Correlation of Medical Comorbidities and Upper Airway Measurements among Dental Patients at Risk of Developing Obstructive Sleep Apnea
Abstract
1. Introduction
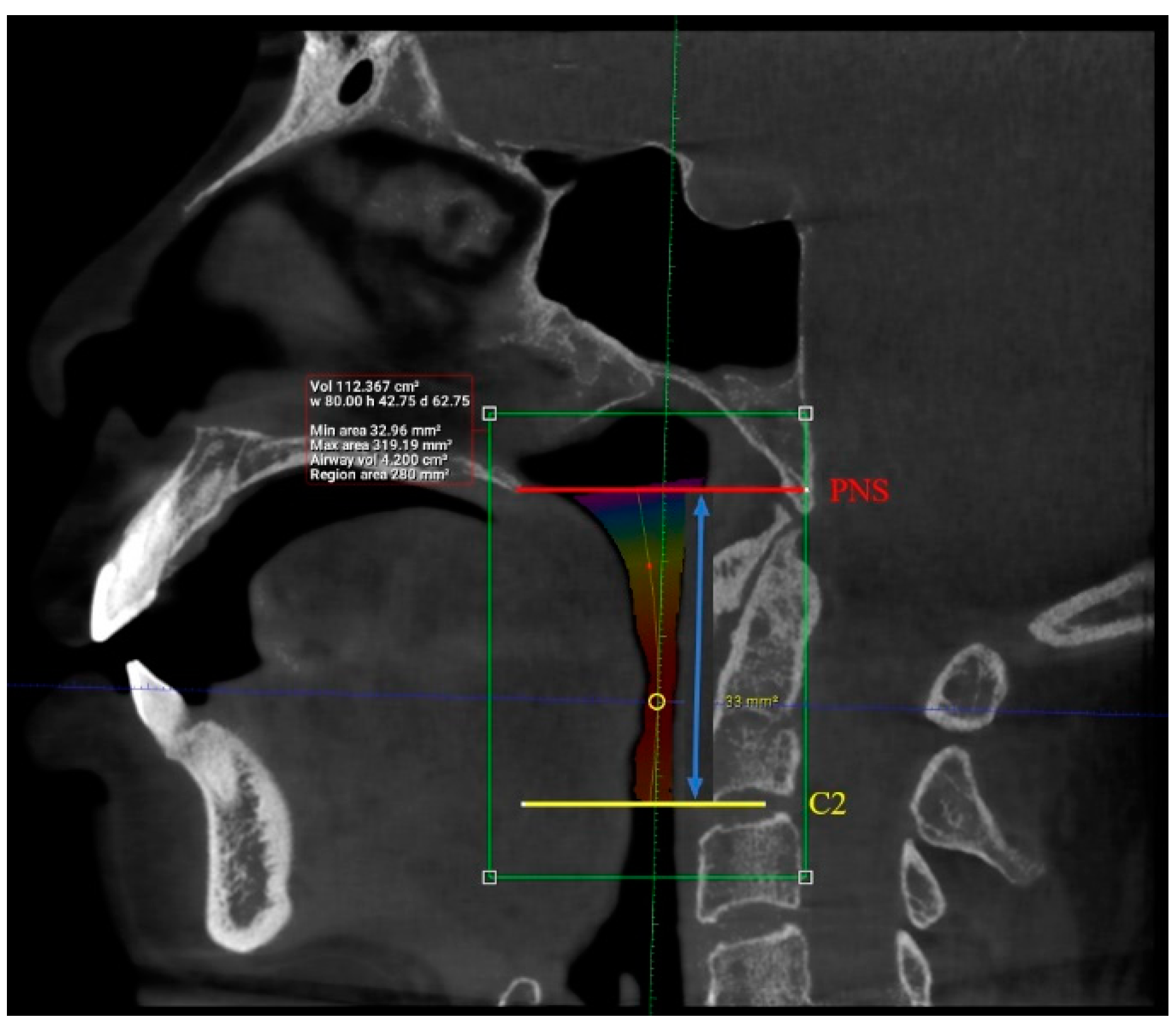
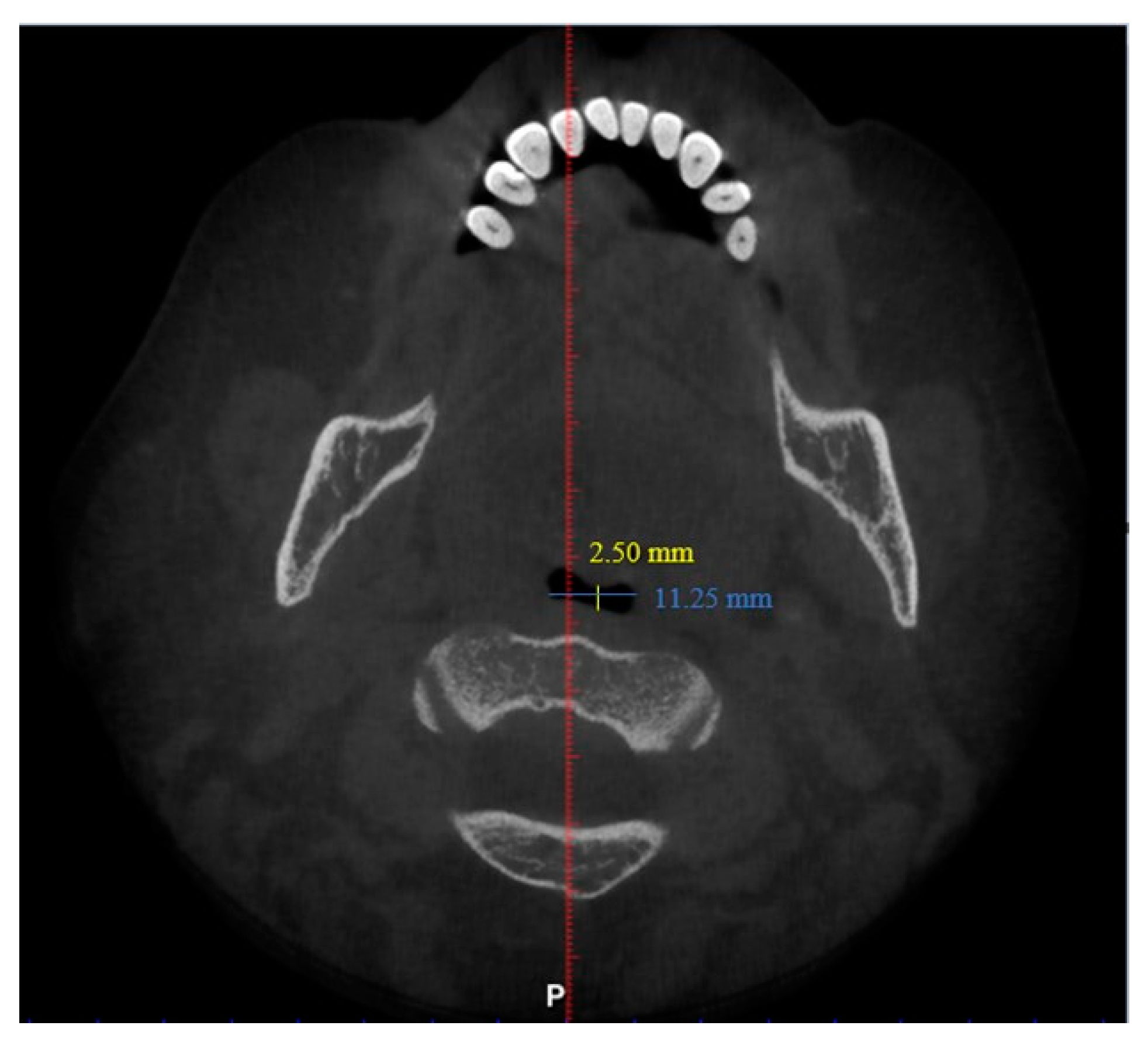
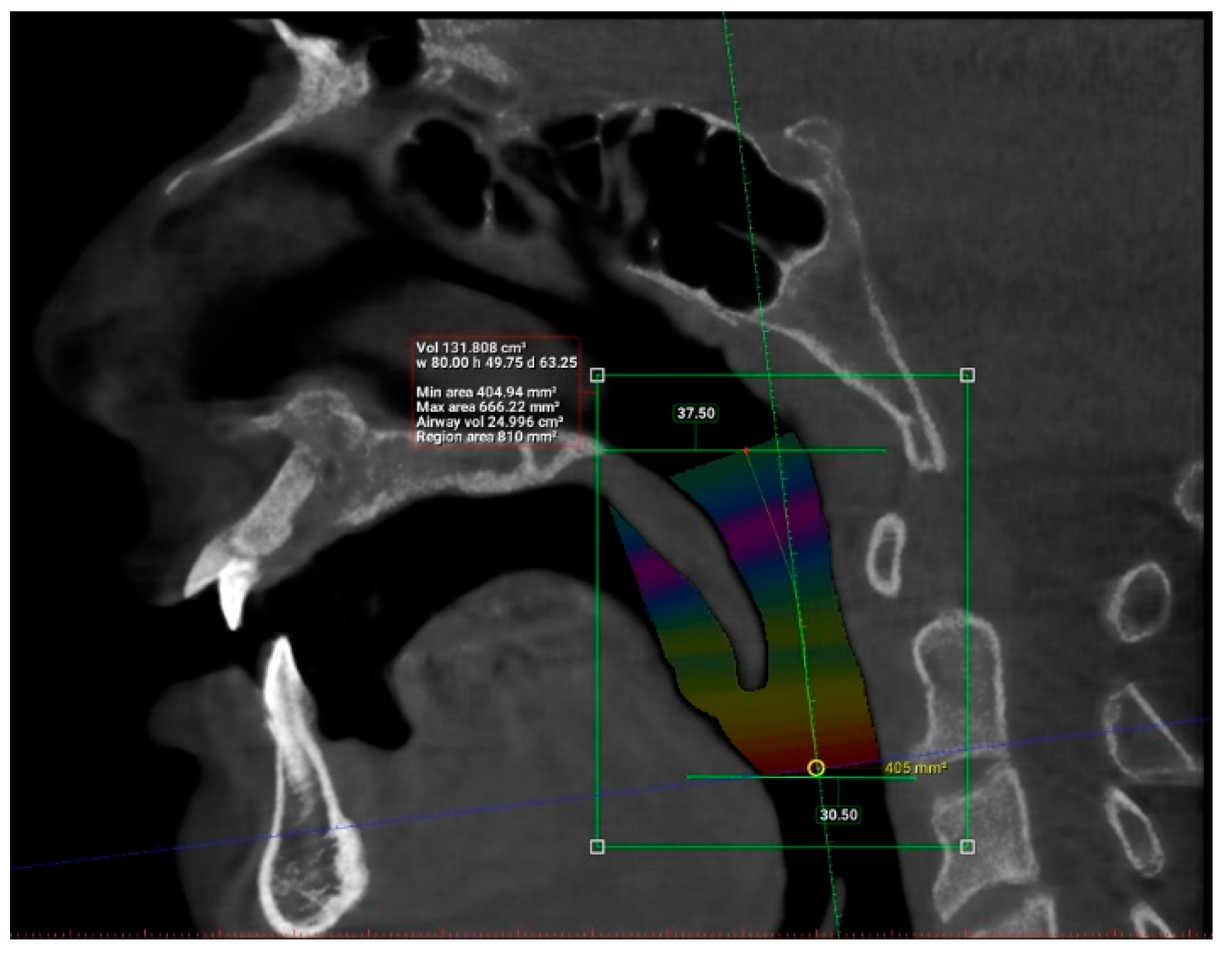
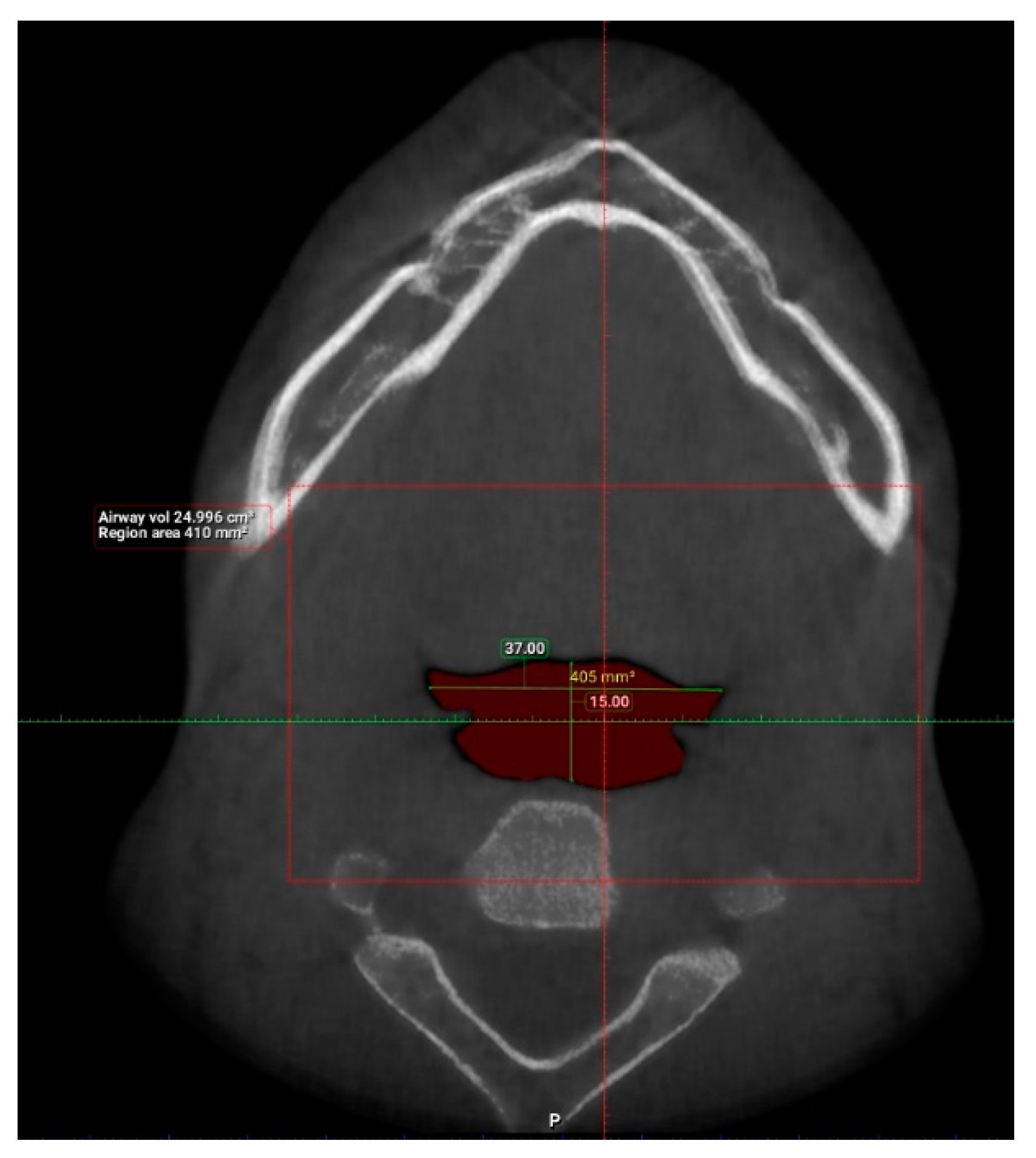
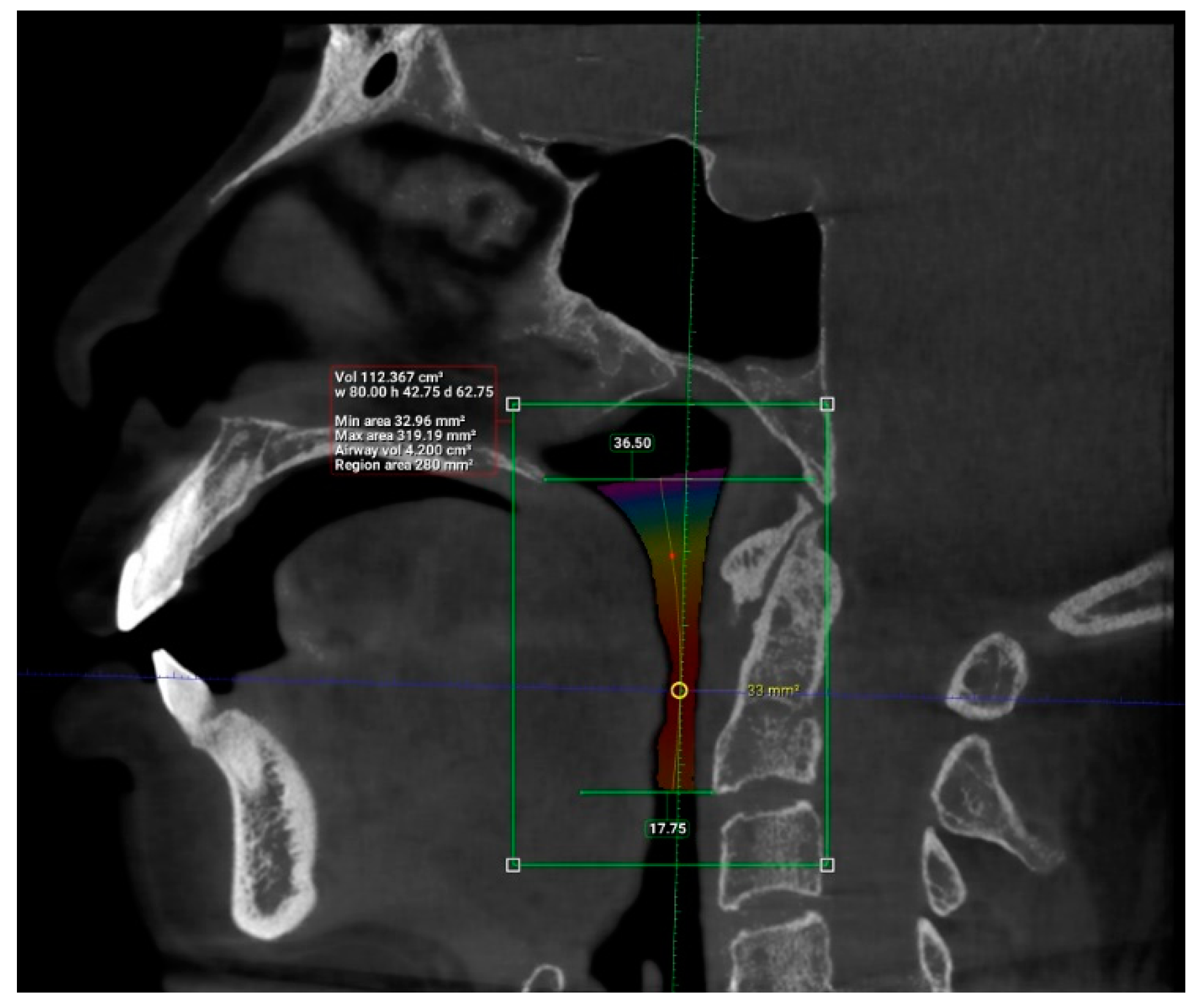
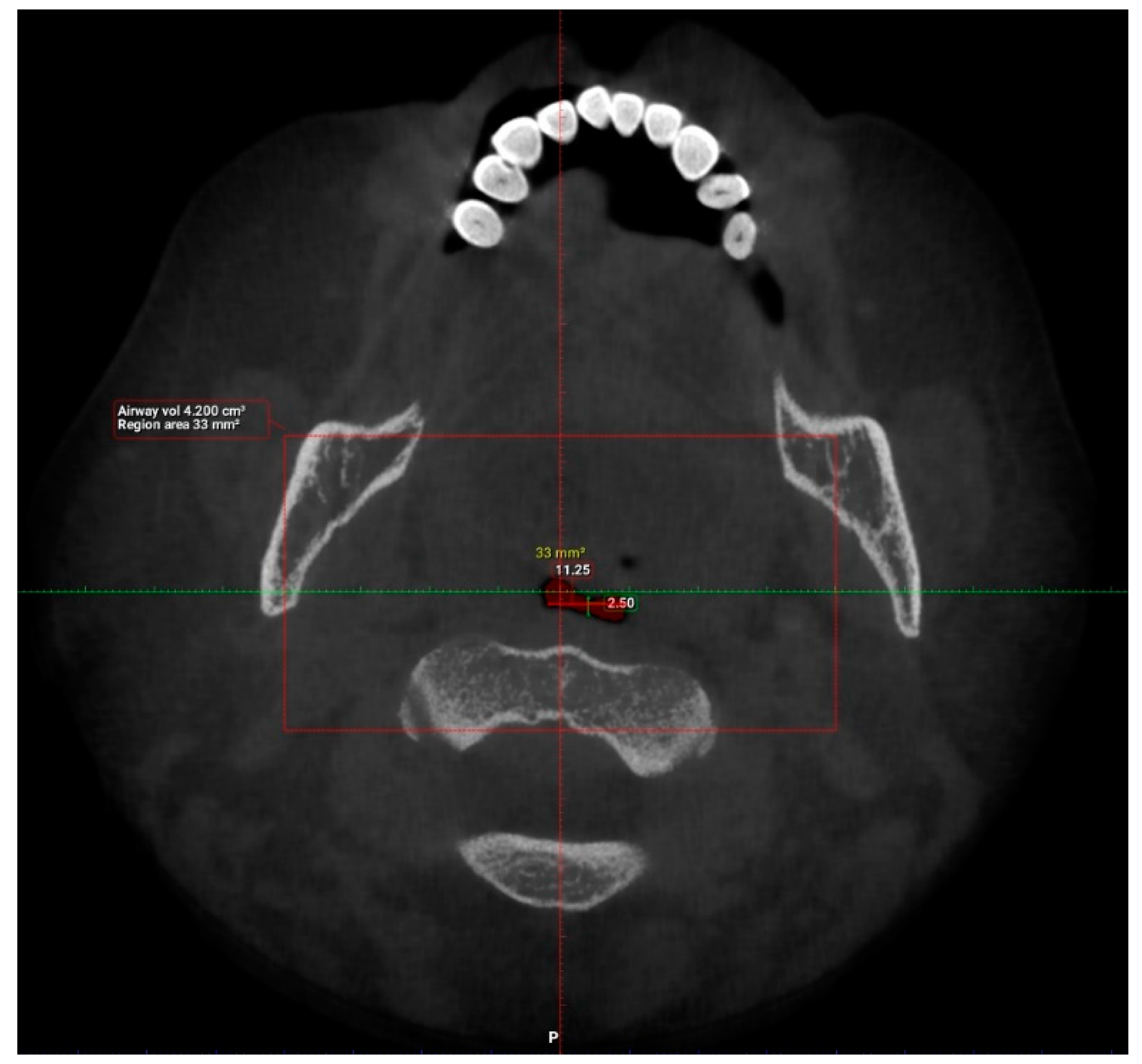
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slowik, J.M.; Collen, J.F. Obstructive Sleep Apnea; StatPearls Publishing: Treasure Island, FL, USA, 2019.
- Lee, W.; Nagubadi, S.; Kryger, M.H.; Mokhlesi, B. Epidemiology of Obstructive Sleep Apnea: A Population-Based Perspective. Expert Rev. Respir. Med. 2008, 2, 349–364. [Google Scholar] [CrossRef] [PubMed]
- STOP-Bang Questionnaire. Available online: http://stopbang.ca/osa/screening.php (accessed on 19 October 2020).
- Pang, K.P.; Terris, D.J. Screening for obstructive sleep apnea: An evidence-based analysis. Am. J. Otolaryngol. 2006, 27, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Calero, E.A.; Escalona, E.E.; Mora, J.M.B.; Carreras, J.M.L.; Reina, E.S. Obstructive Sleep Apnea Syndrome (OSAS). Review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e925–e929. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.J.; Pasirstein, M.; Pierson, R.; Mackley, A.; Hachadoorian, R.; Arens, R.; Maislin, G.; Pack, A.I. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am. J. Respir. Crit. Care Med. 2003, 168, 522–530. [Google Scholar] [CrossRef]
- Pinto, J.A.; Ribeiro, D.K.; Cavallini, A.F.D.S.; Duarte, C.; Freitas, G.S. Comorbidities Associated with Obstructive Sleep Apnea: A Retrospective Study. Int. Arch. Otorhinolaryngol. 2016, 20, 145–150. [Google Scholar] [CrossRef]
- Obstructive Sleep Apnea-Sleep Organization. Available online: https://aasm.org/resources/factsheets/sleepapnea.pdf (accessed on 19 October 2020).
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef]
- Quan, S.F.; Nowara, W.S. The Role of Dentists in the Diagnosis and Treatment of Obstructive Sleep Apnea: Consensus and Controversy. J. Clin. Sleep Med. 2017, 13, 1117–1119. [Google Scholar] [CrossRef]
- Conley, R.S. Evidence for dental and dental specialty treatment of obstructive sleep apnoea. Part 1: The adult OSA patient and Part 2: The paediatric and adolescent patient. J. Oral Rehabil. 2011, 38, 136–156. [Google Scholar] [CrossRef]
- Bahammam, A.S. Sleep medicine in Saudi Arabia: Current problems and future challenges. Ann. Thorac. Med. 2011, 6, 3–10. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E.; Sooronbaev, T.; Mirrakhimov, E.M. Prevalence of obstructive sleep apnea in Asian adults: A systematic review of the literature. BMC Pulm. Med. 2013, 13, 10. [Google Scholar] [CrossRef]
- Lam, B.; Lam, D.C.L.; Ip, M.S.M. Obstructive sleep apnoea in Asia. Int. J. Tuberc. Lung Dis. 2007, 11, 2–11. [Google Scholar] [PubMed]
- Mohd, A.K.; Cheong, L.T.; Syed, A.H. Snoring and breathing pauses during sleep in the Malaysian population. Respirology 2007, 12, 375–380. [Google Scholar] [CrossRef]
- Comorbidities. Available online: http://stopbang.ca/osa/comorbidities.php (accessed on 11 July 2021).
- Symptoms and Signs. Available online: http://stopbang.ca/osa/symptoms.php (accessed on 11 July 2021).
- Buchanan, A.; Cohen, R.; Looney, S.; Kalathingal, S.; Rossi, S.D. Cone-beam CT analysis of patients with obstructive sleep apnea compared to normal controls. Imaging Sci. Dent. 2016, 46, 9–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Worsnop, C.J.; Naughton, M.T.; Barter, C.E.; Morgan, T.O.; Anderson, A.I.; Pierce, R.J. The Prevalence of Obstructive Sleep Apnea in Hypertensives. Am. J. Respir. Crit. Care Med. 2003, 157, 111–115. [Google Scholar] [CrossRef]
- Millman, R.P.; Redline, S.; Carlisle, C.C.; Assaf, A.R.; Levinson, P.D. Daytime hypertension in obstructive sleep apnea. Prevalence and contributing risk factors. Natl. Libr. Med. 1991, 99, 861–866. [Google Scholar] [CrossRef]
- Hora, F.; Napolis, L.M.; Daltro, C.; Kodaira, S.K.; Tufik, S.; Togeiro, S.M.; Nery, L.E. Clinical, anthropometric and upper airway anatomic characteristics of obese patients with obstructive sleep apnea syndrome. Natl. Libr. Med. 2007, 74, 517–524. [Google Scholar] [CrossRef]
- Enciso, R.; Nguyen, M.; Shigeta, Y.; Ogawa, T.; Clark, G.T. Comparison of CBCT Parameters and Sleep Questionnaires in Sleep Apnea Patients and Controls. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 285–293. [Google Scholar] [CrossRef]
- Silverberg, D.S.; Oksenberg, A.; Iaina, A. Sleep related breathing disorders are common contributing factors to the production of essential hypertension but are neglected, underdiagnosed, and undertreated. Am. J. Hypertens. 1997, 10 Pt 1, 1319–1325. [Google Scholar] [CrossRef]
- Torre, M.S.; Campos-Rodrigues, F.; Barbe, F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir. Med. 2013, 1, 61–72. [Google Scholar] [CrossRef]
- Gami, A.S.; Caples, S.M.; Somers, V.K. Obesity and obstructive sleep apnea. Endocrinol. Metab. Clin. N. Am. 2003, 32, 869–894. [Google Scholar] [CrossRef]
- Abel, R.C.; Caples, S.M.; Jimenez, F.L.; Somers, V.K. Interactions Between Obesity and Obstructive Sleep Apnea. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef]
- Jehan, S.; Zizi, F.; Randi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Jean-Louis, G.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. 2017, 1, 00019. [Google Scholar] [PubMed]
- Lugaresi, E.; Cirignotta, F.; Montagna, P. Pathogenic aspects of snoring and obstructive apnea syndrome. Schweiz Med. Wochenschr. 1988, 118, 1333–1337. [Google Scholar] [PubMed]
- Woodhead, C.J.; Davies, J.E.; Allen, M.B. Obstructive sleep apnoea in adults presenting with snoring. Clin. Otolaryngol. Allied Sci. 1991, 16, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Marc. Deviated Septum and Sleep Apnea: Cause, Diagnosis & Treatment. Available online: https://www.deviatedseptumsurgery.org/related-conditions/deviated-septum-and-sleep-apnea/ (accessed on 10 August 2020).
- Andre, D. Obstructive Sleep Apnea Risk May Be Linked to Enlarged Tongue, Tonsillitis. Available online: https://www.belmarrahealth.com/obstructive-sleep-apnea-risk-may-linked-enlarged-tongue-tonsillitis/ (accessed on 10 March 2016).
- Knechtle, B.; Economou, N.T.; Nikolaidis, P.T.; Valentza, L.; Kallianos, A.; Steiropoulas, P.; Koutsompolis, D.; Rosemann, T.; Trakada, G. Clinical Characteristics of Obstructive Sleep Apnea in Psychiatric Disease. J. Clin. Med. 2019, 8, 534. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L.; Howards, M.E.; Barnes, M. Cognition and daytime functioning in sleep-related breathing disorders. Prog. Brain Res. 2011, 190, 53–68. [Google Scholar] [CrossRef]
- Sateira, M.J. Update on sleep and psychiatric disorders. Chest 2009, 135, 1370–1379. [Google Scholar] [CrossRef]
- Nadeem, R.; Mukesh Nida, M.; Waheed, I.; Khan, A.; Ahmed, S.; Naseem, J.; Champeau, D. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: A meta-regression analysis. J. Clin. Sleep Med. 2014, 10, 475–489. [Google Scholar] [CrossRef]
- Lin, Q.C.; Zhang, X.B.; Chen, G.P.; Huang, D.Y.; Din, H.B.; Tang, A.Z. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. Sleep Breath. 2012, 16, 571–578. [Google Scholar] [CrossRef]
- McArdle, N.; Hillman, D.; Beilin, L.; Watts, G. Metabolic risk factors for vascular disease in obstructive sleep apnea: A matched controlled study. Am. J. Respir. Crit. Care Med. 2007, 175, 190–195. [Google Scholar] [CrossRef]
- Tan, K.C.B.; Chow, W.S.; Lam, J.C.M.; Lam, B.; Wong, W.K.; Tam, S.; Ip, M.S. HDL dysfunction in obstructive sleep apnea. Atherosclerosis 2006, 184, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Nyenhuis, S.M.; Weaver, T.E. Obstructive sleep apnea and asthma: Associations and treatment implications. Sleep Med. Rev. 2014, 18, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, X.; Zhang, L. Association between allergic and nonallergic rhinitis and obstructive sleep apnea. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Chirakalwasan, N.; Ruxrungtham, K. The linkage of allergic rhinitis and obstructive sleep apnea. Asian Pac. J. Allergy Immunol. 2014, 32, 276–286. [Google Scholar]
- Michels, D.S.; Rodrigues, A.M.S.; Nakanishi, M.; Sampaio, A.L.L.; Venosa, A.R. Nasal Involvement in Obstructive Sleep Apnea Syndrome. Int. J. Otolaryngol. 2014, 2014, 717419. [Google Scholar] [CrossRef]


| Factor | Frequency | Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 46 | 45.5 |
| Female | 55 | 54.5 |
| Age | ||
| 18–27 years old | 33 | 32.7 |
| 28–37 years old | 24 | 23.8 |
| 38–47 years old | 11 | 10.9 |
| 48–57 years old | 11 | 10.9 |
| 58–67 years old | 16 | 15.8 |
| 68–77 years old | 6 | 5.9 |
| Race | ||
| Malay | 11 | 10.9 |
| Chinese | 76 | 75.2 |
| Indian | 9 | 8.9 |
| Others | 5 | 5.0 |
| Medical Comorbidities | ||
| Hypertension | 16 | 15.8 |
| High Cholesterol | 10 | 9.9 |
| Heart Disease | 2 | 2.0 |
| Diabetes | 6 | 5.9 |
| Asthma | 6 | 5.9 |
| GERD | 2 | 2.0 |
| Cancer | 4 | 4.0 |
| Depression | 3 | 3.0 |
| Deviated Nasal Septum | 3 | 3.0 |
| Tonsillitis | 6 | 5.9 |
| Allergic Rhinitis | 9 | 8.9 |
| Obesity | 9 | 8.9 |
| OSA Symptoms | ||
| Excessive Daytime Sleepiness | 37 | 36.6 |
| Loud Snoring | 57 | 56.4 |
| Stopped Breathing During Sleep | 4 | 4.0 |
| Sudden Awakening from Sleep with Gasping & Choking | 7 | 6.9 |
| Awakening with Dry Mouth/Sore Throat | 33 | 32.7 |
| Morning Headache | 12 | 11.9 |
| Difficulty Concentrating During Daytime | 14 | 13.9 |
| Mood Changes | 18 | 17.8 |
| Night Sweat | 4 | 4.0 |
| Decreased Sex Drive | 7 | 6.9 |
| Excessive Daytime Sleepiness | Loud Snoring | Stopped Breathing during Sleep | Sudden Awakening from Sleep with Gasping & Choking | Awakening with Dry Mouth/Sore Throat | |
| Hypertension | 9 (56.3%) | 7 (43.8%) | 1 (6.3%) | 1 (6.3%) | 5 (31.3%) |
| High Cholesterol | 5 (50.0%) | 4 (40.0%) | 1 (10.0%) | 0 | 2 (20.0%) |
| Heart Disease | 1 (50.0%) | 2 (100.0%) | 0 | 1 (50.0%) | 2 (100.0%) |
| Diabetes | 4 (66.7%) | 2 (33.3%) | 1 (16.7%) | 0 | 3 (50.0%) |
| Asthma | 2 (33.3%) | 1 (16.7%) | 0 | 1 (16.7%) | 0 |
| GERD | 1 (50.0%) | 1 (50.0%) | 0 | 0 | 1 (50.0%) |
| Cancer | 2 (50.0%) | 1 (25.0%) | 0 | 0 | 3 (75.0%) |
| Depression | 2 (66.7%) | 1 (33.3%) | 0 | 1 (33.3%) | 3 (100.0%) |
| Deviated Nasal Septum | 2 (66.7%) | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | 3 (100.0%) |
| Tonsillitis | 3 (50.0%) | 1 (16.7%) | 0 | 1 (16.7%) | 4 (66.7%) |
| Allergic Rhinitis | 2 (22.2%) | 1 (11.1%) | 0 | 2 (22.2%) | 2 (22.2%) |
| Obesity | 3 (33.3%) | 5 (55.6%) | 0 | 0 | 2 (22.2%) |
| Morning Headache | Difficulty Concentrating during Daytime | Mood Changes | Night Sweat | Decreased Sex Drive | |
| Hypertension | 2 (12.5%) | 2 (12.5%) | 2 (12.5%) | 1 (6.3%) | 1 (6.3%) |
| High Cholesterol | 1 (10.0%) | 2 (20.0%) | 2 (20.0%) | 1 (10.0%) | 1 (10.0%) |
| Heart Disease | 0 | 0 | 0 | 0 | 0 |
| Diabetes | 1 (16.7%) | 2 (33.3%) | 1 (16.7%) | 1 (16.7%) | 0 |
| Asthma | 0 | 0 | 0 | 0 | 0 |
| GERD | 0 | 0 | 0 | 0 | 0 |
| Cancer | 0 | 1 (25.0%) | 1 (25.0%) | 1 (25.0%) | 0 |
| Depression | 1 (33.3%) | 2 (66.7%) | 3 (100%) | 1 (33.3%) | 1 (33.3%) |
| Deviated Nasal Septum | 3 (100.0%) | 2 (66.7%) | 1 (33.3%) | 0 | 0 |
| Tonsillitis | 3 (50.0%) | 3 (50.0%) | 3 (50.0%) | 0 | 1 (16.7%) |
| Allergic Rhinitis | 3 (33.3%) | 3 (33.3%) | 3 (33.3%) | 0 | 1 (11.1%) |
| Obesity | 1 (11.1%) | 2 (22.2%) | 2 (22.2%) | 1 (11.1%) | 1 (11.1%) |
| N | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| Length (mm) | 101 | 29.93 | 55.75 | 42.63 | 6.24 |
| Total volume (cm3) | 101 | 94.58 | 173.45 | 131.67 | 17.91 |
| Average volume (cm3) | 101 | 3.34 | 32.09 | 11.10 | 5.21 |
| Antero-posterior (mm) | 101 | 1.00 | 14.50 | 6.44 | 3.19 |
| Width (mm) | 101 | 8.00 | 37.00 | 21.69 | 6.54 |
| Valid N (listwise) | 101 |
| Length (mm) | Total Volume (cm3) | Average Volume (cm3) | Anterior-Posterior (mm) | Width (mm) | ||
|---|---|---|---|---|---|---|
| Hypertension | Odds Ratio | 1.341 | 0.896 | 1.158 | - * | 0.781 |
| 95% CI | 1.056–1.705 | 0.819–0.980 | 0.893–1.503 | - * | 0.648–0.942 | |
| p-value | 0.016 | 0.017 | 0.269 | - * | 0.010 | |
| High Cholesterol | Odds Ratio | 1.077 | 0.965 | 1.077 | 1.022 | 0.960 |
| 95% CI | 0.848–1.367 | 0.891–1.046 | 0.798–1.453 | 0.744–1.403 | 0.799–1.155 | |
| p-value | 0.544 | 0.383 | 0.628 | 0.894 | 0.668 | |
| Heart Disease | Odds Ratio | 2.083 | 0.728 | 1.300 | 0.971 | 0.837 |
| 95% CI | 0.858–5.057 | 0.519–1.020 | 0.539–3.136 | 0.338–2.786 | 0.515–1.361 | |
| p-value | 0.105 | 0.065 | 0.559 | 0.956 | 0.473 | |
| Diabetes | Odds Ratio | 1.163 | 0.919 | 1.177 | 0.918 | 0.845 |
| 95% CI | 0.834–1.622 | 0.816–1.035 | 0.762–1.815 | 0.595–1.415 | 0.659–1.085 | |
| p-value | 0.373 | 0.165 | 0.463 | 0.697 | 0.186 | |
| Asthma | Odds Ratio | 0.841 | 1.047 | 1.297 | 0.941 | 0.770 |
| 95% CI | 0.619–1.142 | 0.951–1.153 | 0.856–1.964 | 0.632–1.402 | 0.585–1.014 | |
| p-value | 0.267 | 0.347 | 0.219 | 0.766 | 0.062 | |
| GERD | Odds Ratio | 22.034 | 0.271 | 0.000 | 0.000 | 0.766 |
| 95% CI | 0.000–2.584 | 0.000–1.020 | 0.000 | 0.000 | 0.000 | |
| p-value | 0.990 | 0.992 | 0.993 | 0.982 | 1.000 | |
| Cancer | Odds Ratio | 1.694 | 0.822 | 1.413 | 0.735 | 0.778 |
| 95% CI | 0.966–2.971 | 0.671–1.006 | 0.789–2.528 | 0.403–1.341 | 0.540–1.122 | |
| p-value | 0.066 | 0.058 | 0.245 | 0.316 | 0.179 | |
| Depression | Odds Ratio | 1.053 | 0.993 | 0.693 | 1.352 | 1.124 |
| 95% CI | 0.693–1.599 | 0.870–1.133 | 0.342–1.402 | 0.770–2.372 | 0.795–1.589 | |
| p-value | 0.810 | 0.918 | 0.308 | 0.294 | 0.507 | |
| Deviated Nasal Septum | Odds Ratio | 1.039 | 0.992 | 0.637 | 1.502 | 1.189 |
| 95% CI | 0.674–1.599 | 0.865–1.139 | 0.310–1.307 | 0.847–2.663 | 0.831–1.702 | |
| p-value | 0.864 | 0.914 | 0.219 | 0.164 | 0.344 | |
| Tonsillitis | Odds Ratio | 1.124 | 1.020 | 0.671 | 1.190 | 1.023 |
| 95% CI | 0.829–1.523 | 0.932–1.115 | 0.365–1.231 | 0.750–1.889 | 0.770–1.358 | |
| p-value | 0.451 | 0.668 | 0.197 | 0.461 | 0.876 | |
| Allergic Rhinitis | Odds Ratio | 0.851 | 1.029 | 0.846 | 1.199 | 1.112 |
| 95% CI | 0.655–1.106 | 0.951–1.113 | 0.577–1.241 | 0.846–1.698 | 0.903–1.368 | |
| p-value | 0.227 | 0.477 | 0.392 | 0.308 | 0.318 | |
| Obesity | Odds Ratio | 1.175 | 0.953 | 1.042 | 1.086 | 0.792 |
| 95% CI | 0.876–1.577 | 0.864–1.051 | 0.687–1.581 | 0.745–1.583 | 0.619–1.014 | |
| p-value | 0.282 | 0.333 | 0.845 | 0.669 | 0.899 |
| Length (mm) | Total Volume (cm3) | Average Volume (cm3) | Anterior-Posterior (mm) | Width (mm) | ||
|---|---|---|---|---|---|---|
| Excessive Daytime Sleepiness | Odds Ratio | 1.159 | 0.979 | 0.843 | 1.243 | 1.010 |
| 95% CI | 0.998–1.345 | 0.934–1.026 | 0.679–1.045 | 1.008–1.533 | 0.893–1.142 | |
| p-value | 0.053 | 0.373 | 0.118 | 0.042 | 0.877 | |
| Loud Snoring | Odds Ratio | 1.154 | - * | 0.711 | 0.908 | 1.052 |
| 95% CI | 1.022–1.304 | - * | 0.515–0.980 | 0.692–1.191 | 0.897–1.233 | |
| p-value | 0.021 | - * | 0.037 | 0.484 | 0.532 | |
| Observed episodes of breathing stopping during sleep | Odds Ratio | 1.118 | 0.997 | 0.626 | 1.018 | 1.185 |
| 95% CI | 0.806–1.551 | 0.901–1.103 | 0.319–1.231 | 0.582–1.780 | 0.887–1.584 | |
| p-value | 0.504 | 0.955 | 0.175 | 0.950 | 0.251 | |
| Abrupt awakening from sleep accompanied by gasping or choking | Odds Ratio | 0.985 | 1.053 | 0.840 | 1.057 | 1.111 |
| 95% CI | 0.764–1.269 | 0.976 | 0.557–1.267 | 0.723–1.545 | 0.876–1.409 | |
| p-value | 0.906 | 1.137 | 0.406 | 0.775 | 0.386 | |
| Awakening with a dry mouth or sore throat | Odds Ratio | 1.119 | 0.982 | 0.928 | - * | 1.029 |
| 95% CI | 0.976–1.283 | 0.937–1.030 | 0.791–1.089 | - * | 0.917–1.155 | |
| p-value | 0.107 | 0.458 | 0.362 | - * | 0.627 | |
| Morning headache | Odds Ratio | 1.108 | 0.988 | 0.796 | 1.333 | 1.118 |
| 95% CI | 0.889–1.382 | 0.921–1.059 | 0.579–1.095 | 0.971–1.828 | 0.929–1.344 | |
| p-value | 0.361 | 0.733 | 0.161 | 0.075 | 0.237 | |
| Difficulty concentrating during the day | Odds Ratio | 1.275 | 1.014 | 0.471 | 2.097 | 1.252 |
| 95% CI | 0.994–1.637 | 0.945–1.089 | 0.291–0.765 | 1.358–3.238 | 0.995–1.576 | |
| p-value | 0.056 | 0.696 | 0.002 | <0.001 | 0.056 | |
| Mood changes | Odds Ratio | 1.101 | 1.015 | 0.729 | 1.477 | 1.133 |
| 95% CI | 0.912–1.330 | 0.958–1.075 | 0.542–0.980 | 1.103–1.979 | 0.960–1.339 | |
| p-value | 0.316 | 0.619 | 0.036 | 0.009 | 0.141 | |
| Night-time sweating | Odds Ratio | 1.421 | 0.916 | 0.533 | 1.599 | 1.160 |
| 95% CI | 0.872–2.316 | 0.786–1.067 | 0.245–1.159 | 0.871–2.937 | 0.847–1.588 | |
| p-value | 0.158 | 0.260 | 0.112 | 0.130 | 0.356 | |
| Decreased sex drive | Odds Ratio | 1.143 | 0.964 | 0.647 | 1.500 | 1.144 |
| 95% CI | 0.841–1.552 | 0.873–1.064 | 0.392–1.066 | 0.995–2.261 | 0.899–1.457 | |
| p-value | 0.393 | 0.466 | 0.087 | 0.053 | 0.274 |
| Excessive Daytime Sleepiness | Loud Snoring | Stopped Breathing During Sleep | Sudden Awakening from Sleep with Gasping & Choking | Awakening with Dry Mouth/Sore Throat | |
| Hypertension | 0.093 | 0.016 | 0.504 | 1.000 | 1.000 |
| High Cholesterol | 0.491 | 0.106 | 0.345 | 1.000 | 0.492 |
| Heart Disease | 1.000 | 0.038 | 1.000 | 0.134 | 0.105 |
| Diabetes | 0.188 | 0.340 | 0.220 | 1.000 | 0.389 |
| Asthma | 1.000 | 1.000 | 1.000 | 0.358 | 0.174 |
| GERD | 1.000 | 0.358 | 1.000 | 1.000 | 0.549 |
| Cancer | 0.622 | 1.000 | 1.000 | 1.000 | 0.101 |
| Depression | 0.552 | 0.488 | 1.000 | 0.196 | 0.033 |
| Deviated Nasal Septum | 0.552 | 0.488 | 0.115 | 0.196 | 0.033 |
| Tonsillitis | 0.666 | 1.000 | 1.000 | 0.358 | 0.087 |
| Allergic Rhinitis | 0.480 | 0.684 | 1.000 | 0.118 | 0.714 |
| Obesity | 1.000 | 0.014 | 1.000 | 1.000 | 0.714 |
| Morning Headache | Difficulty Concentrating during Daytime | Mood Changes | Night Sweat | Decreased Sex Drive | |
| Hypertension | 1.000 | 1.000 | 0.730 | 0.504 | 1.000 |
| High Cholesterol | 1.000 | 0.626 | 1.000 | 0.345 | 0.529 |
| Heart Disease | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Diabetes | 0.541 | 0.193 | 1.000 | 0.220 | 1.000 |
| Asthma | 1.000 | 0.592 | 0.588 | 1.000 | 1.000 |
| GERD | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Cancer | 1.000 | 0.455 | 0.550 | 0.151 | 1.000 |
| Depression | 0.319 | 0.050 | 0.005 | 0.115 | 0.196 |
| Deviated Nasal Septum | 0.001 | 0.050 | 0.449 | 1.000 | 1.000 |
| Tonsillitis | 0.021 | 0.034 | 0.068 | 1.000 | 0.358 |
| Allergic Rhinitis | 0.072 | 0.108 | 0.198 | 1.000 | 0.491 |
| Obesity | 1.000 | 0.608 | 0.660 | 0.316 | 0.491 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.Y.; Eow, P.Y.; Kohli, S.; Math, S.Y. Correlation of Medical Comorbidities and Upper Airway Measurements among Dental Patients at Risk of Developing Obstructive Sleep Apnea. Clin. Pract. 2022, 12, 284-298. https://doi.org/10.3390/clinpract12030034
Lin KY, Eow PY, Kohli S, Math SY. Correlation of Medical Comorbidities and Upper Airway Measurements among Dental Patients at Risk of Developing Obstructive Sleep Apnea. Clinics and Practice. 2022; 12(3):284-298. https://doi.org/10.3390/clinpract12030034
Chicago/Turabian StyleLin, Kar Yi, Pei Ying Eow, Shivani Kohli, and Swarna Yerebairapura Math. 2022. "Correlation of Medical Comorbidities and Upper Airway Measurements among Dental Patients at Risk of Developing Obstructive Sleep Apnea" Clinics and Practice 12, no. 3: 284-298. https://doi.org/10.3390/clinpract12030034
APA StyleLin, K. Y., Eow, P. Y., Kohli, S., & Math, S. Y. (2022). Correlation of Medical Comorbidities and Upper Airway Measurements among Dental Patients at Risk of Developing Obstructive Sleep Apnea. Clinics and Practice, 12(3), 284-298. https://doi.org/10.3390/clinpract12030034






