Cross-Cultural Adaptation of the Brazilian Portuguese-Translated Version of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire in Cancer Patients
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patients and Ethical Procedures
2.3. Procedures for Cross-Cultural Adaptation of the MRSA
2.4. Data Collection and Risk Classification of Sarcopenia
2.5. Statistical Analyses
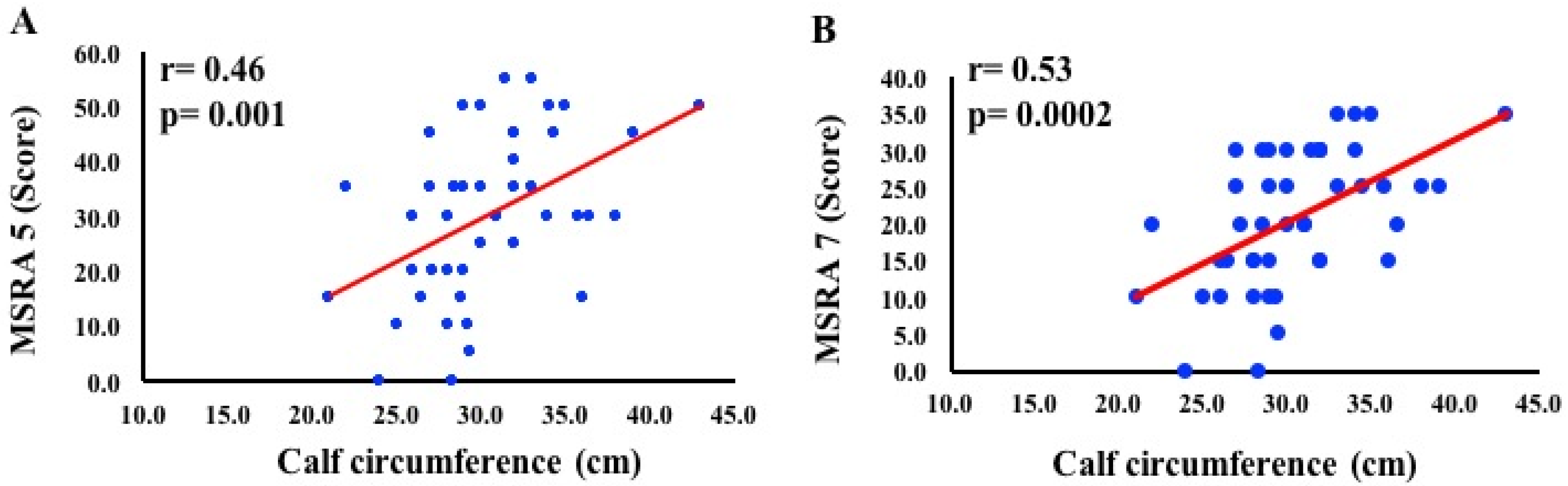
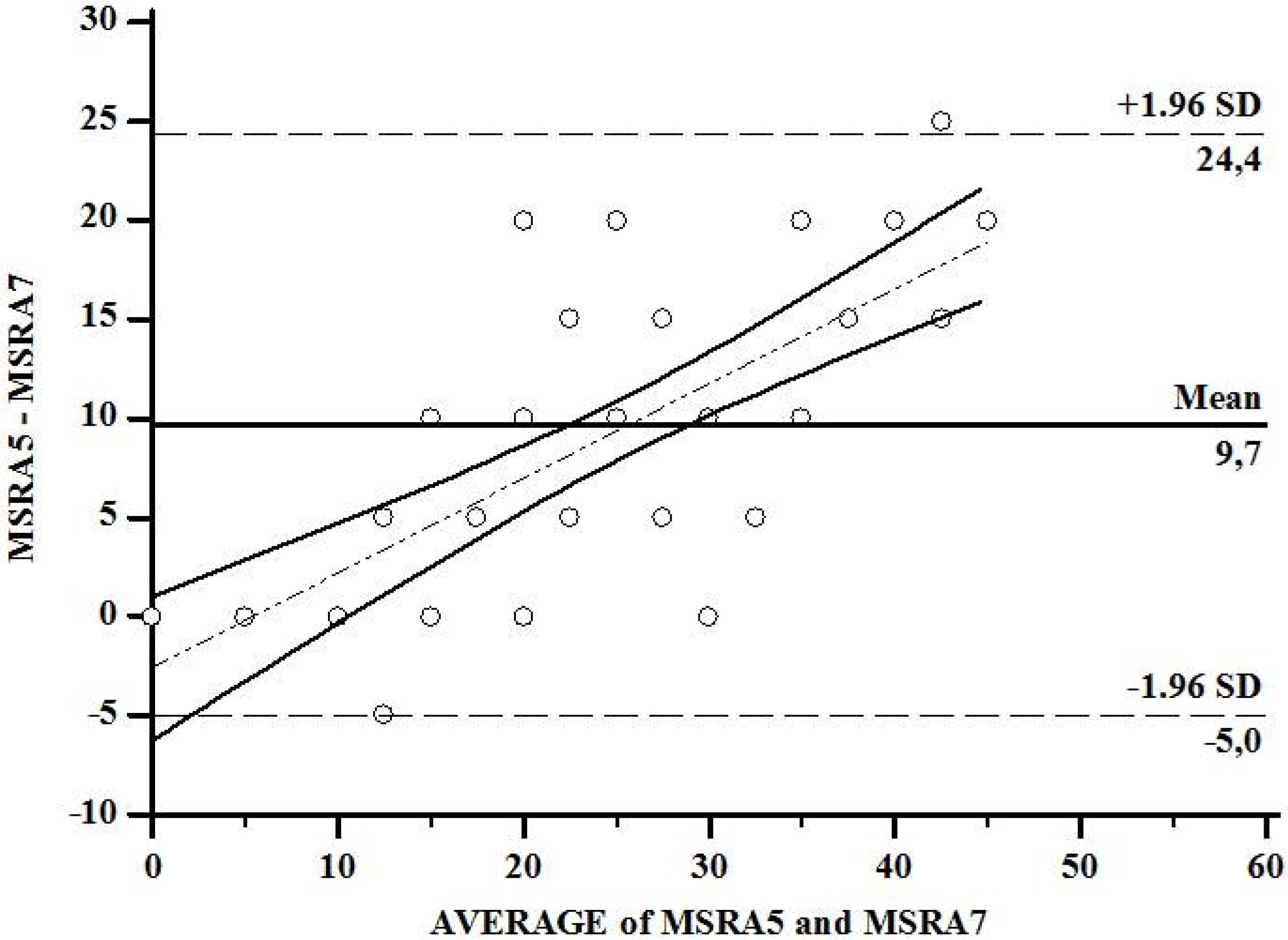
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Disclaimers
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, L.P.; Silveira, M.N.; Mendes, M.C.S.; Costa, F.O.; Macedo, L.T.; de Siqueira, N.S.; Carvalheira, J.B.C. Sarcopenia as an independent prognostic factor in patients with metastatic colorectal cancer: A retrospective evaluation. Clin. Nutr. ESPEN 2019, 32, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, T.G.; Menezes, A.M.B.; Bielemann, R.M.; Malmstrom, T.K.; Gonzalez, M.C. Enhancing SARC-F: Improving Sarcopenia Screening in the Clinical Practice. J. Am. Med. Dir. Assoc. 2016, 17, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; For the GLISTEN Group Investigators; Fantin, F.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; et al. Association between hospitalization-related outcomes, dynapenia and body mass index: The Glisten Study. Eur. J. Clin. Nutr. 2018, 73, 743–750. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Xie, L.; Zhang, L.; Zhou, J.; Lin, J.; Wang, Y.; Li, Y.; Han, Z.; Zhang, D.; et al. Comparing Mini Sarcopenia Risk Assessment With SARC-F for Screening Sarcopenia in Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2019, 20, 53–57. [Google Scholar] [CrossRef]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Carvalho, I.A.d.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef]
- Akarapornkrailert, P.; Muangpaisan, W.; Boonpeng, A.; Daengdee, D. Validation of the Thai version of SARC-F, MSRA-7, and MSRA-5 questionnaires compared to AWGS 2019 and sarcopenia risks in older patients at a medical outpatient clinic. Osteoporos. Sarcopenia 2020, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Krzymińska-Siemaszko, R.; Deskur-Śmielecka, E.; Styszyński, A.; Wieczorowska-Tobis, K. Polish Translation and Validation of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire to Assess Nutritional and Non-Nutritional Risk Factors of Sarcopenia in Older Adults. Nutrients 2021, 13, 1061. [Google Scholar] [CrossRef] [PubMed]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the Process of Cross-Cultural Adaptation of Self-Report Measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, J.; Elosua, P.; Hambleton, R.K. International Test Commission Guidelines for test translation and adaptation: Second edition. Psicothema 2013, 25, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.J.; MNA-International Group; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.; et al. Validation of the Mini Nutritional Assessment short-form (MNA®-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Cronbanch, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 7. [Google Scholar]
- Sayer, A.A.; Syddall, H.; Martin, H.; Patel, H.; Baylis, D.; Cooper, C. The developmental origins of sarcopenia. J. Nutr. Health Aging 2008, 12, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.; Sjøblom, B.; Wentzel-Larsen, T.; Grønberg, B.H.; Baracos, V.E.; Hjermstad, M.J.; Aass, N.; Bremnes, R.M.; Fløtten, Øystein; Jordhøy, M. Muscle mass and association to quality of life in non-small cell lung cancer patients. J. Cachex Sarcopenia Muscle 2017, 8, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Daly, L.; Dolan, R.; Power, D.; Bhuachalla Éadaoin, N.; Sim, W.; Fallon, M.; Cushen, S.; Simmons, C.; McMillan, D.C.; Laird, B.J.; et al. The relationship between the BMI-adjusted weight loss grading system and quality of life in patients with incurable cancer. J. Cachexia Sarcopenia Muscle 2019, 11, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, C.; Martin, L.; Gramlich, L.; Scrimger, R.; Jha, N.; Debenham, B.; Chua, N.; Walker, J.; Baracos, V.E. Prevalence and prognostic significance of malnutrition in patients with cancers of the head and neck. Clin. Nutr. 2020, 39, 901–909. [Google Scholar] [CrossRef] [PubMed]

| Variables | N (%) | Mean ± SD |
|---|---|---|
| Sex Male Female | 21 (46.7) 24 (53.3) | |
| Age (years) | 52.1 ± 16.0 | |
| Cancer type Oncohematological Gastrointestinal tract Others | 22 (48.9) 19 (42.2) 4 (8.9) | |
| Body weight loss (%) | 12 ± 7.2 | |
| Body weight loss time (weeks) | 16.97 ± 14.35 | |
| Body weight loss Non-significant Significant Severe | 13 (28.9) 9 (20.0) 23 (51.1) | |
| Calf circumference (cm) | 30.4 ± 4.2 | |
| Calf circumference No muscle mass loss (CC >31 cm) With muscle mass loss (CC <31 cm) | 20 (44.5) 25 (55.5) | |
| MSRA 7 items—full version (score) No risk of sarcopenia With risk of sarcopenia | 4 (8.9) 41 (91.1) | 20.7 ± 9.2 35.0 ± 0.0 18.4 ± 8.5 |
| MSRA 5 items—short version (score) No risk of sarcopenia With risk of sarcopenia | 7 (15.5) 38 (84.5) | 51.4 ± 2.4 26.5 ± 12.6 |
| Questions | MSRA 7 Items | MSRA 5 Items | p Interaction | ||||
|---|---|---|---|---|---|---|---|
| NS | S | p | NS | S | p | ||
| (n = 4) | (n = 41) | (n = 7) | (n = 38) | ||||
| N | N | N | N | ||||
| 1. How old are you? | 0.307 | 0.089 | 0.161 | ||||
| ≥70 years old | 0 | 13 | 0 | 13 | |||
| <70 years old | 4 | 28 | 7 | 25 | |||
| 2. Were you hospitalized in the last year? | 0.035 * | 0.003 * | 0.006 * | ||||
| Yes, and more than one hospitalization | 0 | 21 | 0 | 21 | |||
| Yes, one hospitalization | 1 | 12 | 2 | 11 | |||
| No | 3 | 8 | 5 | 6 | |||
| 3. What is your activity level? | 0.281 | 0.034 * | 0.052 | ||||
| I’m able to walk less than 1000 meters | 0 | 17 | 0 | 17 | |||
| I’m able to walk more than 1000 meters | 4 | 24 | 7 | 21 | |||
| 4. Do you eat 3 meals per day regularly? | 0.138 | 0.031 * | 0.037 * | ||||
| No, up to twice per week I skip a meal (for example I skip breakfast or I have only milky coffee or soup for dinner) | 0 | 18 | 0 | 18 | |||
| Yes | 4 | 23 | 7 | 20 | |||
| 5. Do you consume any of the following? | 0.138 | - | - | ||||
| Milk or dairy products (yogurt, cheese), but not every day | 0 | 18 | - | - | |||
| Milk or dairy products (yogurt, cheese) at least once per day | 4 | 23 | |||||
| 6. Do you consume any of the following? | 0.280 | - | - | ||||
| Poultry, meat, fish, eggs, legumes, ragout, or ham, but not every day | 0 | 16 | - | - | |||
| Poultry, meat, fish, eggs, legumes, ragout, or ham at least once per day | 4 | 25 | |||||
| 7. Did you lose weight in the last year? | 1.000 | 0.613 | 0.929 | ||||
| >2 kg | 3 | 33 | 5 | 31 | |||
| ≤2 kg | 1 | 8 | 2 | 7 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, L.S.; Souza, B.G.d.A.; de Lima, J.B.; Pimentel, G.D. Cross-Cultural Adaptation of the Brazilian Portuguese-Translated Version of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire in Cancer Patients. Clin. Pract. 2021, 11, 395-403. https://doi.org/10.3390/clinpract11020054
Ribeiro LS, Souza BGdA, de Lima JB, Pimentel GD. Cross-Cultural Adaptation of the Brazilian Portuguese-Translated Version of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire in Cancer Patients. Clinics and Practice. 2021; 11(2):395-403. https://doi.org/10.3390/clinpract11020054
Chicago/Turabian StyleRibeiro, Lays S., Bárbarah G. de A. Souza, Juliana B. de Lima, and Gustavo D. Pimentel. 2021. "Cross-Cultural Adaptation of the Brazilian Portuguese-Translated Version of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire in Cancer Patients" Clinics and Practice 11, no. 2: 395-403. https://doi.org/10.3390/clinpract11020054
APA StyleRibeiro, L. S., Souza, B. G. d. A., de Lima, J. B., & Pimentel, G. D. (2021). Cross-Cultural Adaptation of the Brazilian Portuguese-Translated Version of the Mini Sarcopenia Risk Assessment (MSRA) Questionnaire in Cancer Patients. Clinics and Practice, 11(2), 395-403. https://doi.org/10.3390/clinpract11020054






