Introduction
In human and veterinary therapeutics, approximately 3000 different drugs are used and prescribed in high quantities [1]. The main sub- stance classes are steroidal hormones, anti- microbials and pharmaceuticals and their metabolites [1,2]. These compounds could become problematic in the environment, because >80% of the drugs pass the body without being bio- transformed, and furthermore their elimination in sewage treatment plants is incomplete [1,3]. Additionally, the compounds are constructed for a specific intended effect on living beings, and therefore show intrinsic physico-chemical behavior [4]. Since the compounds are chemically different and peer-reviewed ecotoxicological data is available for less than 1% of human phar- maceuticals, the effects on most trophic levels of aquatic life are hard to predict for a single sub- stance or a mix of various ones [5,6,7,8]. The purpose of this study was to identify the impact of phar- maceuticals commonly found in surface waters on immune cells of harbor seals (Phoca vituli- na) in vitro. Peripheral blood mononuclear cells (PBMCs) from captive seals and a seal B lym- phoma cell line (11B7501) [9] were exposed to selected single substances. Compounds of inter- est were analgesics (ibuprofen, naproxen), psy- choactive substances (carbamazepine, paroxe- tine), antibiotics (erythromycin, sulfamethoxa- zole, trimethoprim), cholesterol lowering com- pounds (gemfibrozil), steroidal hormones (ethynyl estradiol) and caffeine.
Materials and Methods
Sampling and Isolation of Peripheral Blood Mononuclear Cells and Cell Culture
Whole blood samples (7-15 mL) were collected from the extradural intervertebral vein of four female harbor seal adults in captivity (Aquarium du Québec, Quebec, Canada). Blood was kept in heparin tubes at RT for 6 h after sampling until separation with Lympholyte-Mammal (Cedarlane, Burlington, Canada). The PBMCs were resuspended in completed RPMI-1640 and kept at 4°C overnight until the start of the incubation with xenobiotics.
The 11B7501 cell line [9] was maintained at concentrations between 2.0x105-2.0x107 cells/mL in completed RPMI-1640 in a humidi- fied 5% CO2 atmosphere.
Preparation of Xenobiotics
Chemicals (all Sigma-Aldrich) were dis- solved in dimethyl sulfoxide (DMSO), ethanol or water. The final concentration of DMSO or ethanol in the samples never exceeded 0.1%.
In Vitro Exposures
For the in vitro exposures, cells were adjust- ed to the test specific concentration. In case of the lymphoblastic proliferation 200 µL of cells (1.25x106 cells/mL) were incubated with 2 µL of pharmaceutical product. In case of phagocy- tosis and cell cycle and apoptosis, 500 µL of cells (1.0x106 cells/mL and 0.5x106 cells/mL, respectively) were incubated with 5 µL of phar- maceutical product. Viability assays were set up parallel to each of the three assays as an additional control.
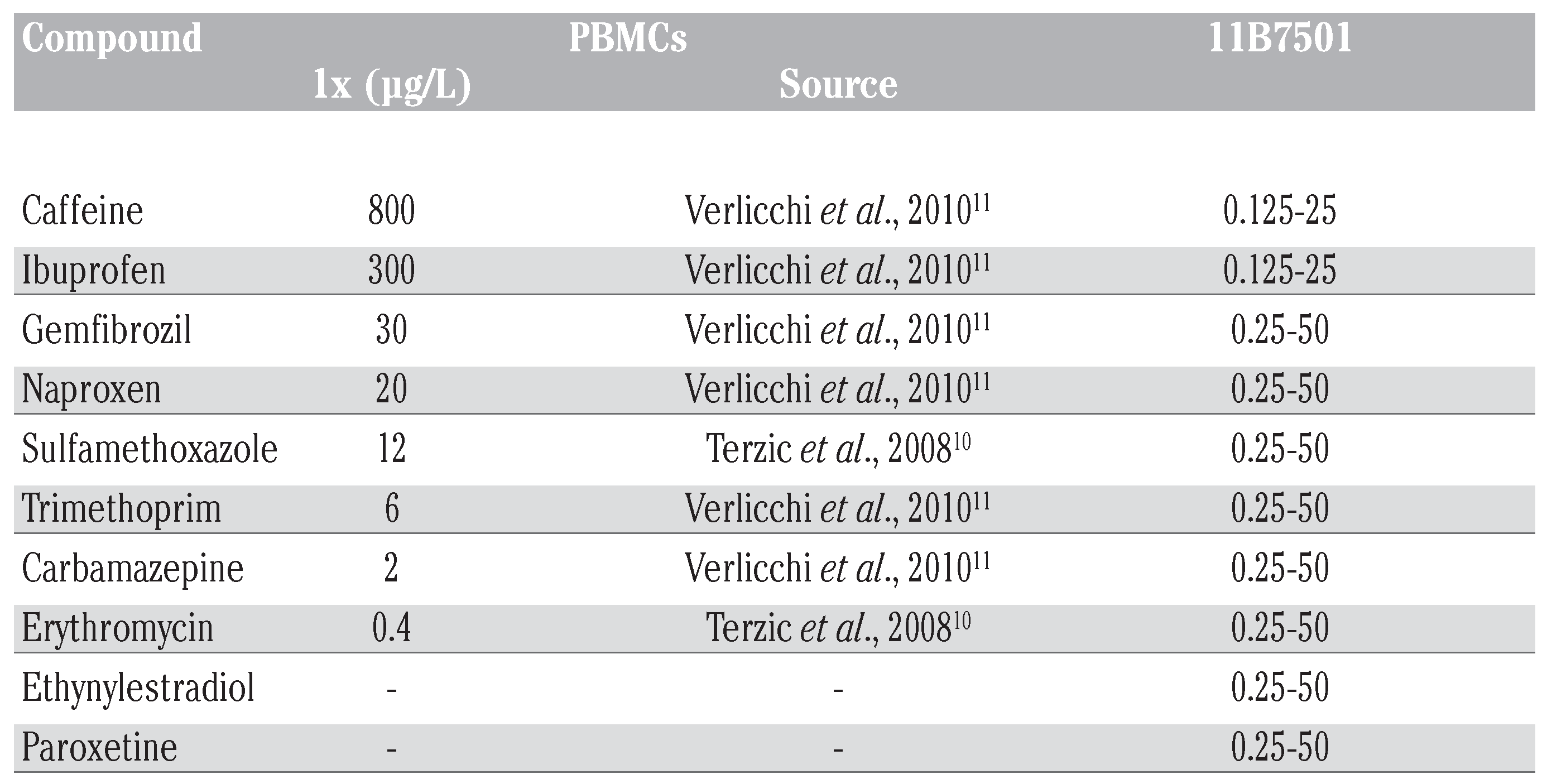
For the experiments with PBMCs, concen- trations of pharmaceuticals were based on environmentally relevant values found in municipal effluents. The concentration 1x refers to the maximum concentration detected in the environment (Table 1), as cited [10,11]. Further concentrations tested were: 0x, 0.01x, 0.1x, 10x, 100x (Table 1).

Table 1.
Concentration range of pharmaceutical compounds tested with PBMCs and the 11B7501 cell line. For the experiments with PBMCs, test concentrations of single sub- stances were chosen according to values found in municipal effluents. The maximum value found in literature was set to be the 1x concentration. Further concentrations test- ed were 0x, 0.01x, 0.1x, 10x and 100x of the 1x value. Concentration ranges of the sin- gle substances for the experiments with the 11B7501 cell line were then increased.
In experiments with the 11B7501 cell line, concentrations of pharmaceuticals were increased to be able to observe a possible neg- ative impact of the single substance on the harbor seal immune cells (Table 1).
Viability Assay
After the respective exposure time, viability of cells was evaluated using 4 µL of a 100 μg/mL of propidium iodide (PI) solution (Sigma- Aldrich) to 500 µL of cell suspension. A FACSCalibur (Becton Dickinson, San Jose, CA, USA) with an air-cooled argon laser providing an excitation at 488 nm was used. For each sample 5 000 events were acquired at a fluo- rescence emission of 620 nm (FL3). The cell population was electronically gated in a FSC/SSC dot plot and the fluorescence fre- quency distribution histogram was obtained using FL3. The percentage of dead cells was determined using a marker. Data collection and analysis were performed with the CellQuest Pro software (Version 4.0.1). The results were expressed in percentage of viable cells.
Phagocytosis Assay
Phagocytosis was measured using carboxy- late coated fluorescent latex beads (Polysciences Inc.; 100 beads:1 cell). After 24 h exposure to single substances of pharmaceuti- cals, cells and beads were incubated for 1.5 h at 37°C to allow attachment and phagocytosis. To remove most free beads after incubation, the suspension was centrifuged on a gradient of 3% BSA (MP Biomedicals) prepared in com- pleted medium. The fluorescence emission was collected at 520 nm (FL1). For each sample 10,000 events were acquired. Using a FSC/SSC dot plot, the adequate population was electron- ically gated and the fluorescence frequency distribution histogram was obtained using FL1. Data was expressed as percentage of cells with phagocytic activity (cells that engulfed>one bead) and phagocytotic efficien- cy (cells that engulfed>three beads). The lym- phocyte population was gated as a negative control.
Lymphocyte Transformation Assay
Change in DNA synthesis after stimulation with a mitogen [Con A for PBMCs, lipopolysac- charide (LPS) for 11B7501 cell line] was meas- ured as the incorporation of methyl-3H-thymi- dine. After 48 h incubation, 1 μCi of methyl-3H- thymidine (PerkinElmer, Shelton, USA) was added and cells were incubated for further 18 h. The cells were harvested onto a glass fiber fil- ter (Tomtec Mach III Cell Harvester) and the amount of radioactivity incorporated was measured with a TriLux counter (Wallac 1450 MicroBeta TriLux Liquid Scintillation Counter & Luminometer) and analyzed with the pro- gram MicroBeta Windows Workstation (Version 4.50.09, PerkinElmer, Shelton, USA). The raw data was expressed as counts per minute and was then converted in percent proliferation of control.
Cell Cycle and Apoptosis Assay
The DNA content of each cell was measured using PI. After fixation of the cells with 70% EtOH following the 24 h exposure and 72 h in RPMI, cells were washed and then resuspend- ed in a PBS solution containing PI (50 μg/mL) and RNAse (100 μg/mL) (all Sigma-Aldrich). PI also binds to double stranded regions of RNA, necessitating treatment with nucleases [12]. Using the FSC/ SSC dot plot, the cell population was electronically gated and the fluorescence frequency distribution histogram using FL2 (585 nm) was obtained. In a second dot plot, the gated lymphocyte population is expressed in FL2-A/FL2-W. Doublets (two G1/G0 cells attached to each other, which seem to have the same DNA content as one cell in the G2/M phase) are discriminated using a second gate. The population lymphocytes minus doublets was analyzed in FL3-A, and apoptosis as well as the phases of the cell cycle were gated inde- pendently. For each sample 5000 events were acquired. The results were expressed in per- centage of cells in different stages of the cell cycle plus apoptotic events.
Statistical Analyses
Differences between controls and treated groups were evaluated by one-way ANOVA fol- lowed by Tukey’s Multiple Comparison post test. The calculations were performed using GraphPad Prism 5 for Windows (GraphPad Software). The level of significance was set at P≤0.05.
Results and Discussion
The experiments conducted with PBMCs exposed to concentrations of pharmaceuticals similar to those found in surface showed no significant impairment of cellular function in the phagocytosis or lymphoblastic proliferation assay (data not shown).
In the experiments with the 11B7501 cell line, three compounds influenced the B lym- phocytes at high concentrations. While the phagocytosis experiment showed no signifi- cant effect with any compound (data not shown), the lymphoblastic proliferation with LPS was modulated in the case of naproxen (>25 µg/mL**) and carbamazepine (>50 µg/mL*) (Figure 1A). The cell cycle and apoptosis assay revealed a significant increase in apoptotic events at 50 µg/mL for ethynylestradiol, while the percentage of cells in the G2/M phase decreased (Figure 1B). For carbamazepine, the percentage of apoptotic events slightly decreased at 50 µg/mL, while the percentage of cells in the G0/G1 phase increased (Figure 1B).
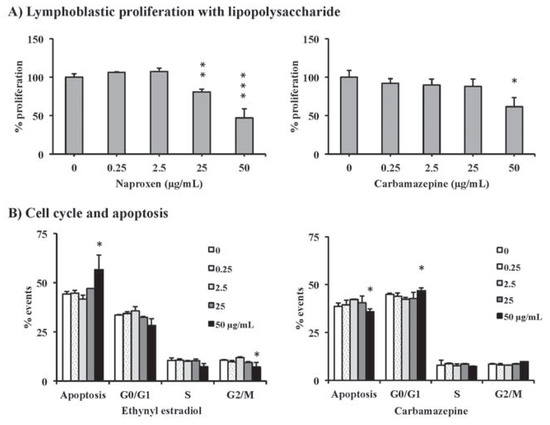
Figure 1.
The lymphoblastic proliferation assay revealed significant effects for naproxen and carbamazepine (A). The cell cycle and apoptosis assay showed significant effects for ethynyl estradiol and carbamazepine (B). Significant differences are expressed by an asterisk (*P<0.05, **P<0.01, ***P<0.001).
The absence of an effect in the lower doses of pharmaceutical compounds does not necessarily conclude to the harmlessness of current environmentally relevant levels of pharmaceu- ticals towards the immune system of marine vertebrates. In contrast, many studies on fish already showed bioaccumulation in various tissues and a significant change in immune parameters, behavior and distribution that were caused by environmentally relevant levels of pharmaceuticals [13,14,15,16]. With fish accumulating pharmaceutical products and seals being pis- civorous, they will likely be exposed to higher concentrations through nutrition, opposed to those concentrations found in the water col- umn. Therefore, the true environmentally rele- vant concentrations for the exposures of marine mammals towards pharmaceuticals still have to be assessed.
Our study shows that more sensitive meth- ods are needed to evaluate the impact of phar- maceuticals on the immune system of marine mammals. Risk assessment in this area is nec- essary, because not only seals but also humans consume fish. If pharmaceuticals in the envi- ronment cause a possible reduction in certain functional activities of the immune system of large vertebrates that may alter the host’s resistance to pathogens, the knowledge of these effects would therefore most certainly have implications on the treatment of munici- pal wastewaters in the future.
Acknowledgments
The study was supported by the Canada Research Chair in Immunotoxicology (MF) and the NSERC Strategic Grant. CK was supported by a scholarship of the German Academic Exchange Service (DAAD). Furthermore, we would like to thank Stéphane Masson and his team at the Aquarium du Québec for providing us with whole blood samples.
References
- Togola, A.; Budzinski, H. Analytical develop- ment for analysis of pharmaceuticals in water samples by SPE and GC-MS. Anal Bioanal Chem 2007, 388, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; Blaise, C.; Fournier, M.; Hansen, P.D. Effects of selected pharmaceutical products on phagocytic activity in Elliptio com- planata mussels. Comp Biochem Phys C 2006, 143, 179–186. [Google Scholar]
- Halling-Sørensen, B.; Nors Nielsen, S.; Lanzky, P.F.; Ingerslev, F.; Holten Lützhøft, H.C.; Jørgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the envi- ronment - A review. Chemosphere 1998, 36, 357–394. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H.; Johnson, D.J.; Reitsma, T.; Brain, R.A.; Wilson, C.J.; Solomon, K.R. Ranking and prioritization of environmen- tal risks of pharmaceuticals in surface waters. Regul Toxicol Pharm 2004, 39, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Aquatic environmental assessment of the top 25 English prescription pharmaceuti- cals. Water Res 2002, 36, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H.; Johnson, D.J.; Wilson, C.J.; Brain, R.A.; Solomon, K.R. Probabilistic haz- ard assessment of environmentally occur- ring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol Lett 2003, 144, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Stuer-Lauridsen, F.; Birkved, M.; Hansen, L.P.; Lutzhoft, H.C.H.; Halling-Sorensen, B. Erratum to “Environmental risk assess- ment of human pharmaceuticals in Denmark after normal therapeutic use” [Chemosphere 40 (2000) 783-793]. Chemosphere 2000, 41, 1509. [Google Scholar] [CrossRef]
- European Commission. White paper. Strategy for a future chemicals policy, COM (2001) 88 Final. Available online: http://ec.europa.eu/enterprise/sectors/che micals/documents/reach/archives/white-paper/index_en.htm (accessed on 12 November 2012).
- Frouin, H.; Fortier, M.; Fournier, M. Toxic effects of various pollutants in 11B7501 lymphoma B cell line from harbour seal (Phoca vitulina). Toxicology 2010, 270, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Terzic, S.; Senta, I.; Ahel, M.; Gros, M.; Petrovi, M.; Barcelo, D.; et al. Occurrence and fate of emerging wastewater contaminants in Western Balkan Region. Sci Total Environ 2008, 399, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barcelo, D. Hospital effluents as a source of emerg- ing pollutants: An overview of micropollu- tants and sustainable treatment options. J Hydrol 2010, 389, 416–428. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujikura, K.; Higashiyama, T.; Takata, K. DNA staining for fluorescence and laser confocal microscopy. J Histochem Cytochem 1997, 45, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, B.; Hitzfeld, B.; Kollner, B.; Dietrich, D.R.; van den Heuvel, M.R. Sex and low-level sampling stress modify the impacts of sewage effluent on the rainbow trout (Oncorhynchus mykiss) immune system. Aquat Toxicol 2005, 73, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Salo, H.M.; Hébert, N.; Dautremepuits, C.; Cejka, P.; Cyr, D.G.; Fournier, M. Effects of Montreal municipal sewage effluents on immune responses of juvenile female rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 2007, 84, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Azzurro, E.; Matiddi, M.; Fanelli, E.; Guidetti, P.; La Mesa, G.; Scarpato, A.; et al. Sewage pollu- tion impact on Mediterranean rocky-reef fish assemblages. Mar Environ Res 2010, 69, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Brooks, B.W. Chapter 8: Active pharmaceutical ingredients and aquatic organisms. In Environmental contaminants in biota: interpreting tissue concentrations; Beyer, W.N., Meador, J.P., Eds.; CRC Press, Taylor and Francis: Boca Raton, FL, 2011; pp. 286–347. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© Copyright C. Lorin-Nebel et al., 2014 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 4.0 License (CC BY-NC 4.0).