requires the referral of patients from another health institution. The other three hospitals only offer Private Health Care Service (PHCS), either through health insurance or disbursement.
Our research followed the components of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [19] protocol for observational studies, as shown in Figure 1.
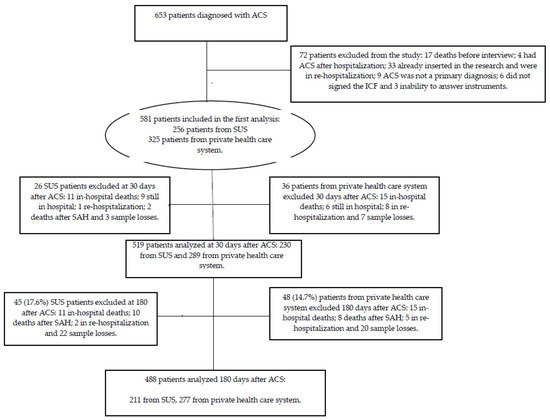
Figure 1.
Study design.
2.2. Study Sample
We adopted the “all-comers”’ sample type. This study enrolled 581 volunteers of both sexes, aged >18 years. They were consecutively diagnosed with ACS, which was characterized by unstable angina (UA), acute myocardial infarction (AMI) without ST-segment elevation (NSTEMI), or AMI with ST-segment elevation (STEMI). Patients who did not agree to participate in the study by signing the informed consent form and/or who were unable to answer the study protocols were excluded from the study. The inclusion and exclusion process is shown in Figure 1.
The diagnosis of ACS was based on the patients’ clinical history, with the onset of consistent symptoms of acute ischemia during the previous 24 h, including or not a series of increases in myocardial necrosis markers. These data were confirmed by electrocardiography, Doppler echocardiography, or cine coronary angiography. In some cases, the diagnosis was confirmed using more than one of the previously cited examinations [20].
Our study was submitted to the Research Ethics Committee involving human beings at the Federal University of Sergipe (CEP/UFS). The committee approved our research (approval no. 302,544). All patients signed the informed consent form.
2.3. Data Collection
Data were collected from October 2013 to March 2016. The study consisted of three stages: (1) initial evaluation after the diagnosis of ACS (hospitalization); (2) follow-up assessment 30 days after ACS; (3) final evaluation at 180 days after ACS. To this end, we used the Case Report Form, which is composed of variables that provide information about patients’ sociodemographic and clinical conditions, levels of physical activity, quality of dietary intake, and QoL. To fill this form, data were obtained through interviews with the patient or one family member when the patients could not respond to the questionnaire by themselves. Their medical records were also analyzed.
The protocols of the medical teams of the hospitals followed national and international guidelines for patients with ACS [1,7,8,9]. At hospital discharge, individuals received general orientation regarding dietary intake, smoking cessation, physical activity, and adherence to drug treatment to prevent disease recurrence. The present study sought to verify the QoL of individuals, with the perspective that the greater the adherence to secondary prevention, the higher the QoL scores would be.
It is important to emphasize that at no time did the team of researchers of the study that originated this article perform interventions on the patients included in the research.
At admission and 180 days after ACS, the International Physical Activity Questionnaire (short version) [21,22] was used to assess adherence to physical activity recommendations. In addition, the Food Frequency Questionnaire [23] was used to collect information on dietary consumption, and the Alternative Healthy Eating Index (2010) [24] adapted from the Food Guide for the Brazilian Population [25] was used to assess their diet quality: the higher the values, the better the state of health. At 180 days after ACS, patients were surveyed about smoking cessation, and information on new cardiovascular events.
In the context of secondary prevention, some classes of medications are labeled as A according to the Specialized Guidelines [1], such as (a) antithrombotics: acetylsalicylic acid (ASA) and/or a P2Y12 inhibitor (Prazygrel, Ticagrelor or Clopidogrel); (b) β-blockers; (c) statins; (d) angiotensin-converting enzyme inhibitors (ACEI)/AT1 Receptor Blockers (ARB) and aldosterone receptor antagonist (spironolactone) in case of heart failure and/or left ventricular dysfunction. We collected the data related to the prescriptions of the medicines mentioned above from the medical records and compared them to the prescriptions, with the patients present at the moment of hospital discharge. Patients were considered adherent at 30 and 180 days post ACS when they reported using all prescribed medications.
About the socioeconomic level of the sample, according to the Brazilian Economic Classification Criterion of the Brazilian Association of Research Companies (ABEP) [26]. For purposes of analysis, the eight economic levels, or levels, or economic classes, established by ABEP, were regrouped and named as follows: A1, A2, and B1 in High Economic Level (A); B2, C1, and C2 in Medium Economic Level (M), and D and E in Low Economic Level (B).
To assess QoL, we applied the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) questionnaire [27], since we used it in research with a specific focus on cardiology [28]. SF-36 consists of a self-administered instrument, which can also be part of an interview, whether face-to-face or by telephone [29], and SF-36 is composed of 36 questions that address eight domains in two major components: physical, which involves functional capacity, physical appearance, pain, and general health, and mental, which covers vitality, social aspects, emotional state, and mental health. We measured these domains in a score ranging from 0 to 100. The higher the score, the better the QoL. SF-36 also includes an item that assesses the individuals’ perception of their own health compared to a year ago [28,29]. As regards the differences in patients in terms of their educational level, we decided to interview them to standardize our investigation. Therefore, a face-to-face interview was carried out at admission and by telephone at 30 and 180 days after the acute event.
2.4. Data Analysis
For data analysis, the patients were divided into two groups (SUS = public and PHCS = private health care groups) according to the type of health care received when they presented ACS. The distribution type of numerical variables was determined using the Kolmogorov–Smirnov test. Data with normal distribution were presented as means and standard deviations and categorical variables as absolute and relative frequencies (%).
The Mann–Whitney and Wilcoxon tests were applied to compare quantitative variables between groups for evaluation at different times and Friedman test for multiple comparisons. The association between groups and categorical variables was also verified using Pearson’s Chi-square test or Fisher’s exact test when appropriate.
To assess the internal consistency of the SF-36, Cronbach’s alpha was calculated, which presented an average of 0.91, representing excellent reliability of the instrument. In addition, we developed a multiple linear regression model with the scores of the SF-36 domains at 180 days after ACS as dependent variables. The following independent variables were adopted: age, sex, educational level, type of health care, presence of comorbidities (systemic arterial hypertension (SAH), diabetes mellitus, dyslipidemia (DLP), overweight, and abdominal obesity), occurrence of a new cardiovascular event within 180 days after ACS, adherence to physical activity, adherence to pharmacotherapy, diet quality index, smoking cessation after 180 days of ACS, and hospitalization time (assessed by percentage variation). A 95% confidence interval was adopted for independent variables associated with the scores of the SF-36 domains. Statistical analyses were carried out using the R Core Team 2016 Program version 3.3.2, with the significance level used being 5%.
3. Results
A total of 581 patients were considered potentially eligible for the study: 256 (44.1%) received medical care from public health care and 325 (55.9%) from the private one. At 30 and 180 days after ACS, we interviewed 519 and 488 patients, respectively.
In general, the patient’s baseline and adherence characteristics in the public health care group (SUS) differed from those in the private health care system. Patients in the public health care group were predominantly younger men, with lower socioeconomic status, higher prevalence of STEMI, alcoholism, smoking, and less adherence to secondary prevention treatment after ACS. Patients treated by the private health care system had more comorbidities, but with a shorter hospital stay. No distinction was found between the groups regarding the occurrence of cardiovascular outcomes at 180 days of ACS (Table 1).

Table 1.
Baseline characteristics, cardiovascular outcomes, and adherence to secondary prevention (adherence to physical activity, medication, smoking cessation, and diet quality) in patients with ACS, according to the Type of Healthcare, Aracaju, Brazil.
In general, patients’ QoL worsened, regardless of the type of healthcare at 30 days after the acute event, except for the emotional aspect. At 180 days after ACS, patients showed improvement in pain, social, and emotional aspects, with worsening of their functional capacity and general health status, compared with those during hospitalization (Table 2).

Table 2.
Quality of life, according to SF–36 domains in patients from public and private health care systems who presented with ACS, Aracaju, Brazil.
Table 3 shows the QoL of patients by type of healthcare. At admission, patients from the SUS had a higher mental health score than those from the private health care system. However, we verified an inverse situation for the emotional aspect. At 30 days after the acute event, patients in the public health care group had lower QoL in terms of physical aspect and pain. Compared with the QoL of patients from the public health care group at 180 days after ACS, the QoL of the patients in the private health care group was superior to all aspects addressed.

Table 3.
Means and standard deviation of SF-36 domains of patients with ACS, according to the type of healthcare, Aracaju, Brazil.
When investigating patients’ perception of their current health compared with that of a year ago, no distinction was found between the groups at the time of hospitalization. However, patients from the public health care group had a worsened perception about their own health compared to patients from the private health care group system, at 30 and 180 days after ACS (Table 4).

Table 4.
Perception of patients with ACS concerning their current health compared to a year ago, according to the type of healthcare, Aracaju, Brazil.
In the multiple linear regression models, the best QoL of patients at 180 days after ACS was mainly associated with male sex and adherence to physical activity for all domains. Moreover, better SF-36 scores were found among individuals with shorter hospital stays, younger age, higher educational level, those who received medical treatment by the private health care system, the ones who did not develop subsequent cardiovascular events, those who had no history of SAH or DLP, and displayed adherence to pharmacotherapy (Table 5).

Table 5.
Multiple linear regression models for QOL of patients at 180 days after ACS, Aracaju, Brazil.
4. Discussion
In this study, in general, the patients’ QoL improved in only three of the eight SF-36 domains 180 days after ACS. Individuals assisted by the private health care network showed better QOL for all domains of the SF-36 when compared to those assisted by the public service. We also found that the better QoL was associated with the male sex and adherence to physical activity for all the evaluated components.
At 180 days after ACS, we associated the absence of a subsequent cardiovascular event and access to the private health care system with higher scores for the six and four SF-36 domains (functional capacity, physical appearance, pain, and general health, and mental, which covers vitality, social aspects, emotional state, and mental health), respectively. We also associated a shorter hospital stay, lower age group, higher educational level, absence of SAH and DLP, and adherence to pharmacotherapy with better QoL.
Studies reported that improvement in QoL is an outcome of aftercare practices, serving as a basis for decision-making in public health policies [11]. Therefore, the results of this study are relevant when considering that the QoL of patients with worse results, after 180 days of ACS, may be associated with longer hospitalization time, shorter adherence to secondary prevention guidelines performed at hospital discharge, and to the public health model. These data were independent predictors of these findings.
We verified that 30 days after ACS, there was a reduction in the scores of seven SF-36 domains, mainly in the ones related to the physical component. However, this may result from the post-hospitalization due to ACS. Thus, we will center our discussion on the results found 180 days after the acute event.
AMI is a highly stressful life-threatening disease that may have consequences on patient well-being for a substantial time, with limited physical functioning, cardiac complications, and deterioration of QoL [31]. Literature shows worse QoL in those who experienced cardiovascular events compared to their healthy counterparts [32].
At 180 days after ACS, QoL improved in only one of the physical components (pain) and worsened in two of them (functional capacity and general health). In a study conducted with ACS patients to verify changes in their QoL and their functional capacity, researchers detected that 8 months after hospital discharge, their functional capacity declined [33]. These data are similar to our study results, where we verified that functional capacity at the previous levels before the acute event was not recovered.
When assessing patients’ QoL by type of healthcare, during hospitalization, similarities were found between the groups in six SF-36 domains. At 180 days after ACS, when compared with patients in the private health care group, those from the public group (SUS) had worse QoL for all assessed items and had worsened perception about their own health. A longer hospitalization time and lower rates of adherence to secondary prevention treatment (less adherence to physical activity, medication therapy, and lower diet quality) in patients from the public health care group (SUS) suggest possible distortions in health care quality between the two groups. Considering these differences in the assistance received and the socioeconomic characteristics intrinsic to patients from the SUS, these factors had a negative influence on the results, culminating in worse QoL scores for this group. These data are consistent with the literature in showing that health care quality and socioeconomic context can influence treatment adherence [16,17], prognosis, and patients’ QoL [13,15].
The results showed an association between worse QoL and increased age, female sex, lower educational level, and a higher prevalence of comorbidities. Three of these characteristics (older age, female sex, and higher prevalence of comorbidities) were more frequent in patients from the private health care group. Despite this, patients who received medical treatment by this service had better QoL, leading once again to questions about the health care models adopted in Sergipe and Brazil as a whole, especially when considering that studies show the negative impact of chronic conditions on worsening QoL in individuals, which is more accentuated with multiple comorbidities [34,35].
The low adherence to secondary prevention by the study patients is a possible sign of health care distortions in Brazil. The benefits of secondary prevention therapies in patients with ACS are evident [36,37], as is the fact that individuals with better adherence to this therapy in intervention studies showed a reduction in hospital readmission rates, cardiovascular mortality, improved health [1] and QOL [38,39].
Therefore, these results have implications for public health policies in Sergipe and, possibly, in Brazil, showing that strategies for improving health care quality are fundamental to create mechanisms for better adherence to secondary prevention and, consequently, better QoL in patients after ACS.
Our analysis had some limitations. First, the advanced vascular unit (UVA in Portuguese) from the public hospital included in this study interrupted patient care in July 2014 and June 2015, contributing to a smaller number of patients treated at this service. Second, results were limited by information on adherence to pharmacotherapy as well as smoking cessation or persistence because we collected these data with simple self-report questions, without using validated measuring instruments.
However, we believe that this study is one of the first to be conducted in Brazil to compare QoL data after hospital discharge in patients with ACS and compare different types of health care. This shows that social factors and possible disparities in health care quality influence QoL after ACS in Sergipe. However, we can speculate that the results presented here reflect the general situation in Brazil.
5. Conclusions
In conclusion, patients receiving medical treatment by the private health care system had better QoL than patients receiving medical care by the public health care system (from SUS), showing a disparity in health care quality. This is a challenge that we must overcome to improve the efficiency and equitability of the health care system.
Author Contributions
I.M.N.B.d.C.C., D.G.d.S. and A.C.S.S. conceptualized the study, coordinated the last follow-up with participants from the study, conducted the statistical analysis, and wrote the manuscript. I.M.N.B.d.C.C., D.G.d.S. and A.C.S.S. conceptualized the study and wrote the manuscript. J.R.S.S. conducted the statistical analysis. J.L.M.O., F.A.d.A., J.d.G.J., L.M.S.M.d.O., R.R.d.A., J.O.C., M.F.C.d.S., L.M.C.P., L.V.S.A., S.M.V., M.A.A.-S., F.J.A., V.B.O., L.S.M. and L.B. wrote the manuscript. Supervision: A.C.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Higher Education Personnel Improvement Coordination (CAPES, Brazil) nº 1793619.
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki, and approved by Research Ethics Committee involving human beings at the Federal University of Sergipe (CEP/UFS). The committee approved our research (approval no. 302,544/date: 06-07-2013). for studies involving humans.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicolau, J.C.; Feitosa-Filho, G.; Petriz, J.L.; Furtado, R.H.M.; Précoma, D.B.; Lemke, W.; Lopes, R.D.; Timerman, A.; Neto, J.A.M.; Neto, L.B.; et al. Brazilian Society of Cardiology Guidelines on Unstable Angina and Acute Myocardial Infarction without STSegment Elevation—2021. Arq. Bras. Cardiol. 2021, 117, 181–264. [Google Scholar] [CrossRef]
- Marasigan, V.; Perry, I.; Bennett, K.; Balanda, K.; Capewell, S.; Flaherty, M.O.; Kabir, Z. Explaining the fall in Coronary Heart Disease mortality in the Republic of Ireland between 2000 and 2015-IMPACT modelling study. Int. J. Cardiol. 2020, 310, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.C.; Oliveira, L.C.S.; Oliveira, J.C.; Barreto, I.D.D.C.; Almeida-Santos, M.A.; Lima, T.C.R.M.; Arcelino, L.A.M.; Silva, I.S.B.S.; Sousa, A.C.S.; Barreto-Filho, J.A.S. Disparidades no Uso de Stents Farmacológicos para Pacientes Diabéticos com Infarto Agudo do Miocárdio com Supradesnivelamento do Segmento ST Assistidos na Rede Pública versus Privada—Registro VICTIM. Arq. Bras. Cardiol. 2019, 112, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Widimsky, P.; Crea, F.; Binder, R.K.; Lüscher, T.F. The year in cardiology 2018: Acute coronary Syndromes. Eur. Heart J. 2019, 40, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Dunlay, S.M.; Barsness, G.W.; Rihal, C.S.; Holmes-Junior, D.R.; Prasad, A. Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am. J. Cardiol. 2019, 124, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Sakalaki, M.; Barywani, S.; Rosengren, A.; Bjorck, L.; Fu, M. Determinants of suboptimal long-term secondary prevention of acute myocardial infarction: The structural interview method and physical examinations. BMC Cardiovasc. Disord. 2019, 19, 243. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 79, 21–129. [Google Scholar] [CrossRef]
- de Bakker, M.; Uijl, I.D.; ter Hoeve, N.; van Domburg, R.T.; Geleijnse, M.L.; Berg-Emons, R.J.V.D.; Boersma, E.; Sunamura, M. The Association between Exercise Capacity and Health-Related Quality of Life during and after Cardiac Rehabilitation in Acute Coronary Syndrome Patients: A substudy of the OPTICARE Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2020, 101, 650–657. [Google Scholar] [CrossRef]
- Soto, M.; Failde, I.; Márquez, S.; Benítez, E.; Ramos, I.; Barba, A.; López, F. Physical and mental component summaries score of the SF-36 in coronary patients. Qual. Life Res. 2005, 14, 759–768. [Google Scholar] [CrossRef]
- WHOQOL Group. The Development of the World Health Organization Quality of Life Assessment Instrument (the WHOQOL). In Quality of Life Assessment: International Perspectives; Springer: Berlin/Heidelberg, Germany, 1994; pp. 41–57. [Google Scholar]
- Ciconelli, M.R.; Bosi, M.; Santos, W.; Meinão, I.; Rodrigues, M. Tradução para língua portuguesa e validação do questionário genérico de avalição de qualidade de vida SF-36 (Brasil SF-36). Rev. Bras. Reumatol. 1999, 39, 143–150. [Google Scholar]
- Pirhonen, L.; Bolin, K.; Olofsson, E.H.; Fors, A.; Ekman, I.; Swedberg, K.; Gyllensten, H. Person-Centred Care in Patients with Acute Coronary Syndrome: Cost-Effectiveness Analysis Alongside a Randomised Controlled Trial. Pharm. Econ. Open. 2019, 3, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Brazil Services and Information. Adequação Do Atendimento Pode Reduzir Mortes Por Infarto. Portal Brasil. Abril de 2012. Available online: http://www.brasil.gov.br/saude/2012/04/adequacao-do-atendimento-pode-reduzir-mortes-por-infarto (accessed on 8 November 2017).
- Brasil. Sistema Único de Saúde (SUS). Portal Ministério da Saúde. Janeiro de 2018. Available online: http://www.portalms.saude.gov.br/sistema-unico-de-saude/sistema-unico-de-saude (accessed on 3 January 2017).
- Costa, I.M.N.B.D.C.; da Silva, D.G.; Filho, J.A.S.B.; Oliveira, J.L.M.; Silva, J.R.S.; Buarque, M.D.B.M.; Nascimento, T.; Jorge, J.D.G.; Almeida, A.S.; Almeida-Santos, M.A.; et al. Diet quality of patients with acute coronary syndrome receiving public and private health care. Nutrition 2019, 59, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Smolderen, K.; Spertus, J.A.; Tang, F.; Oetgen, W.; Borden, W.B.; Ting, H.H.; Chan, P.S. Treatment differences by health insurance among outpatients with coronary artery disease: Insights from the national cardiovascular data registry. J. Am. Coll. Cardiol. 2013, 61, 1069–1075. [Google Scholar] [CrossRef]
- Pons, A.; Whalley, G.; Sneddon, K.; Williams, M.; Coffey, S. Predictors of quality of life after revascularization for ischemic heart disease: A systematic review. Health Sci. Rev. 2022, 2, 100017. [Google Scholar] [CrossRef]
- Malta, M.; Cardoso, L.O.; Bastos, F.I.; Magnanini, M.M.F.; Silva, C.M.F.P. Iniciativa STROBE: Subsídios para a comunicação de estudos observacionais. Rev. Saúde Pública 2010, 44, 559–565. [Google Scholar] [CrossRef]
- Eisen, A.; Giugliano, R.; Braunwald, E. Updates on Acute Coronary Syndrome. JAMA Cardiol. 2016, 1, 718–730. [Google Scholar] [CrossRef]
- Committee IPAQR. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ). Retrieved 15 November 2005. 2010. Available online: https://www.ipaq.ki.se/scoring.pdf (accessed on 14 May 2014).
- Matsudo, S.; Araújo, T.; Marsudo, V.; Andrade, D.; Andrade, E.; Braggion, G. Questionário Internacional de Atividade Física (IPAQ): Estudo de validade e reprodutibilidade no Brasil. Rev. Bras. Ativid. Fís. Saúde 2001, 6, 5–12. [Google Scholar] [CrossRef]
- Slater, B.; Philippi, S.T.; Marchioni, D.M.; Fisberg, R.M. Validação de Questionários de Freqüência Alimentar-QFA: Considerações metodológicas. Rev. Bras. Epidemiol. 2003, 6, 200–208. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–18222. [Google Scholar] [CrossRef]
- Ministério da Saúde. Guia Alimentar Para a População Brasileira: Promovendo a Alimentação Saudável; Ministério da Saúde: Brasília, Brazil, 2006. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_alimentar_populacao_brasileira_2008.pdf (accessed on 8 November 2016).
- ABEP. Associação Brasileira de Empresa e Pesquisa. CCEB. Critério de Classificação Econômica Brasil. 2008. Available online: http://www.abep.org.br (accessed on 17 June 2022).
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Weinberger, M.; Oddone, E.Z.; Samsa, G.P.; Landsman, P.B. Are health-related quality-of-life measures affected by the mode of administration? J. Clin. Epidemiol. 1996, 49, 135–140. [Google Scholar] [CrossRef]
- Almeida, R.M. Remodelamento reverso cirúrgico do ventrículo esquerdo: Seguimento de 111 meses. Rev. Bras. Cirurg. Cardiovasc. 2009, 24, 470–477. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Jalali-Farahani, S.; Amiri, P.; Fakhredin, H.; Torshizi, K.; Cheraghi, L.; Khalili, D.; Azizi, F. Health-related quality of life in men and women who experienced cardiovascular diseases: Tehran Lipid and Glucose Study. Health Qual. Life Outcomes 2021, 19, 225–234. [Google Scholar] [CrossRef]
- Olomu, A.B.; Corser, W.D.; Stommel, M.; Xie, Y.; Holmes-Rovner, M. Do self-report and medical record comorbidity data predict longitudinal functional capacity and quality of life health outcomes similarly? BMC Health Serv. Res. 2012, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Mota, R.E.; Roberts, M.; Abel, G.; Elliott, M.; Lyratzopoulos, G.; Roland, M.; Campbell, J. Common patterns of morbidity and multi-morbidity and their impact on health-related quality of life: Evidence from a national survey. Qual. Life Res. 2015, 24, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Schulman-Marcus, J.; Boden, W.E. A PROMISE Fulfilled That Quality-of-Life Assessments Afford Incremental Value to Coronary Artery Disease Management. Circulation 2016, 133, 1989–1991. [Google Scholar] [CrossRef][Green Version]
- Adriaanse, M.C.; Drewes, H.W.; van der Heide, I.; Struijs, J.N.; Baan, C.A. The impact of comorbid chronic conditions on quality of life in type 2 diabetes patients. Qual. Life Res. 2016, 25, 175–182. [Google Scholar] [CrossRef]
- Kang, K.; Gholizadeh, L.; Han, H.R. Health-related Quality of Life and Its Predictors in Korean Patients with Myocardial Infarction in the Acute Phase. Clin. Nurs. Res. 2021, 30, 161–170. [Google Scholar] [CrossRef]
- Herdy, A.H.; López-Jiménez, F.; Terzic, C.; Milani, M.; Stein, R.; Carvalho, T.; Serra, S.; Araujo, C.G.; Zeballos, P.C.; Anchique, C.V.; et al. Diretriz sul-americana de prevenção e reabilitação cardiovascular. Arq. Bras. Cardiol. 2014, 103, 1–31. [Google Scholar]
- Desai, N.R.; Choudhry, N.K. Impediments to adherence to post myocardial infarction medications. Curr. Cardiol. Rep. 2013, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Oldridge, N.; Thompson, D.R.; Zwisler, A.-D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).