Usefulness and Limits of Tractography for Surgery in the Precentral Gyrus—A Case Report
Abstract
:1. Introduction
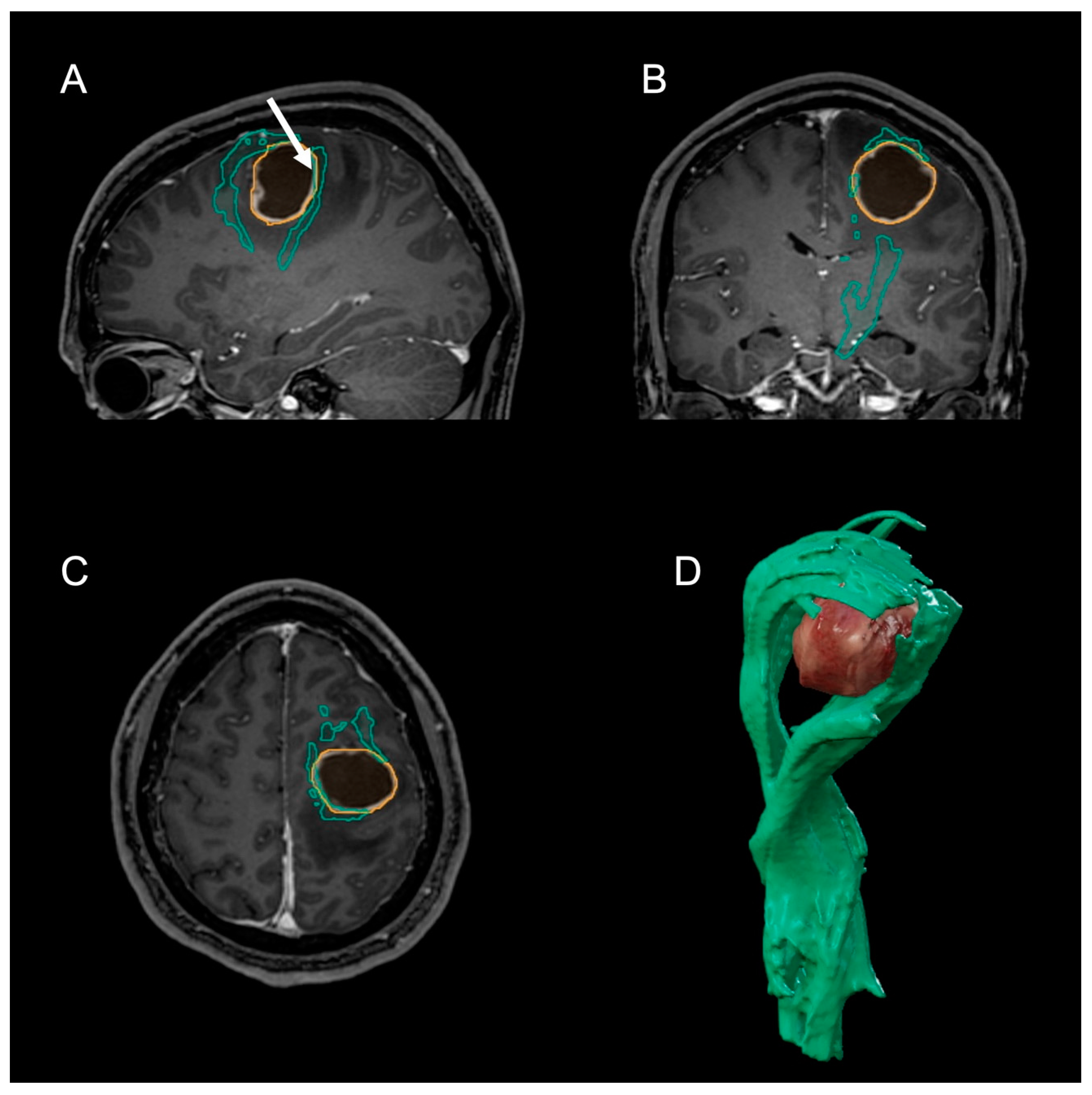
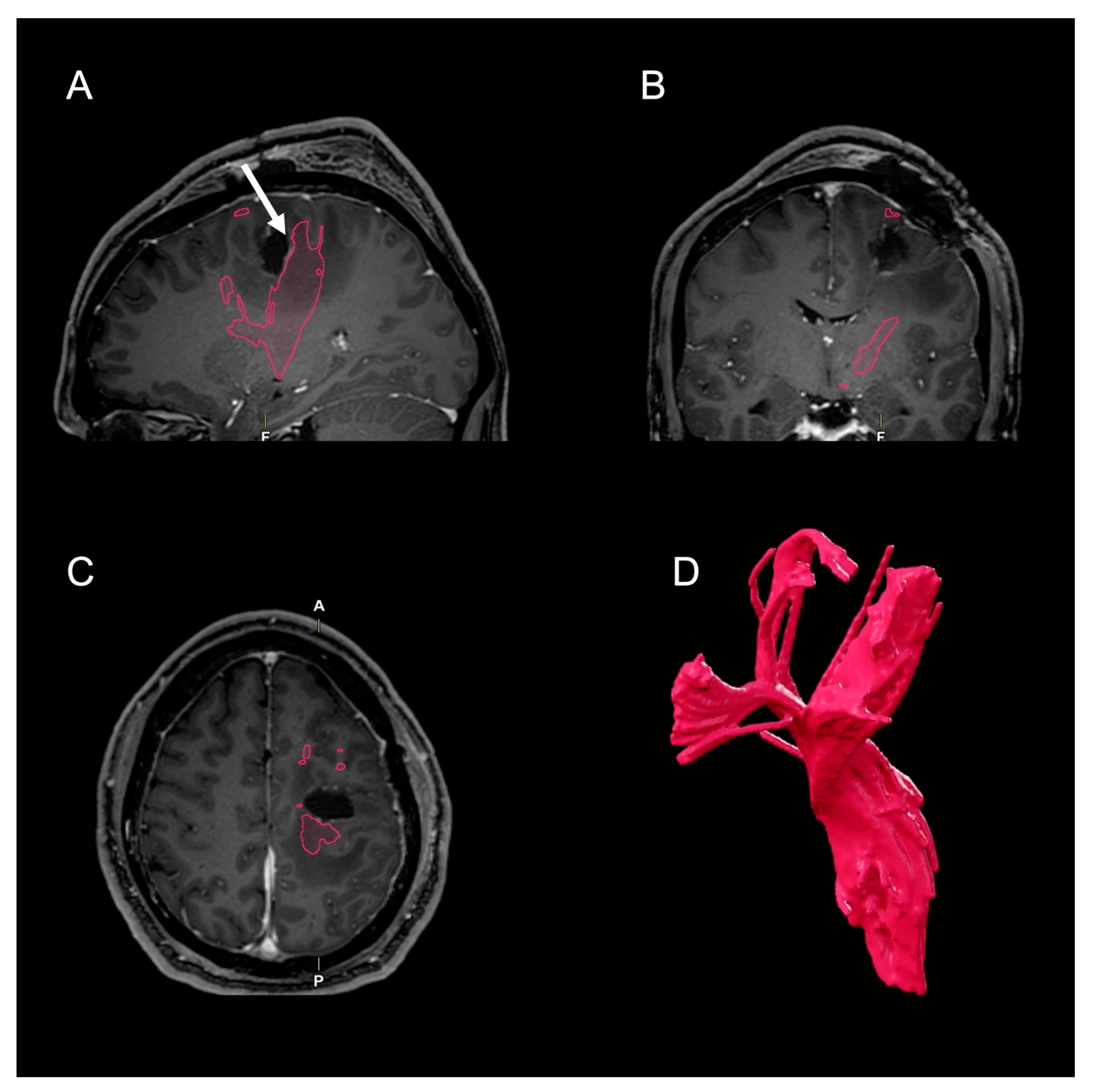
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sollmann, N.; Zhang, H.; Schramm, S.; Ille, S.; Negwer, C.; Kreiser, K.; Meyer, B.; Krieg, S.M. Function-specific Tractography of Language Pathways Based on nTMS Mapping in Patients with Supratentorial Lesions. Clin. Neuroradiol. 2020, 30, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Wende, T.; Hoffmann, K.-T.; Meixensberger, J. Tractography in Neurosurgery: A Systematic Review of Current Applications. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2020, 81, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, T.; Giampiccolo, D.; Schneider, H.; Runge, S.J.; Bährend, I.; Vajkoczy, P.; Picht, T. Specific DTI seeding and diffusivity-analysis improve the quality and prognostic value of TMS-based deterministic DTI of the pyramidal tract. NeuroImage Clin. 2017, 16, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Fratini, A.; Zhang, H.; Zimmer, C.; Meyer, B.; Krieg, S.M. Associations between clinical outcome and tractography based on navigated transcranial magnetic stimulation in patients with language-eloquent brain lesions. J. Neurosurg. 2020, 132, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Buxhoeveden, D.P.; Casanova, M.F. The minicolumn hypothesis in neuroscience. Brain 2002, 125, 935–951. [Google Scholar] [CrossRef] [PubMed]
- Dalamagkas, K.; Tsintou, M.; Rathi, Y.; O’Donnell, L.J.; Pasternak, O.; Gong, X.; Zhu, A.; Savadjiev, P.; Papadimitriou, G.M.; Kubicki, M.; et al. Individual variations of the human corticospinal tract and its hand-related motor fibers using diffusion MRI tractography. Brain Imaging Behav. 2020, 14, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, L.H.; Seidel, K.; Wiest, R.; Beck, J.; Raabe, A. Localization of primary language areas by arcuate fascicle fiber tracking. Neurosurgery 2012, 70, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Rudà, R.; Reifenberger, G.; Frappaz, D.; Pfister, S.M.; Laprie, A.; Santarius, T.; Roth, P.; Tonn, J.C.; Soffietti, R.; Weller, M.; et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro-Oncol. 2018, 20, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wende, T.; Kasper, J.; Wilhelmy, F.; Dietel, E.; Hamerla, G.; Scherlach, C.; Meixensberger, J.; Fehrenbach, M.K. Assessment of a Reliable Fractional Anisotropy Cutoff in Tractography of the Corticospinal Tract for Neurosurgical Patients. Brain Sci. 2021, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.-D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA–09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Silva, L.L.; Tuncer, M.S.; Vajkoczy, P.; Picht, T.; Rosenstock, T. Distinct approaches to language pathway tractography: Comparison of anatomy-based, repetitive navigated transcranial magnetic stimulation (rTMS)–based, and rTMS-enhanced diffusion tensor imaging–fiber tracking. J. Neurosurg. 2021, 136, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Essayed, W.I.; Zhang, F.; Unadkat, P.; Cosgrove, G.R.; Golby, A.J.; O’Donnell, L.J. White matter tractography for neurosurgical planning: A topography-based review of the current state of the art. NeuroImage Clin. 2017, 15, 659–672. [Google Scholar] [CrossRef]
- Zhang, F.; Daducci, A.; He, Y.; Schiavi, S.; Seguin, C.; Smith, R.; Yeh, C.-H.; Zhao, T.; O’Donnell, L.J. Quantitative mapping of the brain’s structural connectivity using diffusion MRI tractography: A review. Neuroimage 2022, 249, 118870. [Google Scholar] [CrossRef]
- Kasper, J.; Hilbert, N.; Wende, T.; Fehrenbach, M.K.; Wilhelmy, F.; Jähne, K.; Frydrychowicz, C.; Hamerla, G.; Meixensberger, J.; Arlt, F. On the Prognosis of Multifocal Glioblastoma: An Evaluation Incorporating Volumetric MRI. Curr. Oncol. 2021, 28, 1437–1446. [Google Scholar] [CrossRef]
- Kasper, J.; Wende, T.; Fehrenbach, M.K.; Wilhelmy, F.; Jähne, K.; Frydrychowicz, C.; Prasse, G.; Meixensberger, J.; Arlt, F. The Prognostic Value of NANO Scale Assessment in IDH-Wild-Type Glioblastoma Patients. Front. Oncol. 2021, 11, 790458. [Google Scholar] [CrossRef]
- Sollmann, N.; Zhang, H.; Fratini, A.; Wildschuetz, N.; Ille, S.; Schröder, A.; Zimmer, C.; Meyer, B.; Krieg, S. Risk Assessment by Presurgical Tractography Using Navigated TMS Maps in Patients with Highly Motor- or Language-Eloquent Brain Tumors. Cancers 2020, 12, 1264. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Schucht, P.; Seidel, K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: Evaluation of a new method. J. Neurosurg. 2014, 120, 1015–1024. [Google Scholar] [CrossRef]
- Schulz, R.; Braass, H.; Liuzzi, G.; Hoerniss, V.; Lechner, P.; Gerloff, C.; Hummel, F.C. White matter integrity of premotor-motor connections is associated with motor output in chronic stroke patients. NeuroImage Clin. 2015, 7, 82–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sander, C.; Reuschel, V.; Eisenlöffel, C.; Nestler, U.; Meixensberger, J. Familial glioblastoma clustering in adult patients: A case report of two non-twin siblings and review of the literature. Int. Med. Case Rep. J. 2019, 12, 205–211. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wende, T.; Wilhelmy, F.; Kasper, J.; Prasse, G.; Franke, C.; Arlt, F.; Frydrychowicz, C.; Meixensberger, J.; Nestler, U. Usefulness and Limits of Tractography for Surgery in the Precentral Gyrus—A Case Report. Clin. Pract. 2022, 12, 231-236. https://doi.org/10.3390/clinpract12020027
Wende T, Wilhelmy F, Kasper J, Prasse G, Franke C, Arlt F, Frydrychowicz C, Meixensberger J, Nestler U. Usefulness and Limits of Tractography for Surgery in the Precentral Gyrus—A Case Report. Clinics and Practice. 2022; 12(2):231-236. https://doi.org/10.3390/clinpract12020027
Chicago/Turabian StyleWende, Tim, Florian Wilhelmy, Johannes Kasper, Gordian Prasse, Christian Franke, Felix Arlt, Clara Frydrychowicz, Jürgen Meixensberger, and Ulf Nestler. 2022. "Usefulness and Limits of Tractography for Surgery in the Precentral Gyrus—A Case Report" Clinics and Practice 12, no. 2: 231-236. https://doi.org/10.3390/clinpract12020027
APA StyleWende, T., Wilhelmy, F., Kasper, J., Prasse, G., Franke, C., Arlt, F., Frydrychowicz, C., Meixensberger, J., & Nestler, U. (2022). Usefulness and Limits of Tractography for Surgery in the Precentral Gyrus—A Case Report. Clinics and Practice, 12(2), 231-236. https://doi.org/10.3390/clinpract12020027






