Disease-Modifying Adjunctive Therapy (DMAT) in Osteoarthritis—The Biological Effects of a Multi-Mineral Complex, LithoLexal® Joint—A Review
Abstract
:1. Introduction
1.1. Epidemiology and Health Burden of Osteoarthritis
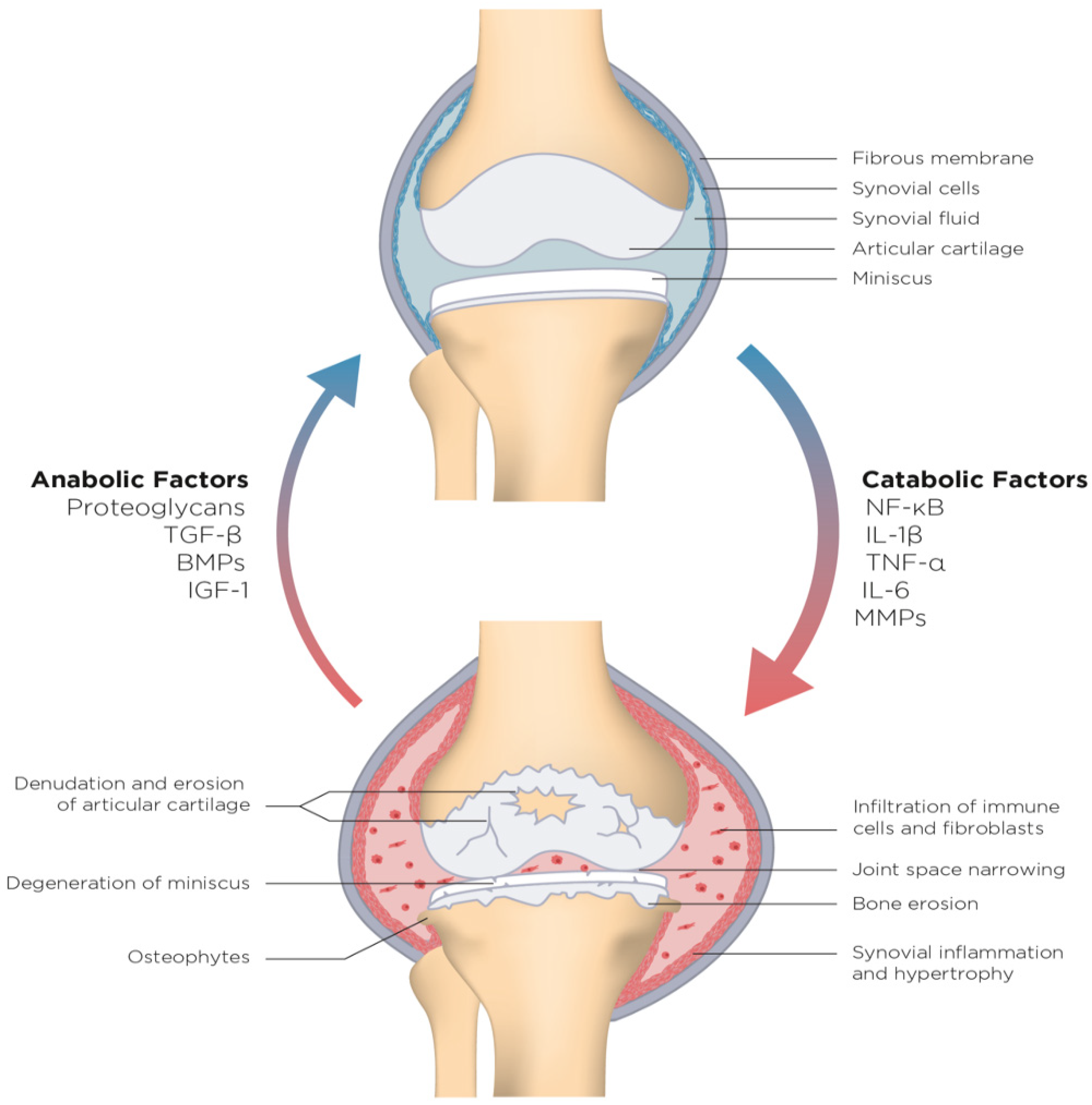
1.2. Understanding Osteoarthritis as an Inflammatory Disease
2. The Role of Adjunctive Therapy in the Management of Osteoarthritis
2.1. Conventional Adjunctive Therapies
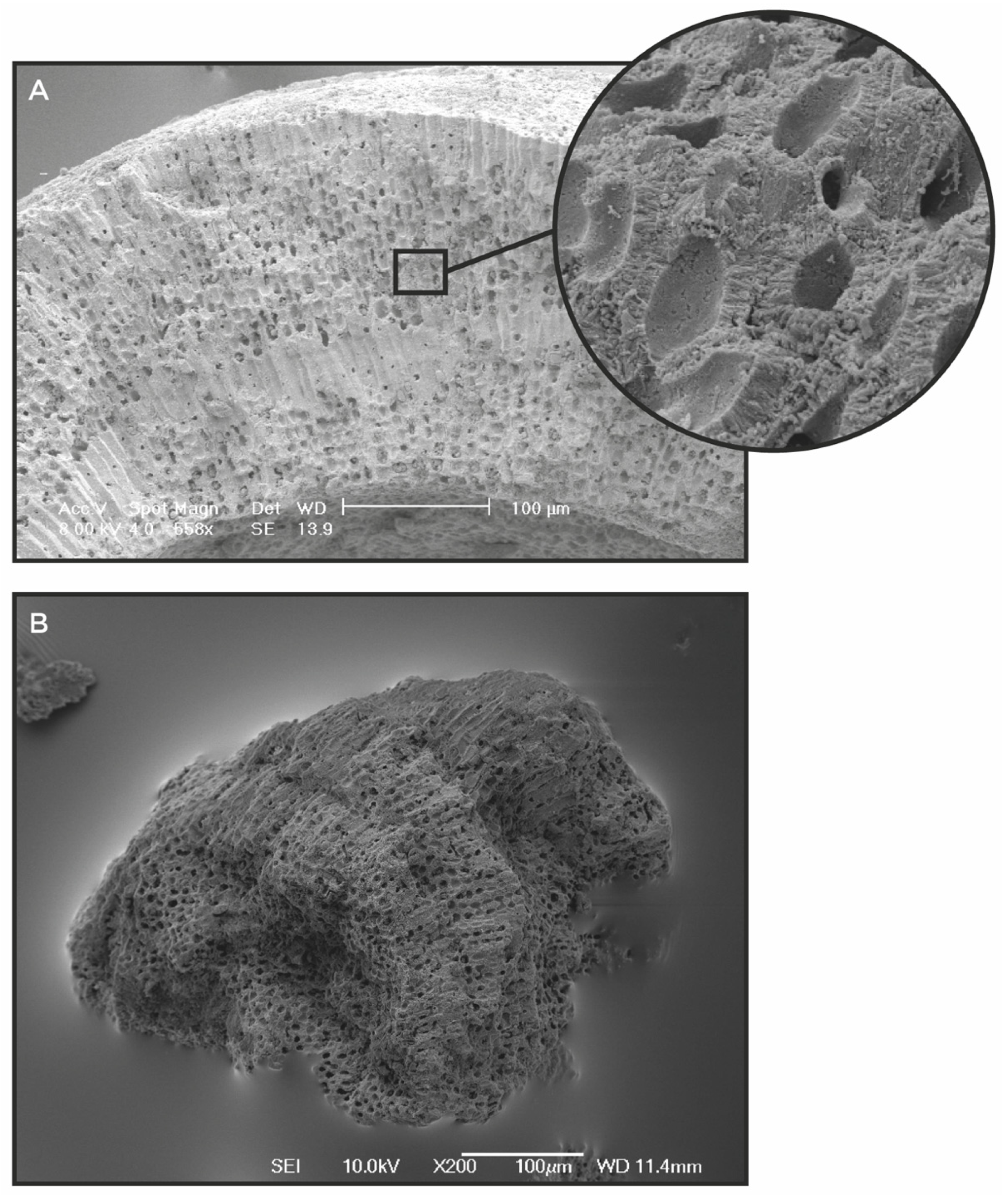
2.2. Natural Multi-Mineral Complexes
2.3. Oral Proteoglycan Replacement Therapy (PRT)
3. Disease-Modifying Adjunctive Therapy (DMAT) in Osteoarthritis: A Novel Frontier
4. LithoLexal® Joint, a Natural DMAT for the Management of Osteoarthritis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abhishek, A.; Doherty, M. Diagnosis and clinical presentation of osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 45–66. [Google Scholar] [CrossRef]
- Felson, D.T.; Naimark, A.; Anderson, J.; Kazis, L.; Castelli, W.; Meenan, R.F. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987, 30, 914–918. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, W.; Wang, Y.; Rong, J.; Bao, C.; Liu, Y.; Zhao, Y.; Wang, C. Body mass index and susceptibility to knee osteoarthritis: A systematic review and meta-analysis. Jt. Bone Spine 2012, 79, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Peleteiro, B.; Araujo, J.; Branco, J.; Santos, R.A.; Ramos, E. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthr. Cartil. 2011, 19, 1270–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Hawker, G.A.; Laporte, A.; Croxford, R.; Coyte, P.C. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology 2005, 44, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Egloff, C.; Hugle, T.; Valderrabano, V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med. Wkly. 2012, 142, w13583. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Chen, C.; Bao, G.F.; Xu, G.; Sun, Y.; Cui, Z.M. Altered Wnt and NF-κB Signaling in Facet Joint Osteoarthritis: Insights from RNA Deep Sequencing. Tohoku J. Exp. Med. 2018, 245, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez Romero, E.A.; Fernandez Carnero, J.; Villafane, J.H.; Calvo-Lobo, C.; Ochoa Saez, V.; Burgos Caballero, V.; Val, S.L.; Pedersini, P.; Martín, D.P. Prevalence of Myofascial Trigger Points in Patients with Mild to Moderate Painful Knee Osteoarthritis: A Secondary Analysis. J. Clin. Med. 2020, 9, 2561. [Google Scholar] [CrossRef] [PubMed]
- Porcheret, M.; Healey, E.H.E.; Dziedzic, K.; Corp, N.; Howells, N.; Birrell, F. Osteoarthritis: A modern approach to diagnosis and management. In Reports on the Rheumatic Diseases; Series 6 (Hands On No 10); Arthritis Research UK: Chesterfield, UK, 2011. [Google Scholar]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Pfander, D.; Heinz, N.; Rothe, P.; Carl, H.D.; Swoboda, B. Tenascin and aggrecan expression by articular chondrocytes is influenced by interleukin 1beta: A possible explanation for the changes in matrix synthesis during osteoarthritis. Ann. Rheum. Dis. 2004, 63, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Goldring, M.B.; Birkhead, J.; Sandell, L.J.; Kimura, T.; Krane, S.M. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J. Clin. Investig. 1988, 82, 2026–2037. [Google Scholar] [CrossRef]
- Shabestari, M.; Kise, N.J.; Landin, M.A.; Sesseng, S.; Hellund, J.C.; Reseland, J.E.; Eriksen, E.F.; Haugen, I.K. Enhanced angiogenesis and increased bone turnover characterize bone marrow lesions in osteoarthritis at the base of the thumb. Bone Jt. Res. 2018, 7, 406–413. [Google Scholar] [CrossRef]
- Shabestari, M.; Shabestari, Y.R.; Landin, M.A.; Pepaj, M.; Cleland, T.P.; Reseland, J.E.; Eriksen, E.F. Altered protein levels in bone marrow lesions of hip osteoarthritis: Analysis by proteomics and multiplex immunoassays. Int. J. Rheum. Dis. 2020, 23, 788–799. [Google Scholar] [CrossRef]
- Franz, A.; Joseph, L.; Mayer, C.; Harmsen, J.F.; Schrumpf, H.; Frobel, J.; Ostapczuk, M.S.; Krauspe, R.; Zilkens, C. The role of oxidative and nitrosative stress in the pathology of osteoarthritis: Novel candidate biomarkers for quantification of degenerative changes in the knee joint. Orthop. Rev. 2018, 10, 7460. [Google Scholar] [CrossRef] [Green Version]
- Goldring, M.B.; Otero, M.; Tsuchimochi, K.; Ijiri, K.; Li, Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann. Rheum. Dis. 2008, 67 (Suppl. S3), iii75–iii82. [Google Scholar] [CrossRef] [Green Version]
- Burrage, P.S.; Brinckerhoff, C.E. Molecular targets in osteoarthritis: Metalloproteinases and their inhibitors. Curr. Drug Targets 2007, 8, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.; Kim, H.A.; Roach, H.I. Apoptosis in osteoarthritis. Rheum. Dis. Clin. N. Am. 2004, 30, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, P.E.; Haudenschild, D.R.; Samuels, J.; Abramson, S.B. Pathogenesis of Osteoarthritis. In Kelley and Firestein’s Textbook of Rheumatology, 10th ed.; Firestein, G.S., Budd, R.C., Gabriel, S.E., McInnes, I.B., O’Dell, J.R., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 1685–1704. [Google Scholar]
- Scanzello, C.R.; Plaas, A.; Crow, M.K. Innate immune system activation in osteoarthritis: Is osteoarthritis a chronic wound? Curr. Opin. Rheumatol. 2008, 20, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Villafane, J.H. Does “time heal all wounds” still have a future in osteoarthritis? Clin. Exp. Rheumatol. 2018, 36, 513. [Google Scholar] [PubMed]
- Clegg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; Hooper, M.M.; Bradley, J.D.; Bingham, C.O., III; Weisman, M.H.; Jackson, C.G.; et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N. Engl. J. Med. 2006, 354, 795–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandel, S.; Juni, P.; Tendal, B.; Nuesch, E.; Villiger, P.M.; Welton, N.J.; Reichenbach, S.; Trelle, S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: Network meta-analysis. BMJ 2010, 341, c4675. [Google Scholar] [CrossRef] [Green Version]
- Rozendaal, R.M.; Koes, B.W.; van Osch, G.J.; Uitterlinden, E.J.; Garling, E.H.; Willemsen, S.P.; Ginai, A.Z.; Verhaar, J.A.N.; Weinans, H.; Bierma-Zeinstra, S.M.A. Effect of glucosamine sulfate on hip osteoarthritis: A randomized trial. Ann. Intern. Med. 2008, 148, 268–277. [Google Scholar] [CrossRef]
- Arden NK, H.M. Management of osteoarthritis. In Rheumatology, 2, 7th ed.; Hochberg, M.C.S.A., Gravallese, E.M., Smolen, J.S., Weinblatt, M.E., Weisman, M.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1582–1590. [Google Scholar]
- De Carvalho, R.T.; Salgado, L.T.; Amado Filho, G.M.; Leal, R.N.; Werckmann, J.; Rossi, A.L.; Campos, A.P.C.; Karez, C.S.; Farina, M. Biomineralization of calcium carbonate in the cell wall of Lithothamnion crispatum (Hapalidiales, Rhodophyta): Correlation between the organic matrix and the mineral phase. J. Phycol. 2017, 53, 642–651. [Google Scholar] [CrossRef]
- Krayesky-Self, S.; Richards, J.L.; Rahmatian, M.; Fredericq, S. Aragonite infill in overgrown conceptacles of coralline Lithothamnion spp. (Hapalidiaceae, Hapalidiales, Rhodophyta): New insights in biomineralization and phylomineralogy. J. Phycol. 2016, 52, 161–173. [Google Scholar] [CrossRef]
- Aslam, M.N.; Kreider, J.M.; Paruchuri, T.; Bhagavathula, N.; DaSilva, M.; Zernicke, R.F.; Goldstein, S.A.; Varani, J. A mineral-rich extract from the red marine algae Lithothamnion calcareum preserves bone structure and function in female mice on a Western-style diet. Calcif. Tissue Int. 2010, 86, 313–324. [Google Scholar]
- Brennan, O.; Sweeney, J.; O’Meara, B.; Widaa, A.; Bonnier, F.; Byrne, H.J.; O’Gorman, D.M.; O’Brien, F.J. A Natural, Calcium-Rich Marine Multi-mineral Complex Preserves Bone Structure, Composition and Strength in an Ovariectomised Rat Model of Osteoporosis. Calcif. Tissue Int. 2017, 101, 445–455. [Google Scholar] [CrossRef]
- Slevin, M.M.; Allsopp, P.J.; Magee, P.J.; Bonham, M.P.; Naughton, V.R.; Strain, J.J.; Duffy, M.E.; Wallace, J.M.; Mc Sorley, E.M. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. J. Nutr. 2014, 144, 297–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviello, G.; Amu, S.; Saunders, S.P.; Fallon, P.G. A mineral extract from red algae ameliorates chronic spontaneous colitis in IL-10 deficient mice in a mouse strain dependent manner. Phytother. Res. 2014, 28, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.N.; Paruchuri, T.; Bhagavathula, N.; Varani, J. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr. Cancer Ther. 2010, 9, 93–99. [Google Scholar] [CrossRef]
- Hampton, A.L.; Aslam, M.N.; Naik, M.K.; Bergin, I.L.; Allen, R.M.; Craig, R.A.; Kunkel, S.L.; Veerapaneni, I.; Paruchuri, T.; Patterson, K.; et al. Ulcerative Dermatitis in C57BL/6NCrl Mice on a Low-Fat or High-Fat Diet With or Without a Mineralized Red-Algae Supplement. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 487–496. [Google Scholar] [PubMed]
- Ono, H.K.; Yoshimura, S.; Hirose, S.; Narita, K.; Tsuboi, M.; Asano, K.; Nakane, A. Salmon cartilage proteoglycan attenuates allergic responses in mouse model of papaininduced respiratory inflammation. Mol. Med. Rep. 2018, 18, 4058–4064. [Google Scholar] [PubMed] [Green Version]
- Mitsui, T.; Sashinami, H.; Sato, F.; Kijima, H.; Ishiguro, Y.; Fukuda, S.; Yoshihara, S.; Hakamada, K.-I.; Nakane, A. Salmon cartilage proteoglycan suppresses mouse experimental colitis through induction of Foxp3+ regulatory T cells. Biochem. Biophys. Res. Commun. 2010, 402, 209–215. [Google Scholar] [CrossRef]
- Thom, E. Nourkrin: Objective and subjective effects and tolerability in persons with hair loss. J. Int. Med. Res. 2006, 34, 514–519. [Google Scholar] [CrossRef]
- Sashinami, H.; Asano, K.; Yoshimura, S.; Mori, F.; Wakabayashi, K.; Nakane, A. Salmon proteoglycan suppresses progression of mouse experimental autoimmune encephalomyelitis via regulation of Th17 and Foxp3(+) regulatory T cells. Life Sci. 2012, 91, 1263–1269. [Google Scholar] [CrossRef]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Chamberland, A.; Wang, E.; Jones, A.R.; Collins-Racie, L.A.; LaVallie, E.R.; Huang, Y.; Liu, L.; Morris, E.A.; Flannery, C.R.; Yang, Z. Identification of a novel HtrA1-susceptible cleavage site in human aggrecan: Evidence for the involvement of HtrA1 in aggrecan proteolysis in vivo. J. Biol. Chem. 2009, 284, 27352–27359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta 2012, 1824, 133–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Masutani, T.; Tomonaga, A.; Watanabe, K.; Yamamoto, T.; Ito, K.; Tsuboi, M.; Yamaguchi, H.; Nagaoka, I. Influence on improvement of osteoarthritis by oral intake of proteoglycan extracted from salmon nasal cartilage. In Proceedings of the International Conference and Exhibition on Nutraceuticals and Functional Foods, Sapporo, Japan, 14–17 November 2011. Abstract P112. [Google Scholar]
- Tomonaga, A.; Takahashi, T.; Tanaka, Y.T.; Tsuboi, M.; Ito, K.; Nagaoka, I. Evaluation of the effect of salmon nasal proteoglycan on biomarkers for cartilage metabolism in individuals with knee joint discomfort: A randomized double-blind placebo-controlled clinical study. Exp. Ther. Med. 2017, 14, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondeau, N. Building the momentum in stroke protection using the nutraceutical potential of omega-3 alpha-linolenic acid for patients and public Health. J. Community Med. Health Educ. 2016, 6 (Suppl. S1), 135. [Google Scholar]
- O’Gorman, D.M.; O’Carroll, C.; Carmody, R.J. Evidence that marine-derived, multi-mineral, Aquamin inhibits the NF-kappaB signaling pathway in vitro. Phytother. Res. 2012, 26, 630–632. [Google Scholar] [CrossRef]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Lee, A.S.; Ellman, M.B.; Yan, D.; Kroin, J.S.; Cole, B.J.; van Wijnen, A.J.; Im, H.J. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 2013, 527, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Ryan, S.; O’Gorman, D.M.; Nolan, Y.M. Evidence that the marine-derived multi-mineral Aquamin has anti-inflammatory effects on cortical glial-enriched cultures. Phytother. Res. 2011, 25, 765–767. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.T.; Martin, C.; Doolan, A.M.; Molloy, M.G.; Dinan, T.G.; Gorman, D.; Nally, K. The Marine-derived, Multi-mineral formula, AquaPT Reduces TNF-α Levels in Osteoarthritis Patients. J. Nutr. Health Food Sci. 2014, 2, 1–3. [Google Scholar]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [Green Version]
- Frestedt, J.L.; Walsh, M.; Kuskowski, M.A.; Zenk, J.L. A natural mineral supplement provides relief from knee osteoarthritis symptoms: A randomized controlled pilot trial. Nutr. J. 2008, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frestedt, J.L.; Kuskowski, M.A.; Zenk, J.L. A natural seaweed derived mineral supplement (Aquamin F) for knee osteoarthritis: A randomised, placebo controlled pilot study. Nutr. J. 2009, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Romero, E.A.; Pecos-Martin, D.; Calvo-Lobo, C.; Garcia-Jimenez, D.; Ochoa-Saez, V.; Burgos-Caballero, V.; Fernandez-Carnero, J. Clinical features and myofascial pain syndrome in older adults with knee osteoarthritis by sex and age distribution: A cross-sectional study. Knee 2019, 26, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Aguilar, E.; Marcos-Pasero, H.; Ikonomopoulou, M.P.; Loria-Kohen, V. Food Implications in Central Sensitization Syndromes. J. Clin. Med. 2020, 9, 4106. [Google Scholar] [CrossRef]
- Heffernan, S.M.; McCarthy, C.; Eustace, S.; FitzPatrick, R.E.; Delahunt, E.; De Vito, G. Mineral rich algae with pine bark improved pain, physical function and analgesic use in mild-knee joint osteoarthritis, compared to Glucosamine: A randomized controlled pilot trial. Complement. Ther. Med. 2020, 50, 102349. [Google Scholar] [CrossRef]
- Zeng, C.; Li, H.; Wei, J.; Yang, T.; Deng, Z.H.; Yang, Y.; Zhang, Y.; Yang, T.-B.; Lei, G.-H. Association between Dietary Magnesium Intake and Radiographic Knee Osteoarthritis. PLoS ONE 2015, 10, e0127666. [Google Scholar]
- Shimaya, M.; Muneta, T.; Ichinose, S.; Tsuji, K.; Sekiya, I. Magnesium enhances adherence and cartilage formation of synovial mesenchymal stem cells through integrins. Osteoarthr. Cartil. 2010, 18, 1300–1309. [Google Scholar] [CrossRef] [Green Version]
- Shmagel, A.; Onizuka, N.; Langsetmo, L.; Vo, T.; Foley, R.; Ensrud, K.; Valen, P. Low magnesium intake is associated with increased knee pain in subjects with radiographic knee osteoarthritis: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2018, 26, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yue, J.; Yang, C. Unraveling the role of Mg(++) in osteoarthritis. Life Sci. 2016, 147, 24–29. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.L.; Talyor, R.W.; Young, D.A. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann. Rheum. Dis. 2010, 69, 1502–1510. [Google Scholar] [CrossRef] [Green Version]
- Sanchez Romero, E.A.; Melendez Oliva, E.; Alonso Perez, J.L.; Martin Perez, S.; Turroni, S.; Marchese, L.; Villafañe, J. Relationship between the Gut Microbiome and Osteoarthritis Pain: Review of the Literature. Nutrients 2021, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Pedersini, P.; Turroni, S.; Villafane, J.H. Gut microbiota and physical activity: Is there an evidence-based link? Sci. Total Environ. 2020, 727, 138648. [Google Scholar] [CrossRef] [PubMed]
- Beane KER, M.C.; Wang, X.; Pan, J.H.; Le, B.; Cicalo, C.; Jeon, S.; Kim, Y.J.; Lee, J.H.; Shin, E.; Li, Y.; et al. Effects of dietry fibers, micronutrients, and phytonutrients on gut microbiome: A review. Appl. Biol. Chem. 2021, 64, 36. [Google Scholar] [CrossRef]
- Dougados, M. Symptomatic slow-acting drugs for osteoarthritis: What are the facts? Jt. Bone Spine 2006, 73, 606–609. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eriksen, E.F.; Lech, O.; Nakama, G.Y.; O’Gorman, D.M. Disease-Modifying Adjunctive Therapy (DMAT) in Osteoarthritis—The Biological Effects of a Multi-Mineral Complex, LithoLexal® Joint—A Review. Clin. Pract. 2021, 11, 901-913. https://doi.org/10.3390/clinpract11040104
Eriksen EF, Lech O, Nakama GY, O’Gorman DM. Disease-Modifying Adjunctive Therapy (DMAT) in Osteoarthritis—The Biological Effects of a Multi-Mineral Complex, LithoLexal® Joint—A Review. Clinics and Practice. 2021; 11(4):901-913. https://doi.org/10.3390/clinpract11040104
Chicago/Turabian StyleEriksen, Erik Fink, Osvandre Lech, Gilberto Yoshinobu Nakama, and Denise M. O’Gorman. 2021. "Disease-Modifying Adjunctive Therapy (DMAT) in Osteoarthritis—The Biological Effects of a Multi-Mineral Complex, LithoLexal® Joint—A Review" Clinics and Practice 11, no. 4: 901-913. https://doi.org/10.3390/clinpract11040104
APA StyleEriksen, E. F., Lech, O., Nakama, G. Y., & O’Gorman, D. M. (2021). Disease-Modifying Adjunctive Therapy (DMAT) in Osteoarthritis—The Biological Effects of a Multi-Mineral Complex, LithoLexal® Joint—A Review. Clinics and Practice, 11(4), 901-913. https://doi.org/10.3390/clinpract11040104




