Recommendations for the Analysis of Hemocyte-Related Immunocompetent Oxidative Activity in the Freshwater Snail Lymnaea stagnalis †
Introduction
Materials and Methods
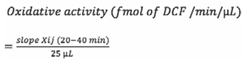
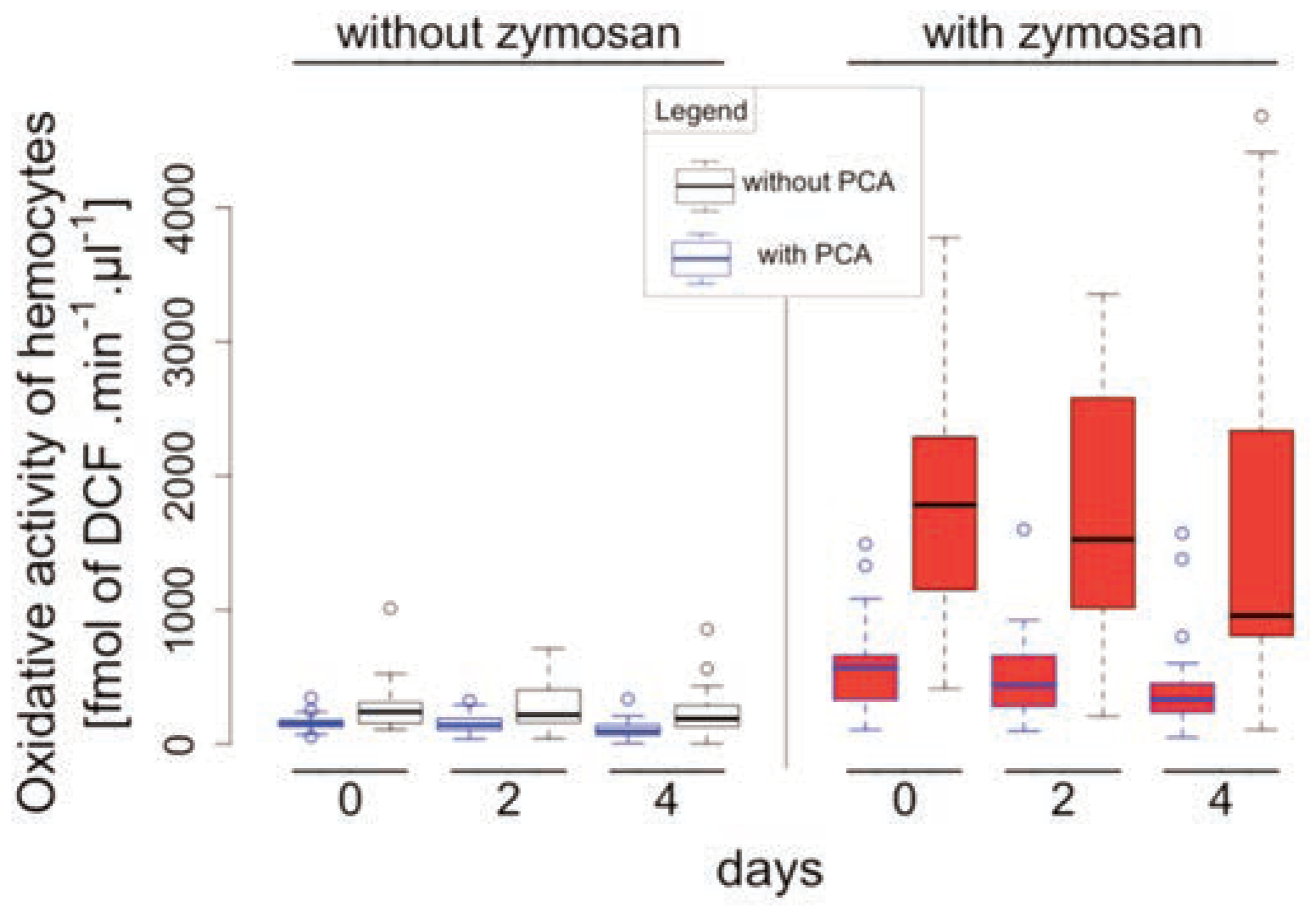
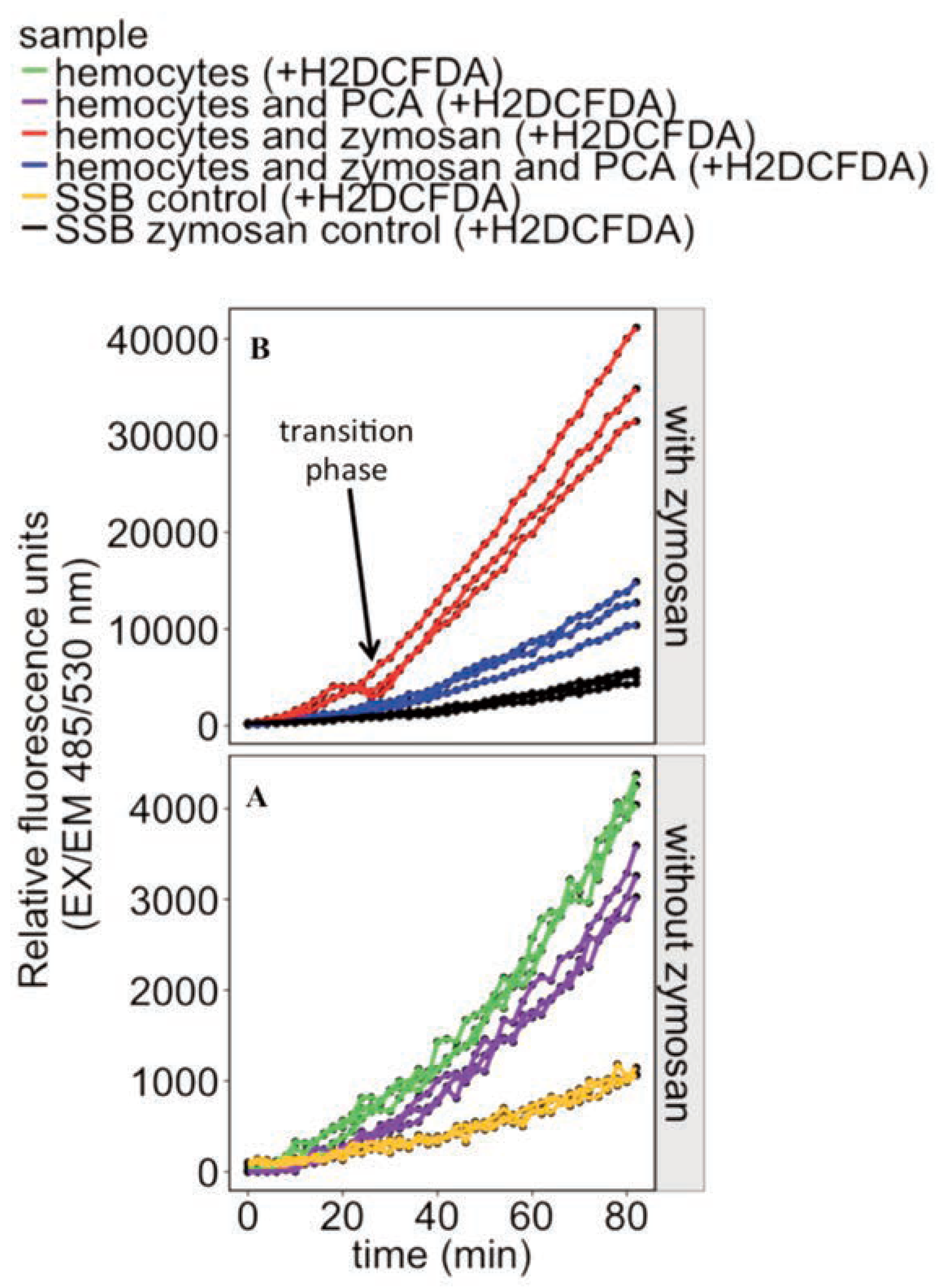
Results and Discussion
Baseline activities
Repeated collections
Conclusions
Funding
References
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev 2007, 87, 245–313. [Google Scholar]
- Buggé, D.M.; Hégaret, H.; Wikfors, G.H.; Allam, B. Oxidative burst in hard clam (Mercenaria mercenaria) haemocytes. Fish Shellfish Immun 2007, 23, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Donaghy, L.; Hong, H.-K.; Jauzein, C.; Choi, K.-S. The known and unknown sources of reactive oxygen and nitrogen species in haemocytes of marine bivalve molluscs. Fish Shellfish Immun 2015, 42, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Benna, J.E.; Chanock, S.J.; Smith, R.M. The NADPH oxidase of leukocytes: The respiratory burst oxidase. Cold Spring Harbor Monogr Archiv 1997, 34, 737–783. [Google Scholar]
- Donaghy, L.; Kraffe, E.; Le Goïc, N.; Lambert, C.; Volety, A.K.; Soudant, P. Reactive oxygen species in unstimulated hemocytes of the Pacific oyster Crassostrea gigas: A mitochondrial involvement. PLoS ONE 2012, 7, e46594. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Soudant, P.; Jegaden, M.; Delaporte, M.; Labreuche, Y.; Moal, J.; et al. In itro modulation of reactive oxygen and nitrogen intermediate (ROI/RNI) production in Crassostrea gigas hemocytes. Aquaculture 2007, 270, 413–421. [Google Scholar] [CrossRef]
- Manduzio, H.; Rocher, B.; Durand, F.; Galap, C.; Leboulenger, F. The point about oxidative stress in molluscs. ISJ 2005, 2, 91–104. [Google Scholar]
- Bugge, D.M.; Hegaret, H.; Wikfors, G.H.; Allam, B. Oxidative burst in hard clam (Mercenaria mercenaria) haemocytes. Fish Shellfish Immunol 2007, 23, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hahn, U.K.; Bender, R.C.; Bayne, C.J. Production of reactive oxygen species by hemocytes of Biomphalaria glabrata: Carbohydrate-specific stimulation. Develop Comparat Immunol 2000, 24, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Adema, C.; van Deutekom-Mulder, E.; van der Knaap, W.; Sminia, T. NADPH-oxidase activity: The probable source of reactive oxygen intermediate generation in hemocytes of the gastropod Lymnaea stagnalis. J Leuk Biol 1993, 54, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Boisseaux, P.; Delignette-Muller, M.-L.; Abbaci, K.; Thomas, H.; Garric, J. Analysis of hemocytes in Lymnaea stagnalis: Characterization and effects of repeated hemolymph collections. Fish Shellfish Immun 2016. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, A.; Adema, C.; Van der Knaap, W.; Sminia, T. The effect of haemolymph extraction on distribution of lysosomal enzymes in Lymnaea stagnalis haemocytes: A cytochemical study. Comparat Haematol Int 1992, 2, 61–67. [Google Scholar] [CrossRef]
- Sminia, T. Structure and function of blood and connective tissue cells of the fresh water pulmonate Lymnaea stagnalis studied by electron microscopy and enzyme histochemistry. Zeitschr Zellforsch mikroskop Anat 1972, 130, 497–526. [Google Scholar]
- Moss, B.; Allam, B. Fluorometric measurement of oxidative burst in lobster hemocytes and inhibiting effect of pathogenic bacteria and hypoxia. J Shellfish Res 2006, 25, 1051–1057. [Google Scholar]
- Russo, J.; Lefeuvre-Orfila, L.; Lagadic, L. Hemocyte-specific responses to the peroxidizing herbicide fomesafen in the pond snail Lymnaea stagnalis (Gastropoda, Pulmonata). Environ Pollut 2007, 146, 420–427. [Google Scholar]
- A’t Hart, B.; Simons, J.M.; Rijkers, G.T.; Hoogvliet, J.C.; Van Dijk, H.; Labadie, R.P. Reaction products of 1-naphthol with reactive oxygen species prevent NADPH oxidase activation in activated human neutrophils, but leave phagocytosis intact. Free Radical Biol Med 1990, 8, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using {lme4}. J Stat Softw 2015, 67, 1–48. [Google Scholar]
- Lacoste, A.; Malham, S.K.; Gélébart, F.; Cueff, A.; Poulet, S.A. Stress-induced immune changes in the oyster Crassostrea gigas. Dev Comparat Immunol 2002, 26, 1–9. [Google Scholar]
- Winston, G.W.; Moore, M.N.; Kirchin, M.A.; Soverchia, C. Production of reactive oxygen species by hemocytes from the marine mussel, Mytilus edulis: Lysosomal localization and effect of xenobiotics. Comparat Biochem Physiol Part C 1996, 113, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Koutsogiannaki, S.; Franzellitti, S.; Fabbri, E.; Kaloyianni, M. Oxidative stress parameters induced by exposure to either cadmium or 17 -estradiol on Mytilus galloprovincialis hemocytes. The role of signaling molecules. Aquat Toxicol 2014, 146, 186–195. [Google Scholar]
© Copyright P. Boisseaux et al., 2016. Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 4.0 License (CC BYNC 4.0).
Share and Cite
Boisseaux, P.; Noury, P.; Delignette-Muller, M.-L.; Thomas, H.; Garric, J. Recommendations for the Analysis of Hemocyte-Related Immunocompetent Oxidative Activity in the Freshwater Snail Lymnaea stagnalis. J. Xenobiot. 2016, 6, 6585. https://doi.org/10.4081/xeno.2016.6585
Boisseaux P, Noury P, Delignette-Muller M-L, Thomas H, Garric J. Recommendations for the Analysis of Hemocyte-Related Immunocompetent Oxidative Activity in the Freshwater Snail Lymnaea stagnalis. Journal of Xenobiotics. 2016; 6(2):6585. https://doi.org/10.4081/xeno.2016.6585
Chicago/Turabian StyleBoisseaux, P., P. Noury, M.-L. Delignette-Muller, H. Thomas, and J. Garric. 2016. "Recommendations for the Analysis of Hemocyte-Related Immunocompetent Oxidative Activity in the Freshwater Snail Lymnaea stagnalis" Journal of Xenobiotics 6, no. 2: 6585. https://doi.org/10.4081/xeno.2016.6585
APA StyleBoisseaux, P., Noury, P., Delignette-Muller, M.-L., Thomas, H., & Garric, J. (2016). Recommendations for the Analysis of Hemocyte-Related Immunocompetent Oxidative Activity in the Freshwater Snail Lymnaea stagnalis. Journal of Xenobiotics, 6(2), 6585. https://doi.org/10.4081/xeno.2016.6585



