Extensive Left Ventricular Thrombosis with Concomitant Pulmonary Embolism
Abstract
1. Introduction
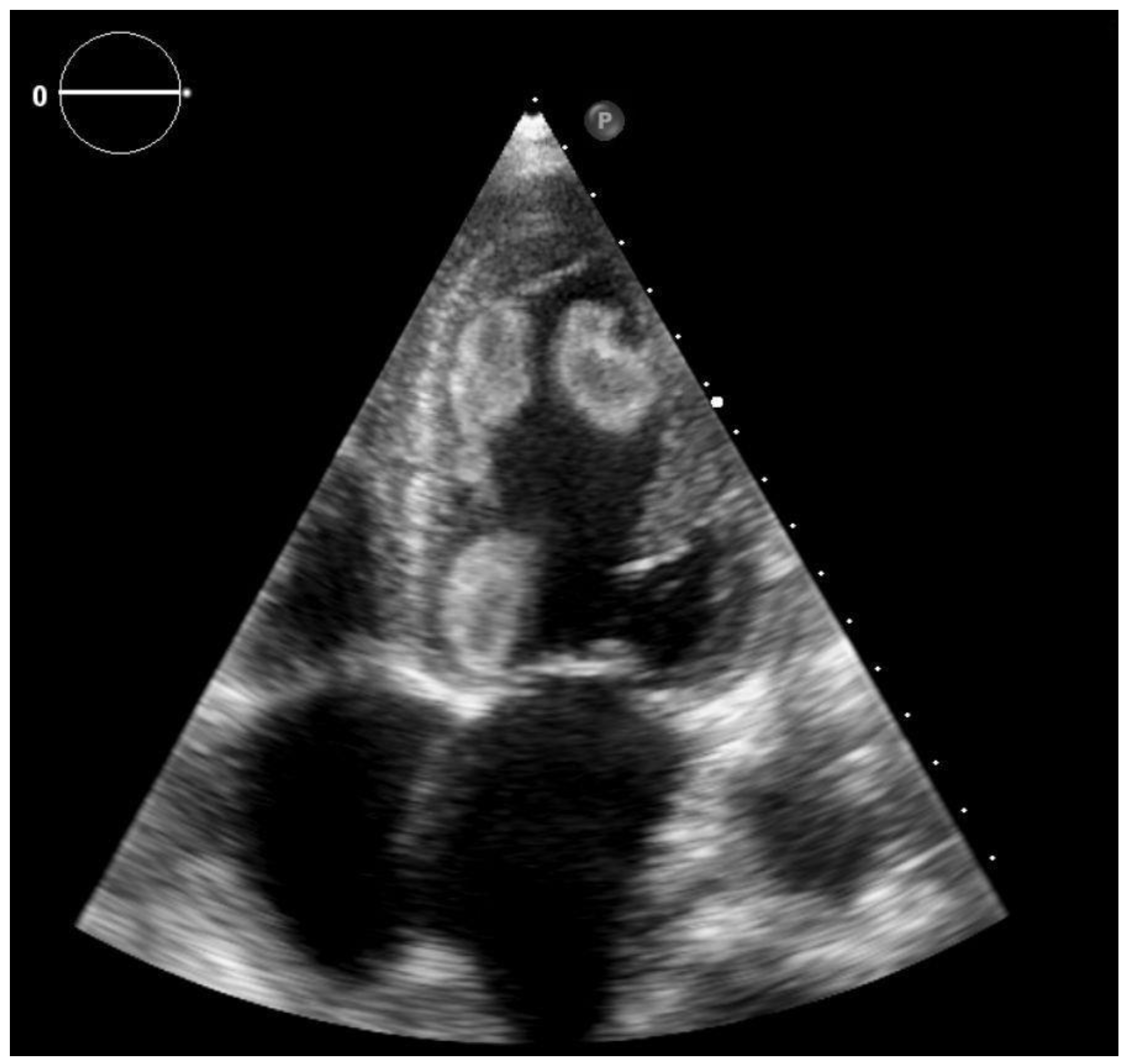
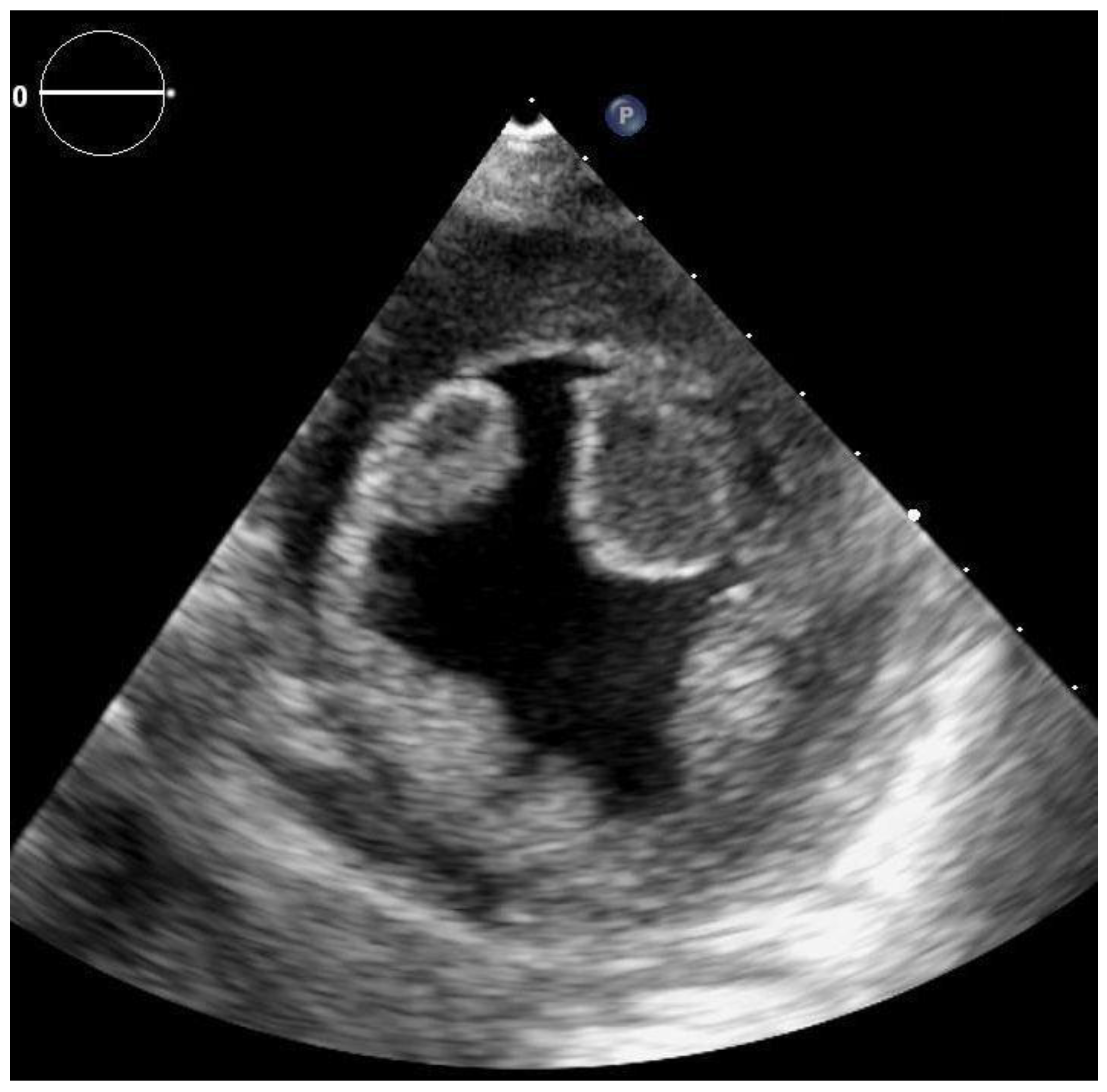
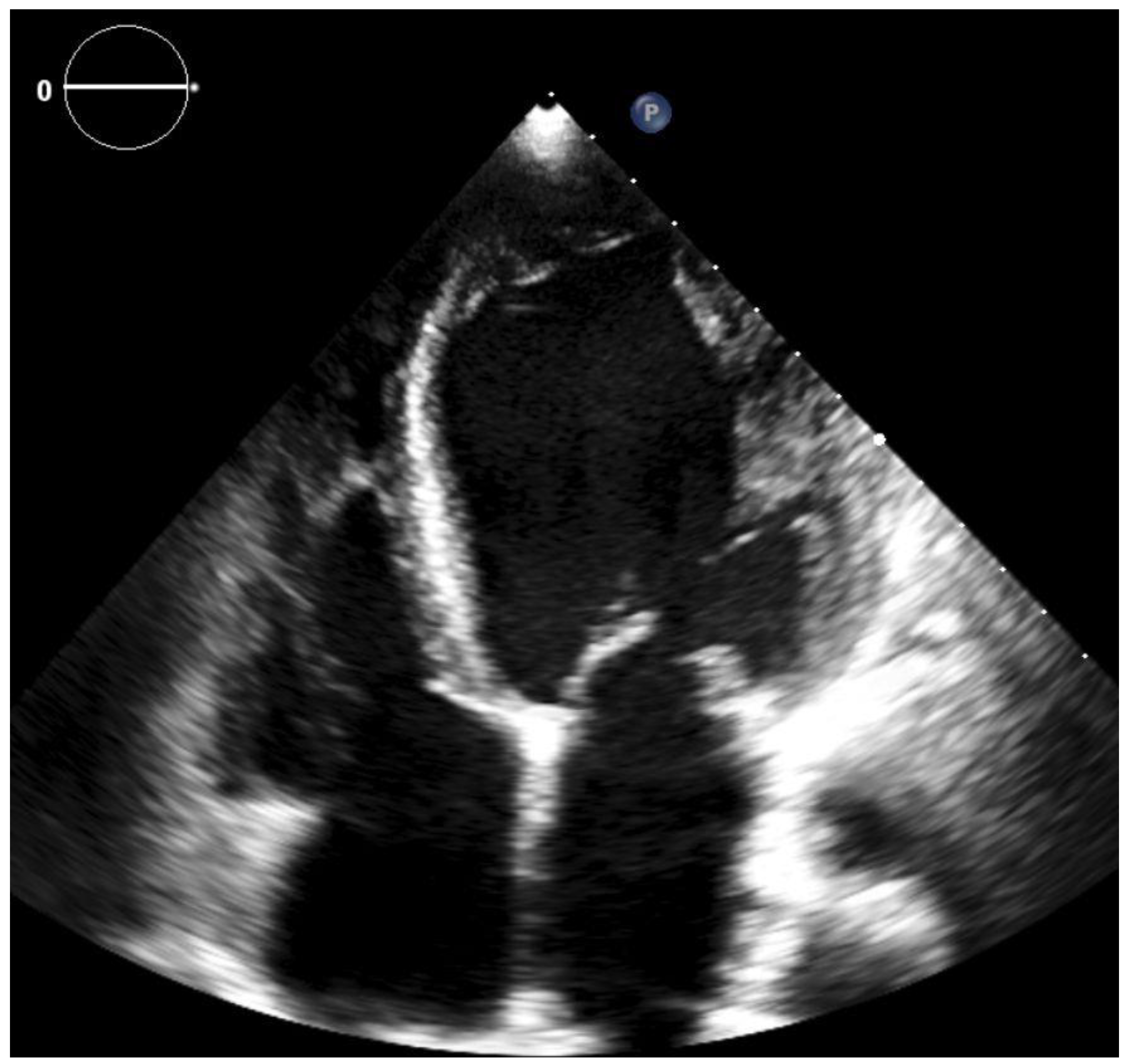
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jiang, X.Y.; Jing, L.D.; Jia, Y.H. Clinical characteristics and risk factors of left ventricular thrombus after acute myocardial infarc-tion: A matched case-control study. Chin. Med. 2015, 128, 2415–2419. [Google Scholar] [CrossRef] [PubMed]
- Delewi, R.; Zijlstra, F.; Piek, J. Left ventricular thrombus formation after acute myocardial infarction. Heart 2012, 98, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Silverstein, B.V.; Khuddus, M.A.; Bray, C.L.; Lee, A.C. Giant Left Ventricular Thrombus in a Patient with Acute Ischemic Stroke: A Case Report and Minireview. Case Rep. Cardiol. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pol, D.; Perera, P.; Zaman, S. Multiorgan embolization of a left ventricular thrombus. BMJ Case Rep. 2019, 12, e227626. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Van Der Geest, R.J.; Swoboda, P.P.; Crandon, S.; Fent, G.J.; Foley, J.R.; Dobson, L.E.; Al Musa, T.; Onciul, S.; Vijayan, S.; et al. Left ventricular thrombus formation in myocardial infarction is associated with altered left ventricular blood flow energetics. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Cevik, C.; Shah, N.; Wilson, J.M.; Stainback, R.F. Multiple left ventricular thrombi in a patient with left ventricular noncom-paction. Tex. Heart Inst. J. 2012, 39, 550–553. [Google Scholar] [PubMed]
- McCarthy, C.P.; Murphy, S.; Venkateswaran, R.V.; Singh, A.; Chang, L.L.; Joice, M.G.; Rivero, J.M.; Vaduganathan, M.; Januzzi, J.L.; Bhatt, D.L. Left ventricular thrombus. contemporary etiologies, treatment strategies, and outcomes. J. Am. Coll. Cardiol. 2019, 73, 2007–2009. [Google Scholar] [CrossRef] [PubMed]
- Habash, F.; Vallurupalli, S. Challenges in management of left ventricular thrombus. Ther. Adv. Cardiovasc. Dis. 2017, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Chan, M.H.H.; Paradies, V.; Yellon, R.L.; Ho, H.H.; Chan, M.Y.; Chin, C.W.L.; Tan, J.W.; Hausenloy, D.J. Incidence and predictors of left ventricular thrombus by cardiovascular magnetic resonance in acute ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: A meta-analysis. J. Cardiovasc. Magn. Reson. 2018, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mine, T.; Sato, I.; Miyake, H. Multiple left ventricular thrombi in a patient with dilated cardiomyopathy and cerebral infarction: A case report. J. Med. Case Rep. 2014, 8, 306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodrigues, P.; Sousa, M.J.; Caiado, L.; Cabral, S.; Meireles, A.; Santos, M.; Palma, P.; Torres, S. Intracardiac thrombus and Murphy’s law: Reflections on a clinical dilemma. Rev. Port. Cardiol. 2016, 35, 233.e1–233.e3. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Park, J.-H.; Lee, J.-H.; Kim, J.; Seong, I.-W. Shape and Mobility of a Left Ventricular Thrombus Are Predictors of Thrombus Resolution. Korean Circ. J. 2019, 49, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Gliga, M.; Gomotârceanu, A.; Podeanu, D.; Dogaru, G. Multiple renal infarctions due to thromboembolism. Importance of ultrasound in diagnosis. Case report. Med. Ultrason. 2012, 14, 71–73. [Google Scholar] [PubMed]
- Tomasoni, D.; Sciatti, E.; Bonelli, A.; Vizzardi, E.; Metra, M. Direct oral anticoagulants for the treatment of left ventricular throm-bus-a new indication? A meta-summary of case reports DOACs in left ventricular thrombosis. J. Cardiovasc. Pharmacol. 2020, 75, 530–534. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdás, A.; Podoleanu, C.; Frigy, A. Extensive Left Ventricular Thrombosis with Concomitant Pulmonary Embolism. Clin. Pract. 2021, 11, 303-308. https://doi.org/10.3390/clinpract11020043
Magdás A, Podoleanu C, Frigy A. Extensive Left Ventricular Thrombosis with Concomitant Pulmonary Embolism. Clinics and Practice. 2021; 11(2):303-308. https://doi.org/10.3390/clinpract11020043
Chicago/Turabian StyleMagdás, Annamária, Cristian Podoleanu, and Attila Frigy. 2021. "Extensive Left Ventricular Thrombosis with Concomitant Pulmonary Embolism" Clinics and Practice 11, no. 2: 303-308. https://doi.org/10.3390/clinpract11020043
APA StyleMagdás, A., Podoleanu, C., & Frigy, A. (2021). Extensive Left Ventricular Thrombosis with Concomitant Pulmonary Embolism. Clinics and Practice, 11(2), 303-308. https://doi.org/10.3390/clinpract11020043




