Introduction
Estuaries are among the most anthropogenically degraded habitats on Earth. These shallow waters are particularly exposed to the impacts of pollution and global warming [1,2]. The European flounder, Platichthys flesus, is a northern temperate catadromous fish widely distributed over Europe, that is considered as a sentinel species for the monitoring of estuarine water quality [3,4]. The southern distribution limit of P. flesus is presently located along the Portuguese coast, and the European flounder is currently rare in the surroundings of Lisbon, where it was abundant a few decades ago [5]. The southern limits of numerous other cold-water marine species are also shifting North along the Portuguese coast, this trend being probably linked to recent warming [6].
The concept of energy-limited tolerance to stress, that focuses on the bioenergetic effects of stressors on organisms and their immediate consequences on fitness, appears particularly relevant for estuarine species that have to cope with a broad range of stressors [7]. This approach provides useful tools to: i) investigate the vulnerability of wild populations facing stressors; and ii) improve the assessment of ecological risk in complex systems.
In this study, bioenergetic markers of P. flesus were assessed in natura in three contrasted environments: the highly polluted Seine estuary (France) that displays a chronicle diffuse contamination, the moderately contaminated Vilaine estuary (France)[8] and the Mondego estuary (Portugal) that represents the southern limit of the species’ distribution range and can be considered as a warm environment for the flounder [9]. The chemical signature of the three estuaries was determined by measuring the concentration of a persistent organic pollutant, the polychlorinated biphenyl (PCB) CB153 in fish liver and muscle, that is a pertinent proxy of the contamination level of estuarine systems [10]. This was followed with molecular markers evaluations in fish liver from the three locations, considering the expression level of several genes involved in detoxification (Cytochrome P450) and bioenergetics (Cytochome C Oxydase, ATP synthase, 12S, 18S). The modulation of gene expression is an important component of acclimatization and/or adaptation of organisms to environmental changes [11,12], but can also provide reliable indicators of sub-lethal stress [13]. The final objective of the study was to explore the variability in bioenergetic responses of flounder populations among the three environmentally contrasted estuaries, to assess the possible impacts of pollution and warming on fish collected in respectively the Seine and Mondego estuaries in comparison to fish from a weakly stressed system, the Vilaine.
Materials and Methods
Fish collection and rearing
Thirty 0+ juvenile P. flesus were captured by trawl, in September-October 2009, in two estuaries located in the core of the distribution range of the European flounder (i.e., Seine and Vilaine, France), as well as in the southernmost population of this species (i.e., Mondego, Portugal) (Figure 1). Fish of similar size were quickly euthanized by cerebral dislocation. The liver and a piece of white muscle were sampled and immediately frozen in liquid nitrogen, then stored at –80°C until chemical and gene expression analyses.
Figure 1.
Localization of the sampling sites (estuaries of the Seine, Vilaine and Mondego).
Lipid storage and chemical analysis
All tissue samples were kept at –20°C until freeze-drying. The water content of samples was estimated from the weight loss during freeze-drying. Extraction and cleanup of fish tissues (liver and muscle) were performed [14]. The lipid storage was assessed by gravimetric method and the concentration in CB153 was subsequently analysed by gas chromatography with an electron capture detector, on a HP 5890 series II equipped with a CP-Sil19 capillary column [15].
Gene expression
Five gene expression markers were evaluated: CYP450 1A1 (detoxification process), Cytochrome C oxidase sub-unit 2 and ATP synthase F0 (COII & ATP F0: mitochondrial respiratory chain), 12S and 18S (ribosomal activities in the mitochondria and in the cytoplasm, respectively). Respectively 21, 26 and 25 fish from the Seine, Vilaine and Mondego were analysed. Total RNA was extracted from 150 mg of liver tissue using TRI Reagent® (Ambion), according to the manufacturer’s recommendations. An additional DNAse treatment using DNASE RQ1 (Promega) was included to eliminate traces of genomic DNA. The concentration of extracted RNA was determined with a ND-1000 spectrophotometrer (Nanodrop®) and total RNA quality was analyzed by capillary electrophoresis with the Agilent 2100 bioanalyser (Agilent technologies) with an Agilent RNA 6000 Nano Assay Kit. For each sample, 1 µg total RNA was reverse transcribed into cDNA using Revert Aid H minus First Strand cDNA Synthesis Kit® (Fermentas), and a reference cDNA sample was produced by mixing equal amounts of RNA from individual samples.
All the primers used in the quantitative PCR amplifications are available in the literature [4,8,10,16]. Two classical housekeeping genes were screened (Alpha-tubulin, Elongation Factor 1), and their variation coefficients were analyzed directly from their cycle threshold (Ct) values to define the gene displaying the most stable expression level. The Alpha-tubulin, a sub-unit protein of microtubules and thus a major constituent of the cytoskeleton, was finally retained as housekeeping gene, since it displayed the highest stability. PCR efficiency (E) was estimated for each primer pair by using a serial dilution (from 1/20 to 1/380) of reverse transcription products. Standard curves were generated for each primer pair and E was determined using the formula:
E = 10(−1/ slope) − 1.
For each primer pair, the dissociation curve generated a unique peak, and the PCR efficiency ranged between 99 and 100%. Real-Time PCRs were carried out in triplicate in a final volume of 10 μL using 4.86 μL of cDNA (1/40 dilution) with 5 μL of Absolute QPCR SYBR Green ROX Mix (Thermo Scientific) and 0.07 μL of each primer (70 nM). PCR amplifications were performed in a 7300 Real-Time PCR System (Applied Biosystems™) as follows: initial enzyme activation at 94°C for 15 min, followed by 50 cycles of 94°C for 15 s and 60°C for 1 min. For each reaction, a dissociation curve was calculated from 95 to 60°C by decreasing temperature by 0.5°C each 10 s in order to assess the specificity of the amplification. Each run included a reference cDNA control, a no template control, and a water control. The relative quantification of gene expression was calculated according to the comparative Ct method. The relative quantification value of each sample, normalized to the Alpha-tubulin gene and relative to the reference cDNA sample, was expressed as 2–ΔΔCt, where ΔCt= (Ct (candidate gene) – Ct (Alpha-tubulin)) and ΔΔCt=ΔCt of cDNA sample – ΔCt of reference cDNA control, with E = 100%.
Data analysis
A Shapiro-Wilk test of normality and a Levene’s test of homogeneity of variances were performed in R 2.13.2 (www.rproject.org/). Since data did not follow a normal distribution after logarithmic transformation, a Kruskal-Wallis Rank Sum Test using kruskal.test was followed by a multiple comparison test between treatments with kruskalmc function.
Results and Discussion
Fish total length (Mean±SD in cm) was 13.60±1.7 in the Seine, 13.28±0.96 in the Vilaine and 13.41±2.76 in the Mondego.
Lipid storage and contaminant level in fish tissue
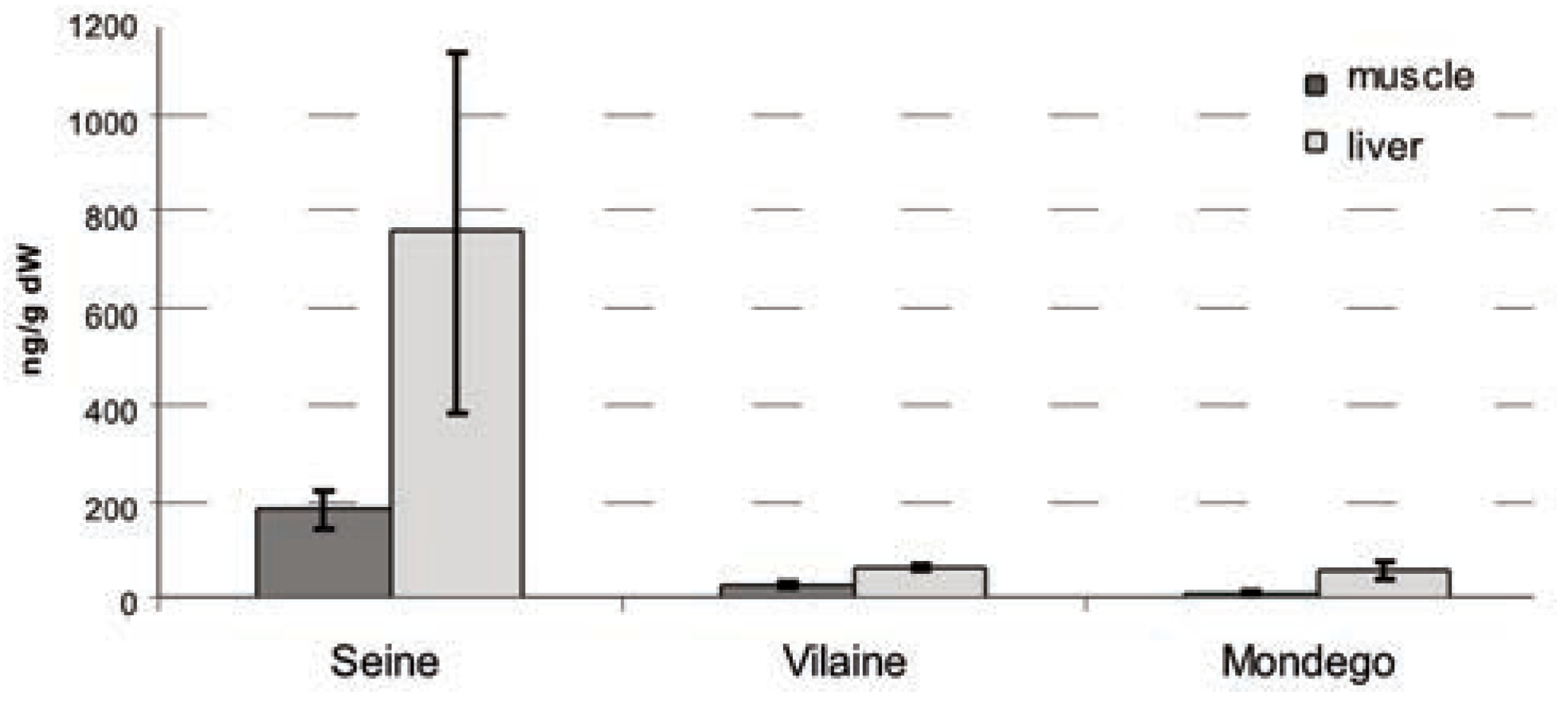
The high PCB concentration observed in fish tissues (Figure 2) in the Seine (liver CB 153: 800 ng.g–1 dw) confirms previous measurements in large and polluted French estuaries (i.e., the Gironde & Loire: flounder liver CB 153: 300 ng.g–1 dw) [10]. On the other hand, the contaminant load measured in fish from the Vilaine and Mondego (liver CB 153 <100 ng. g–1 dw) is comparable to that observed in the pristine Ster estuary, France (liver CB 153: 100 ng.g–1 dw) [10]. These results suggest that the level of chemical stress is high in the Seine estuary and probably limited in the Vilaine and Mondego systems.
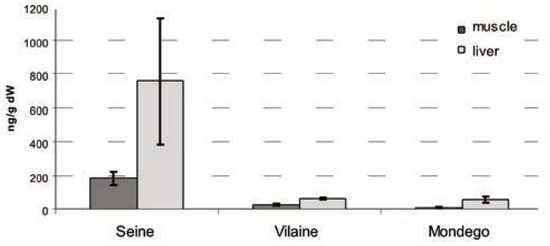
Figure 2.
Mean (and standard deviation) of CB153 concentrations observed in flounder muscle and liver, for three Atlantic estuaries, expressed in ng.g–1 of dry weight.
Contrasted patterns of lipid storage were observed by gravimetric method. The Seine estuary displayed a two times lower lipid content in liver (Mean±SD: 10.36±2.46) compared to the Vilaine and Mondego (19.05±1.91 and 18.21±4.43, respectively), and the Seine and Mondego showed a two times lower lipid content in muscle (2.35±0.96 and 2.83±0.71, respectively) compared to the Vilaine (5.96±1.02). These results probably underline a reduced ability of flounder to store energy in the highly contaminated system (the Seine) and in the southern estuary (the Mondego) compared to the Vilaine, a moderately stressed system. This also confirms the general decrease in lipid content detected in juvenile flounder muscle in polluted vs pristine estuaries [17], and also a reduced capacity of southern vs northern fish populations to store energy [18].
Detoxification and bioenergetic markers
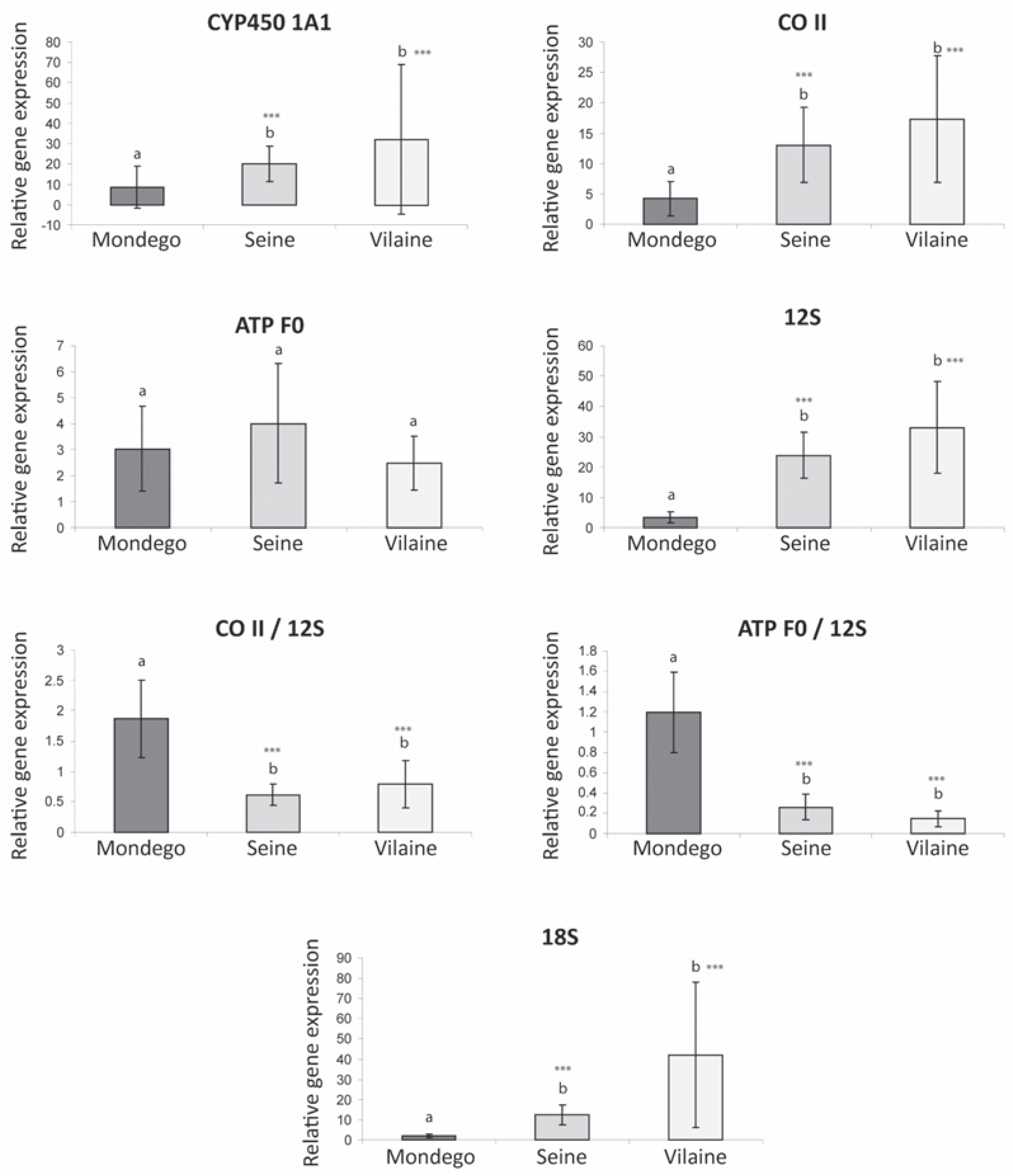
A significant general increase of the gene expression level was clearly detected for four of the five analyzed genes in the Seine and Vilaine, vs the Mondego populations: CYP450 1A1, CO II, 12S and 18S (Figure 3). In addition to this gene upregulation a higher inter-individual variability (i.e., standard deviation) was found for the same four genes in Vilaine vs Seine samples. The level of inter-individual variation could be a relevant indicator of population health in ecotoxicology [19]. Considering that the physiological status of the sampled fish was probably similar in the two northern estuaries that display a similar thermal regime (immature young of the year, identical feeding status linked to a high abundance of preys in estuaries at the beginning of autumn), we suggest that the higher phenotypic diversity detected in the less stressed environment (Vilaine estuary) could reflect a higher genetic variability and possibly a better adaptive ability of this population. In contrast, the heavily polluted flounder population of the Seine estuary that displays a lower inter-individual variability could be possibly at risk. The same conclusion could also be formulated for the Mondego population, which also displayed a low inter-individual variability in gene expression levels for CYP450 1A1, COII, 12S and 18S; this might be also related to a differential physiological status of the fish sampled in this estuary.
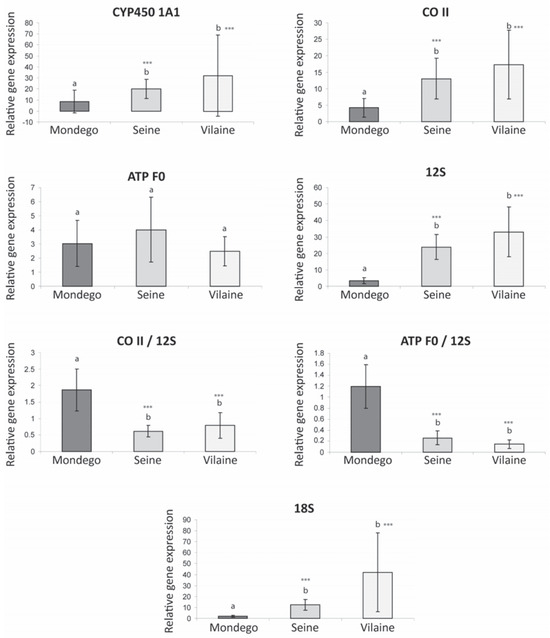
Figure 3.
Expression level of six transcripts (CYP450, CO II, ATP F0, 12S, 18S) in the flounder liver tissues from different estuaries of the Atlantic coast (mean and SD) and ratios of expression levels (CO II/12S, ATP F0/12S). ***Significant difference (P<0.001) between the considered population and the Mondego population.
The CYP450 is involved in the detoxification process and particularly in phase I of xenobiotics metabolism and is thus classically upregulated in contaminated estuaries [20]. Surprisingly, in the present study, the upregulation of CYP450 observed is relatively limited in the highly polluted Seine system in comparison to the Vilaine estuary. However organisms chronically exposed to organic pollutants can develop a resistance conducting to a reduced inducibility of the CYP450 [21]. On the other hand, the expression level of CYP450 could be related to the level of contaminants in the other estuaries, with a lower signal in the weakly contaminated Mondego and a higher signal in the Vilaine where the presence of PAH was detected in a previous investigation [10].
The lower expression level of three major genes involved in bioenergetics (CO II: oxidative phosphorylation; 12S: proxy of the mitochondrial density; 18S: proxy of protein biosynthesis) in the Mondego population compared to those from the Seine and Vilaine underlines a general reduction of the metabolism in the southern peripheral population vs northern ones. On the other hand, no significant difference was detected among populations in the expression of ATP F0 (last complex of the oxidative phosphorylation). Furthermore, both ratios CO II/12S and ATP F0/12S showed higher values in the Mondego vs the Vilaine and Seine samples, that could indicate a higher activity per mitochondria, i.e., an increase of the mitochondrial power output in the southern population. Gene expression changes do not necessarily equate to actual physiological changes in enzyme activity [22], however positive correlations were observed between proxies of molecular bioenergetics (levels of gene expression and enzymatic activities) in estuarine fish populations of Fundulus heteroclitus [23].
Thus, in the present study, the molecular analysis of fish responses to estuarine conditions highlights contrasting patterns of gene expression in the Mondego population, localized at the southern limit of the species’ range, compared to northern populations. These results support the hypothesis that cold-acclimated northern populations tend to display higher levels of metabolism compared to southern ones, as demonstrated in killifish along a latitudinal gradient (greater Cytochrome C Oxidase enzyme activity and CO II gene expression in most tissues in northern fish) [23]. Results obtained in both European flounder and killifish confirm the concept of oxygen-limitation of thermal tolerance in ectotherms:[24] this model predicts that the metabolic increment elicited by rising temperature must be kept small in order to maintain aerobic scope and thus the individual performance. Furthermore, the prediction of this model is also supported at the level of the whole organism in two studies on killifish [23] and cod [25], which underline a greater metabolic rate in northern fish than in southern ones.
Finally, the present study suggests that the European flounder may minimize the mitochondrial density in liver and maximize the activity per mitochondria in the southernmost region of its distribution range. This acclimation and/or adaptation mechanism may probably allow: i) preventing an excessive increase of the metabolic rate in hot water periods; and ii) maintaining a sufficient ATP production during cold water periods, when southern fish are probably unable to increase the mitochondrial density in tissues, comparatively to northern fish [26].
Conclusions
Energy-limited tolerance to stress can be considered as a framework to integrate effects of stressors [7] in European flounder populations. The high values observed in fish energy reserve, metabolic activity and inter-individual variability in gene expression levels confirm that the moderately polluted Vilaine population is probably facing a low ecological risk. In the Seine estuary, the high fish load in contaminants associated with a decrease in energy reserve and a loss of inter-individual variability indicate a highly stressed system, probably displaying a significant ecological risk. In the southern population of the Mondego, the suggested general reduction of the energy metabolism in this warm environment is classically considered in the literature as a common adaptive response of southern fish populations. Thus this suggests that juvenile flounder from the Mondego may experience only a moderate fitness loss in response to warming; however, the low inter-individual variability in gene expression may suggest a limited genetic variability, inducing a reduced ability of the southern population to evolve and adapt to future changes. Moreover, enzymatic activities linked to aerobiosis (Citrate synthase and Cytochrome C Oxydase) were measured in the three previous populations in a common garden experiment; they confirmed the inter-population variation in bioenergetics observed in the present study and suggested that much of this variation is possibly due to local adaptation [27].
Funding
this work was financed by the ANR program: VMCS-EVOLFISH (Paris), the Région Bretagne and the Institution d’Aménagement de la Vilaine (IAV, La Roche Bernard).
References
- Rabalais, N.N.; Turner, R.E.; Diaz, R.J.; Justic, D. Gobal change and eutrophication of coastal waters. ICES J Mar Sci 2009, 66, 1528–1537. [Google Scholar] [CrossRef]
- Gillanders, B.M.; Elsdon, T.S.; Halliday, I.A.; Jenkins, G.P.; Robins, J.B.; Valesini, F.J. Potential effects of climate change on Australian estuaries and fish utilizing estuaries: A review. Mar Freshwater Res 2011, 62, 1115–1131. [Google Scholar] [CrossRef]
- Laroche, J.; Gauthier, O.; Quiniou, L.; Devaux, A.; Bony, S.; Evrard, E.; et al. Variation patterns in individual fish responses to chemical stress among estuaries, seasons and genders: The case of the European flounder (Platichthys flesus) in the Bay of Biscay. Env Sci Poll Res 2013, 20, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, C.; Galland, C.; Pichereau, V.; Sanchez, W.; Riso, R.; Labonne, M.; et al. Assessment of the European flounder responses to chemical stress in the English Channel, considering biomarkers and life history traits. Mar Poll Bull 2015, 95, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.N.; Costa, M.J.; Salgado, J.P. Does the Tagus estuary fish community reflect environmental changes? Clim Res 2001, 18, 119–126. [Google Scholar] [CrossRef]
- Assis, J.; Castilho Coelho, N.; Alberto, F.; Valero, M.; Raimondi, P.; et al. High and distinct range-edge genetic diversity despite local bottlenecks. PLoS ONE 2013, 8, e68646. [Google Scholar] [CrossRef]
- Sokolova, I.M. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr Comp Biol 2013, 53, 597–608. [Google Scholar] [CrossRef]
- Lavergne, E.; Pedron, N.; Calves, I.; Claireaux, G.; Mazurais, D.; Zambonino-Infante, J.; et al. Does the chronic chemical contamination of a European flounder population decrease its thermal tolerance? Mar Poll Bull 2015, 95, 658–664. [Google Scholar] [CrossRef]
- Martinho, F.; van der Veer, H.; Cabral, H.N.; Pardal, M.A. Juvenile nursery colonization patterns for the European flounder (Platichthys flesus): A latitudinal approach. J Sea Res 2013, 84, 61–69. [Google Scholar] [CrossRef]
- Evrard, E.; Devaux, A.; Bony, S.; Burgeot, T.; Riso, R.; Budzinski, H.; et al. Responses of the European flounder Platichthys flesus to the chemical stress in estuaries: Load of contaminants, gene expression, cellular impact and growth rate. Biomarkers 2010, 15, 11–127. [Google Scholar] [CrossRef]
- Whitehead, A.; Crawford, D.L. Neutral and adaptive variation in gene expression. PNAS USA 2006, 103, 5425–5430. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.; Nielsen, E.E.; Williams, T.D.; Hemmer-Hansen, J.; Chipman, J.K.; Kruhøffer, M.; et al. Adaptive differences in gene expression in European flounder (Platichthys flesus). Mol Ecol 2007, 16, 4674–4683. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; Hofman, G.E. Defining the limits of physiological plasticity: How gene expression can assess and predict the consequences of ocean change. Philos T R Soc 2012, 367, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Bodiguel, X.; Loizeau, V.; Le Guellec, A.M.; Roupsard, F.; Philippon, X.; Mellon-Duval, C. Influence of sex, maturity and reproduction on PCB and p,p’DDE concentrations and repartitions in the European hake (Merluccius merluccius, L.) from the Gulf of Lions (N.W. Mediterranean). Sci Total Environ 2009, 408, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Jaouen-Madoulet, A.; Abarnou, A.; Le Guellec, A.M.; Loizeau, V.; Leboulenger, F. Validation of an analytical procedure for polychlorinated biphenyls, coplanar polychlorinated biphenyls and polycyclic aromatic hydrocarbons in environmental samples. J Chromatogr A 2000, 886, 153–173. [Google Scholar] [CrossRef]
- Leaver, M.J.; Diab, A.; Boukouvalaa, E.; Williams, T.D.; Chipman, J.K.; Moffat, C.F.; et al. Hepatic gene expression in flounder chronically exposed to multiply polluted estuarine sediment: Absence of classical exposure ‘biomarker’ signals and induction of inflammatory, innate immune and apoptotic pathways. Aquat Toxicol 2009, 96, 234–245. [Google Scholar] [CrossRef]
- Kerambrun, E.; Henry, F.; Cornille, V.; Courcot, L.; Amara, R. A combined measurement of metal bioaccumulation and condition indices in juvenile European flounder, Platichthys flesus, from Ruropean estuaries. Chemosphere 2013, 91, 498–505. [Google Scholar] [CrossRef]
- Fangue, N.A.; Mandic, M.; Richards, J.G.; Schulte, P.M. Swimming performance and energetics as a function of temperature in killifish Fundulus heteroclitus. Physiol Biochem Zool 2008, 81, 389–401. [Google Scholar] [CrossRef]
- Devin, S.; Giamberini, L.; Pain-Devin, S. Variation in variance means more than mean variations: What does variability telle us about population health status? Environ Int 2014, 73, 282–287. [Google Scholar] [CrossRef]
- Williams, T.D.; Gensberg, K.; Minchin, S.D.; Chipman, J.K. A DNA expression array to detect toxic stress response in European flounder (Platichthys flesus). Aquat Toxicol 2003, 65, 141–157. [Google Scholar] [CrossRef]
- Brammel, B.F.; Price, D.J.; Birge, W.J.; Elskus, A.A. Lack of CYP1A responsiveness in species inhabiting chronically contaminated habitats: Two varieties of resistance? Comp Biochem Physiol C 2013, 157, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, M.; Rytkönen, K.T. Functional genomics in aquatic toxicology-do not forget the function. Aquat Toxicol 2011, 105, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Fangue, N.A.; Richards, J.G.; Schulte, P.M. Do mitochondrial properties explain intraspecific variation in thermal tolerance? J Exp Biol 2009, 212, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Bock, C.; Knust, R.; Lannig, G.; Lucassen, M.; Mark, F.C.; et al. Cod and climate in a latitudinal cline: Physiological analyses of climate effects in marine fishes. Clim Res 2008, 37, 253–270. [Google Scholar] [CrossRef]
- Sylvestre, E.L.; Lapointe, D.; Dutil, J.D.; Guderley, H. Thermal sensitivity of metabolic rates and swimming performance in two latitudinally separated populations of cod, Gadus morhua. J Comp Physiol B 2007, 177, 447–460. [Google Scholar] [CrossRef]
- Dhillon, R.S.; Schulte, P.M. Intraspecific variation in the thermal plasticity of mitochondria in killifish. J Exp Biol 2011, 214, 3639–3648. [Google Scholar] [CrossRef]
- Pédron, N. Structure génétique, réponses bioénergétiques et traits de vie, de populations de flets (Platichthys flesus) soumises au réchauffement climatique, sur un gradient latitudinal. PhD Thesis, Université de Bretagne Occidentale, Brest, France, 2016. [Google Scholar]
© Copyright E. Borcier et al., 2016 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 4.0 License (CC BYNC 4.0).