Arterial Thrombosis in an Unusual Site (Ulnar Artery) after COVID-19 Vaccination—A Case Report
Abstract
:1. Background
2. Case Presentation
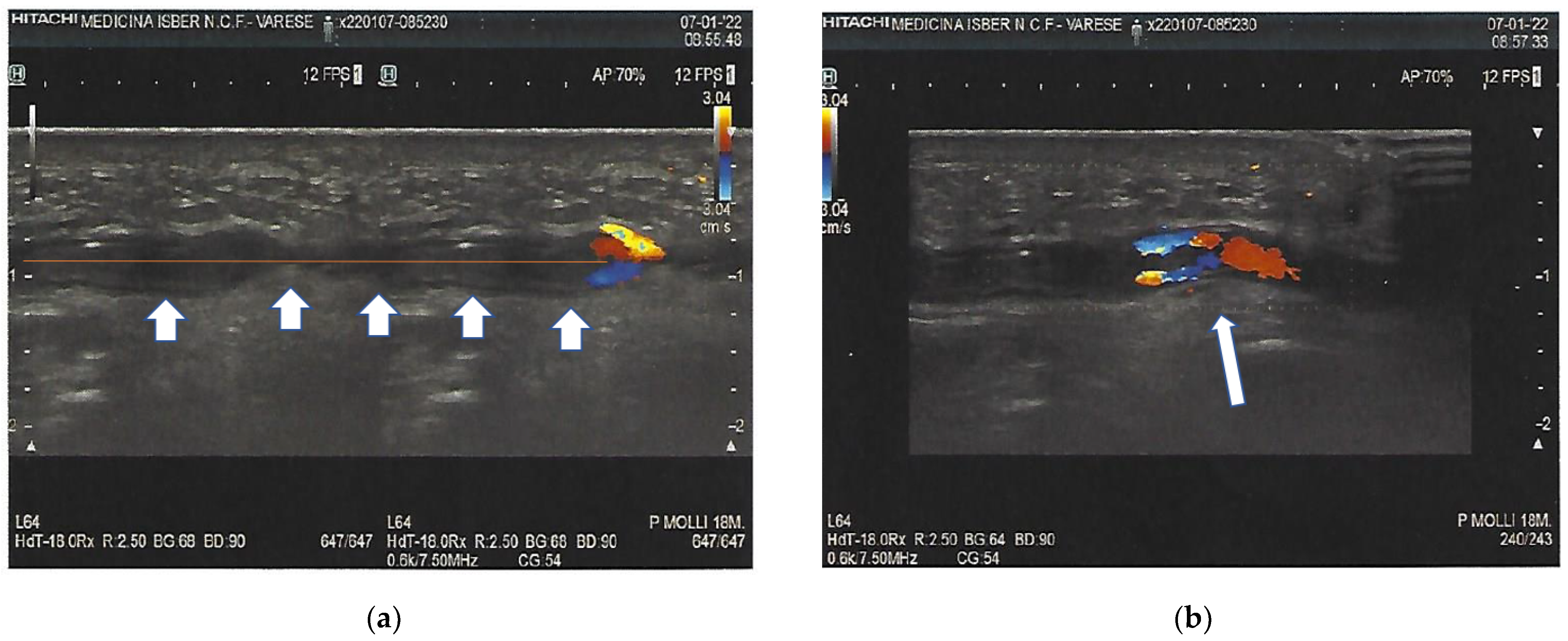
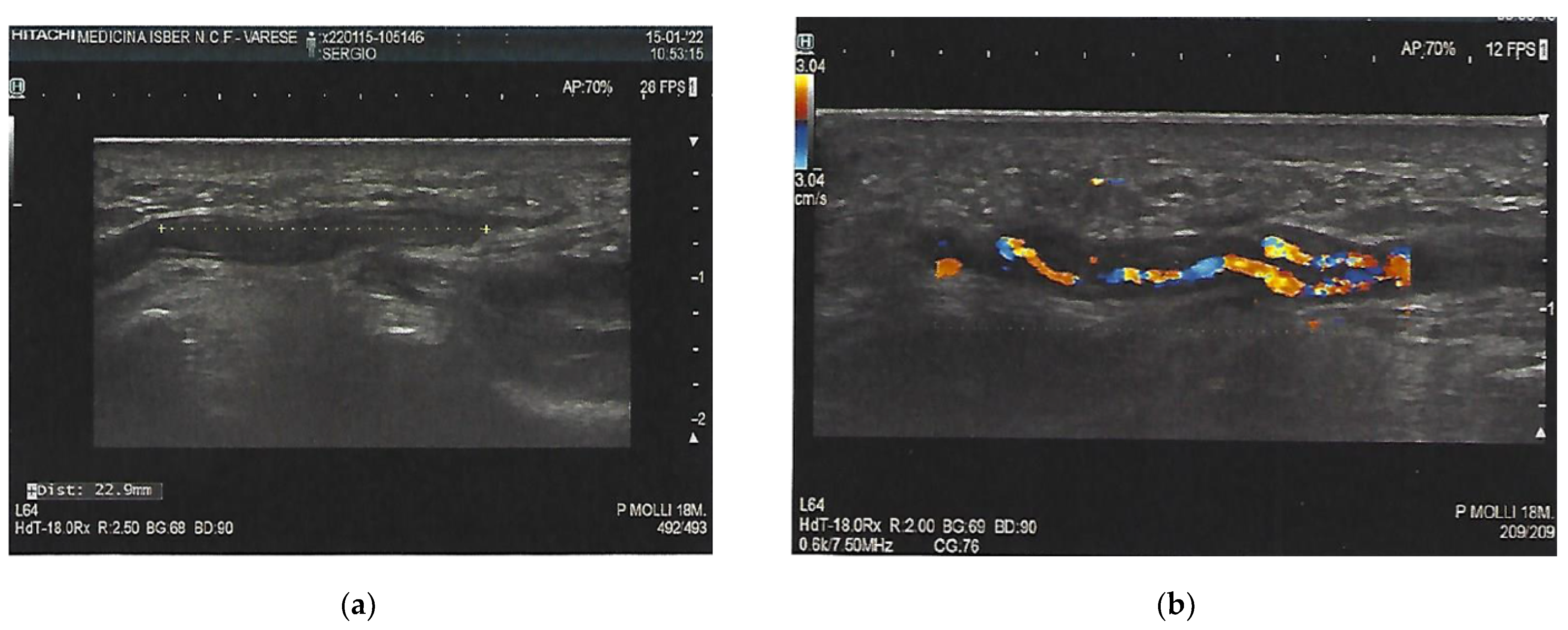
3. Investigations
4. Differential Diagnosis
5. Treatment
6. Outcome and Follow-Up
7. Discussion
8. Site of Thrombosis
9. Learning Points/Take-Home Messages
10. Patient’s Perspective and General Considerations
11. Comment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 against SARS-CoV-2: A preliminary report of a phase ½, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Smadja, D.M.; Yue, Q.-Y.; Chocron, R.; Sanchez, O.; Louet, A.L.-L. Vaccination against COVID-19: Insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur. Respir. J. 2021, 58, 2100956. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, N.B. Occlusive vascular disorders of the upper extremity. Hand Clin. 1993, 9, 139–143. [Google Scholar] [CrossRef]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, I.; Lewington, S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.vigifarmaco.it (accessed on 9 February 2022).
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, 2 March to 21 April 2021. JAMA 2021, 325, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine–Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Fuchs, M.; Grossmann, A.; Walter, M.; Thiele, T.; Storch, A.; Wittstock, M. Adenovirus-Vectored COVID-19 Vaccine–Induced Immune Thrombosis of Carotid Artery. Neurology 2021, 97, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Pastori, D.; Cangemi, R.; Pignatelli, P.; Loffredo, L. Hypercoagulation and Antithrombotic Treatment in Coronavirus 2019: A New Challenge. Thromb. Haemost. 2020, 120, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.; Nadeem, A.J. Coronavirus (COVID-19) vaccine-induced immune thrombotic thrombocytopenia (VITT). In StatPearls; StatPearls Publishing: Treasure Island, CA, USA, 2021. [Google Scholar]
- Violi, F.; Cammisotto, V.; Pastori, D.; Pignatelli, P. Thrombosis in pre- and post-vaccination phase of COVID-19. Eur. Heart J. Suppl. 2021, 23, E184–E188. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Available online: www.adrreports.eu (accessed on 26 February 2022).
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.F.; Uzzo, M.L.; Argo, A.; Zerbo, S. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front. Immunol. 2021, 12, 729251. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Ali, K.; Connell, D.; Mordi, I.; George, J.; Lang, E.; Lang, C. COVID-19-Associated Cardiovascular Complications. Diseases 2021, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Seddon, M.; Shahzad, K.; Lunevicius, R. Post-COVID-19 vaccination occurrence of splenic infarction due to arterial thrombosis. BMJ Case Rep. 2021, 14, e243846. [Google Scholar] [CrossRef]

| Test | Value | Normal Range |
|---|---|---|
| Hemoglobin (g/L) | 143 | 130–175 |
| White blood cells (per mm3) | 6200 | 4800–10,800 |
| Platelet count (per mm3) | 244,000 | 130,000–400,000 |
| Prothrombin time (PT) (INR) | 1.0 | 0.8–1.2 |
| Activate partial thromboplastine time (aPTT) (sec) | 32.2 | 22.7–32.5 |
| D-dimers (mcg/mL) | 0.25 | <0.50 |
| Lupus anticoagulant | 0.50 | <1.20 |
| Antiphospholipid antibodies IgG (U/mL) | 3.1 | <12 |
| SARS-CoV-2 RT-PCR test | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sessa, A.; Gattamorta, M.; Punginelli, M.; Maggioni, G. Arterial Thrombosis in an Unusual Site (Ulnar Artery) after COVID-19 Vaccination—A Case Report. Clin. Pract. 2022, 12, 237-242. https://doi.org/10.3390/clinpract12030028
Sessa A, Gattamorta M, Punginelli M, Maggioni G. Arterial Thrombosis in an Unusual Site (Ulnar Artery) after COVID-19 Vaccination—A Case Report. Clinics and Practice. 2022; 12(3):237-242. https://doi.org/10.3390/clinpract12030028
Chicago/Turabian StyleSessa, Aurelio, Marco Gattamorta, Maurizia Punginelli, and Gianluigi Maggioni. 2022. "Arterial Thrombosis in an Unusual Site (Ulnar Artery) after COVID-19 Vaccination—A Case Report" Clinics and Practice 12, no. 3: 237-242. https://doi.org/10.3390/clinpract12030028
APA StyleSessa, A., Gattamorta, M., Punginelli, M., & Maggioni, G. (2022). Arterial Thrombosis in an Unusual Site (Ulnar Artery) after COVID-19 Vaccination—A Case Report. Clinics and Practice, 12(3), 237-242. https://doi.org/10.3390/clinpract12030028






