Personalised Prophylaxis in a Child with Haemophilia A and Type 1 Diabetes
Abstract
1. Introduction
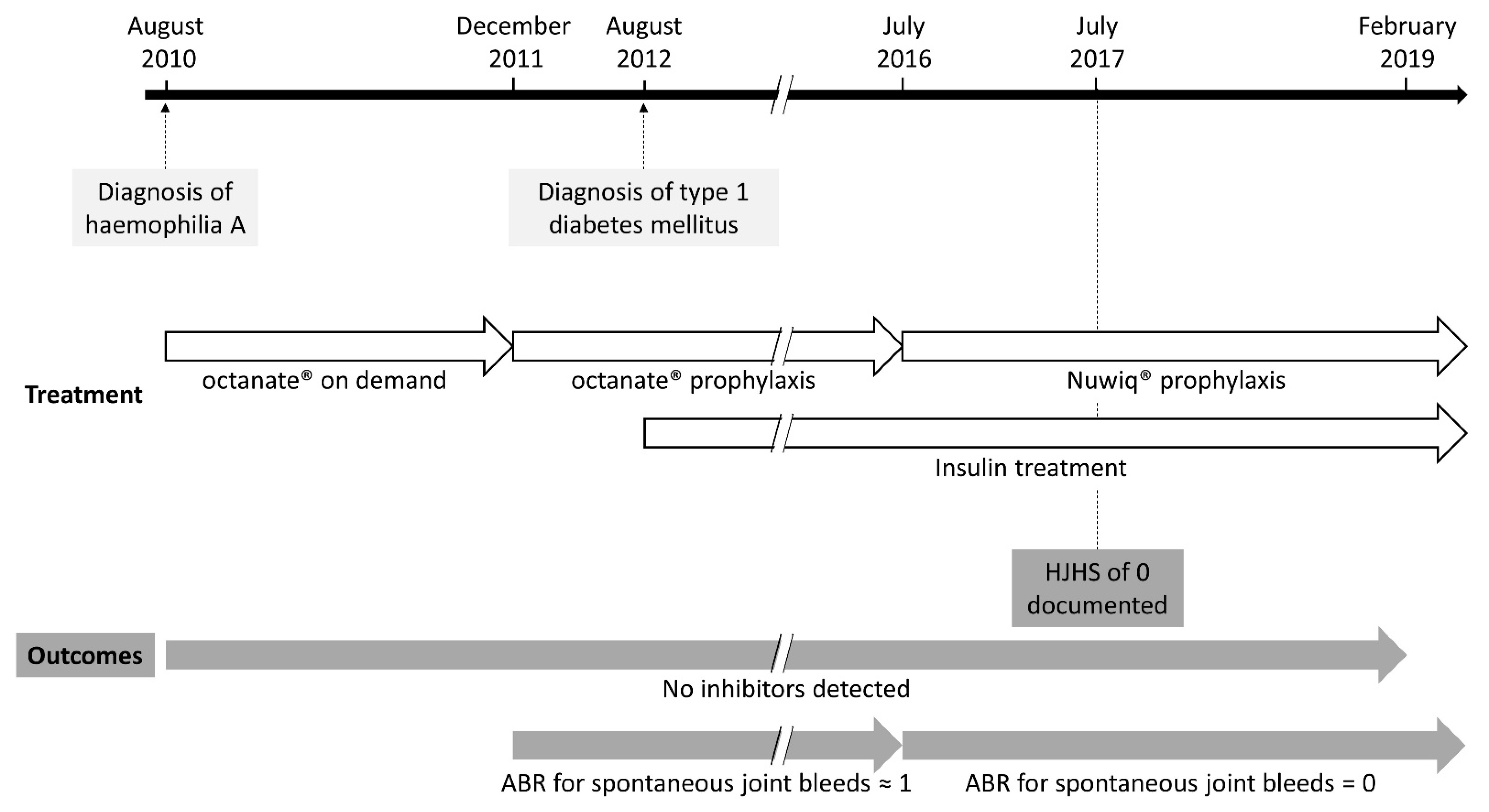
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the management of hemophilia. Haemophilia 2013, 19, e1–e47. [Google Scholar] [CrossRef] [PubMed]
- Valentino, L.A.; Khair, K. Prophylaxis for hemophilia A without inhibitors: Treatment options and considerations. Expert Rev. Hematol. 2020, 13, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Ar, M.C.; Baslar, Z.; Soysal, T. Personalized prophylaxis in people with hemophilia A: Challenges and achievements. Expert Rev. Hematol. 2016, 9, 1203–1208. [Google Scholar] [CrossRef]
- Simurda, T.; Stanciakova, L.; Stasko, J.; Dobrotova, M.; Kubisz, P. Yes or no for secondary prophylaxis in afibrinogenemia? Blood Coagul. Fibrinolysis 2015, 26, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, M.; Napolitano, M.; Giuffrida, G.; Coluccia, A.; Siragusa, S.; Calafiore, V.; Lassandro, G.; Giordano, P. Status of Recombinant Factor VIII Concentrate Treatment for Hemophilia a in Italy: Characteristics and Clinical Benefits. Front. Med. 2019, 6, 261. [Google Scholar] [CrossRef]
- Lissitchkov, T.; Rusen, L.; Georgiev, P.; Windyga, J.; Klamroth, R.; Gercheva, L.; Nemes, L.; Tiede, A.; Bichler, J.; Knaub, S.; et al. PK-guided personalized prophylaxis with Nuwiq® (human-cl rhFVIII) in adults with severe haemophilia A. Haemophilia 2017, 23, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J. Optimal treatment strategies for hemophilia: Achievements and limitations of current prophylactic regimens. Blood 2015, 125, 2038–2044. [Google Scholar] [CrossRef]
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef]
- Gringeri, A.; Lundin, B.; von Mackensen, S.; Mantovani, L.; Mannucci, P.M.; Esprit Study Group. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J. Thromb. Haemost. 2011, 9, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Royal, S.; Schramm, W.; Berntorp, E.; Giangrande, P.; Gringeri, A.; Ludlam, C.; Kroner, B.; Szucs, T.; European Haemophilia Economics Study Group. Quality-of-life differences between prophylactic and on-demand factor replacement therapy in European haemophilia patients. Haemophilia 2002, 8, 44–50. [Google Scholar] [CrossRef]
- O’Hara, J.; Sima, C.S.; Frimpter, J.; Paliargues, F.; Chu, P.; Presch, I. Long-term outcomes from prophylactic or episodic treatment of haemophilia A: A systematic review. Haemophilia 2018, 24, e301–e311. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.R.; Geraghty, S.; Manco-Johnson, M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia 2001, 7, 392–396. [Google Scholar] [PubMed]
- Thornburg, C.D.; Duncan, N.A. Treatment adherence in hemophilia. Patient Prefer. Adherence 2017, 11, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Lee Mortensen, G.; Strand, A.M.; Almén, L. Adherence to prophylactic haemophilic treatment in young patients transitioning to adult care: A qualitative review. Haemophilia 2018, 24, 862–872. [Google Scholar] [CrossRef]
- Schrijvers, L.H.; van der Beijlevelt Zande, M.; Peters, M.; Lock, J.; Cnossen, M.H.; Schuurmans, M.J.; Fischer, K. Adherence to prophylaxis and bleeding outcome in haemophilia: A multicentre study. Br. J. Haematol. 2016, 174, 454–460. [Google Scholar] [CrossRef] [PubMed]
- García-Dasí, M.; Aznar, J.A.; Jiménez-Yuste, V.; Altisent, C.; Bonanad, S.; Mingot, E.; Lucía, F.; Giménez, F.; López, M.F.; Marco, P.; et al. Adherence to prophylaxis and quality of life in children and adolescents with severe haemophilia A. Haemophilia 2015, 21, 458–464. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.M.; Witkop, M.L.; Lambing, A.; Anderson, T.L.; Munn, J.; Tortella, B. Better adherence to prescribed treatment regimen is related to less chronic pain among adolescents and young adults with moderate or severe haemophilia. Haemophilia 2014, 20, 506–512. [Google Scholar] [CrossRef]
- de Moerloose, P.; Urbancik, W.; van den Berg, H.M.; Richards, M. A survey of adherence to haemophilia therapy in six European countries: Results and recommendations. Haemophilia 2008, 14, 931–938. [Google Scholar] [CrossRef]
- Hajducek, D.M.; Chelle, P.; Hermans, C.; Iorio, A.; McEneny-King, A.; Yu, J.; Edginton, A. Development and evaluation of the population pharmacokinetic models for FVIII and FIX concentrates of the WAPPS-Hemo project. Haemophilia 2020, 26, 384–400. [Google Scholar] [CrossRef]
- Liesner, R.J.; Abraham, A.; Altisent, C.; Belletrutti, M.J.; Carcao, M.; Carvalho, M.; Chambost, H.; Chan, A.K.C.; Dubey, L.; Ducore, J.; et al. Simoctocog alfa (Nuwiq) in previously untreated patients with severe haemophilia A: Final results of the NuProtect study. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
- Schrijvers, L.H.; Kars, M.C.; van der Beijlevelt Zande, M.; Peters, M.; Schuurmans, M.J.; Fischer, K. Unravelling adherence to prophylaxis in haemophilia: A patients’ perspective. Haemophilia 2015, 21, 612–621. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, M.S.; Santillan, J.; Lesser, J.; Ortiz, J.P.; Forzani, L. Personalised Prophylaxis in a Child with Haemophilia A and Type 1 Diabetes. Clin. Pract. 2021, 11, 287-292. https://doi.org/10.3390/clinpract11020041
Cruz MS, Santillan J, Lesser J, Ortiz JP, Forzani L. Personalised Prophylaxis in a Child with Haemophilia A and Type 1 Diabetes. Clinics and Practice. 2021; 11(2):287-292. https://doi.org/10.3390/clinpract11020041
Chicago/Turabian StyleCruz, Maria Sol, Josefina Santillan, Julieta Lesser, Juan Pablo Ortiz, and Laura Forzani. 2021. "Personalised Prophylaxis in a Child with Haemophilia A and Type 1 Diabetes" Clinics and Practice 11, no. 2: 287-292. https://doi.org/10.3390/clinpract11020041
APA StyleCruz, M. S., Santillan, J., Lesser, J., Ortiz, J. P., & Forzani, L. (2021). Personalised Prophylaxis in a Child with Haemophilia A and Type 1 Diabetes. Clinics and Practice, 11(2), 287-292. https://doi.org/10.3390/clinpract11020041




