Introduction
In the present study, the effects of copper (Cu) and cadmium (Cd) to the Pacific oyster Crassostrea gigas gametes were investigated. Exposure to metals concentrations of 1, 5, 10, 20, 40 µg.L−1 for Cu and 100, 200, 400, 800, 1600 µg.L−1 for Cd for 30 min in seawater caused significant adverse effects on the fertilization success of sperm [effective concentration (EC)50=20 µg.L−1 for Cu, EC50=830 µg.L−1 for Cd] and oocytes (EC50=57 µg.L−1 and 1350 µg.L−1, respectively). Lowest observed effect concentrations (LOEC) for spermiotoxicity assay were 1 µg.L−1 for Cu and 100 µg.L−1 for Cd. However, LOEC values for fertilization success of Cu-exposed and Cd-exposed oocytes were obtained at higher concentrations: 10 µg.L−1 and 400 µg.L−1 for Cu and Cd respectively. It can therefore be concluded that Cu was more toxic than Cd to oyster C. gigas gametes where spermatids were seemingly more sensitive than oocytes. The study showed that these metals could adversely affect reproduction at environmentally realistic concentrations.
Background
Heavy metals have long been recognized as hazardous pollutants for aquatic organisms in particular at an early developmental stage. Copper (Cu) and cadmium (Cd) are among the most dangerous metals for bivalve [1,2] shrimp [3] and sea urchin larvae [4]. Although Cu is an essential micronutrient for aquatic animals, it can be toxic to marine organisms at elevated concentrations. Background Cu levels in surface water are usually low (0.03-0.23 µg.L−1), but can exceed 100 µg.L−1 in severely polluted coastal areas [5]. Solomon [6] reported that when parental sea scallops were exposed to environmentally realistic concentrations of Cu at 10-20 µg.L−1, sperm and oocyte production was decreased. In contrast to Cu, Cd is a nonessential element which can accumulate in polluted environments reaching µg/L [7]. Cd has been shown to be highly toxic to bivalves embryos from Ruditapes decussatus and M. gal-loprovincialis [8]. Bioassays play a crucial role in assessing the hazard of pollutants in marine organisms. Pacific oysters, particularly in their highly sensitive early life stages, are commonly used to assess the toxicity of a large variety of pollutants [1,9,10]. However, previous studies on heavy metal embryotoxicity in the Pacific oyster have been mainly focused on the effect of metals on embryogenesis (from fertilization to D-shape larvae) with only limited information available on Cu or Cd toxicity to sperm and oocytes fertilizing capabilities. In the present paper, we studied the fertilizing potency of spermotozoa and oocytes of C. gigas exposed to Cu and Cd. Our aims were to establish a doseresponse relationship for each tested metal and to assess the pollutant sensitivity of both gamete types in comparison to embryos.
Materials and Methods
Analytical grade of copper sulfate, cadmium chloride and formalin solution were purchased from Sigma-Aldrich Chemical (St. Quentin Fallavier, France). Seawater was collected from Arcachon Bay (SW of France), an area where oysters reproduce naturally. Immediately after sampling, seawater was filtered using 0.2 µm- pore membrane filter. Filtered seawater (FSW) was stocked at 4°C in the dark and was used within 3 days. Mature oysters (Crassostrea gigas) came from a commercial hatchery specialized in the year-round production of mature oysters (Guernsey Sea Farms, UK). All oysters were used within 3 days.
Stock solutions of metals (500 mg.L−1 for Cd and 250 mg.L−1 for Cu) were prepared in Milli- Q water. Test concentrations of either metal were prepared by diluting the stock solution in FSW. The test concentrations were 1, 5, 10, 20,40 µg.L−1 for Cu and 100, 200, 400, 800, 1600 µg.L−1 for Cd. In each experiment, FSW was used as negative control. All glasswares were acid-washed before the experiments. Experimental solutions were acidified with 1% final v/v 65% nitric acid and were then analyzed for Cd or Cu content by ICP-AES (Varian Vista ProAxial, Agilent Technologies, USA) using standard conditions (Table 1). Detection limits were 1 µg.L−1 for water samples.

Table 1.
Copper and cadmium concentrations (µg.L−1) determined for exposure solutions.
The spermiotoxicity and oocyte toxicity tests have been described in details previously [10]. Briefly, sperms cells and oocytes were exposed to metals for 30-min before they were used for fertilization. Two fertilization assays were then conducted. For Assay (1), 1.0 mL of exposed sperm solution was added to 10 mL of FSW containing unexposed oocytes. For Assay (2) 1.0 mL of unexposed sperm solution was added to 10 mL of FSW containing exposed oocytes. Embryos were incubated at 24°C for 2 h until the 2-4 cell-stage was attained in the control treatment. To calculate the fertilization rate (FR), unfertilized oocytes were scored under an inverted microscope (Olympus, magnification x 200) among 100 oocytes.
Data are expressed as means±SEM (standard error mean). Differences in fertilization success were assessed for significance by one- way analysis of variance and Tukey post hoc test. The EC50 defined here as the toxicant concentrations causing 50% unsuccessful fertilization or abnormal larval development were calculated by PRISM 5 software (GraphPad Software, CA, USA).
Results and Discussion
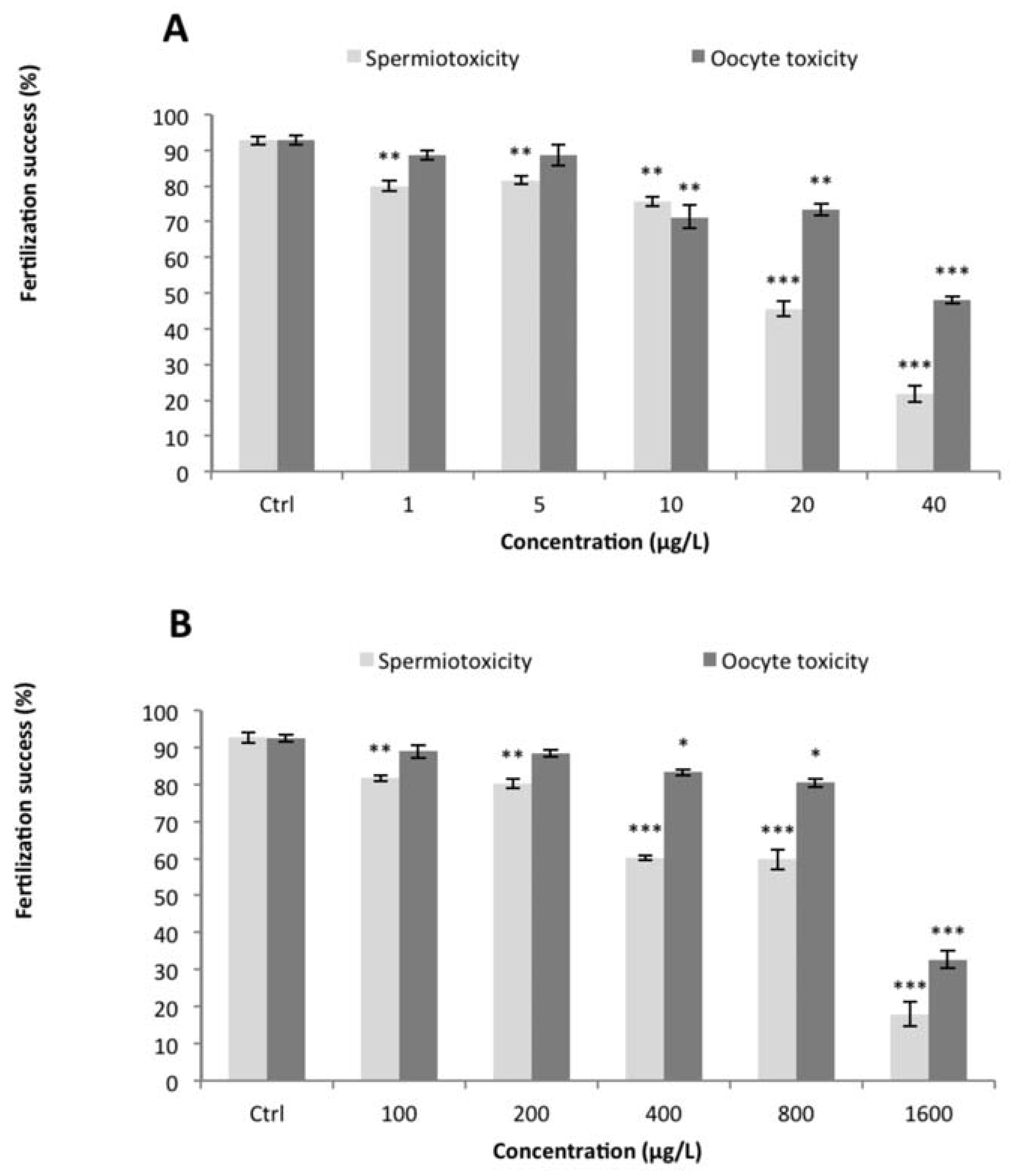
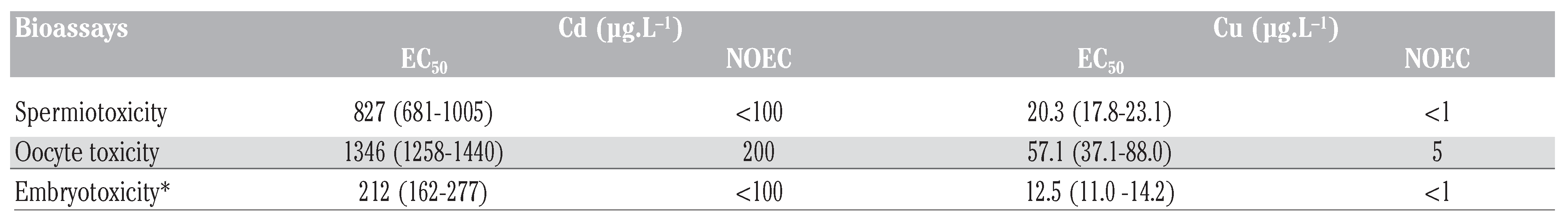
Nominal and measured concentrations of Cu and Cd for the different applied treatments were determined (Table 1). Measured concentrations were within 10-23% of the nominal concentrations. Therefore, nominal concentrations were used for presentation and calculation of toxicity parameters. The background level of unfertilized eggs in the various controls was low as well as the response variability between replicates (ca 7%). A significant decrease of the fertilization rate was observed after sperm exposure (Assay 1) to the lowest Cu and Cd concentrations tested (1 µg.L−1 and 100 µg.L−1, respectively) as compared to the control (P<0.05) (Figure 1). The fertilization success was reduced to 20% at the highest concentration tested for both Cu and Cd. Calculated EC50 were 20 µg.L−1 for Cu and 827 µg.L−1 for Cd (Table 2). The non-observed effect concentrations (NOECs) for Cu and Cd in this assay was below 1 and 100 µg.L−1 respectively. For oocyte exposure (Assay 2), Cu inhibited fertilization from 10 µg.L−1 concentration and Cd impaired fertilization at concentration of 400 µg.L−1 (Figure 1). The EC50 and NOEC data for fertilization rate are summarized in Table 2.
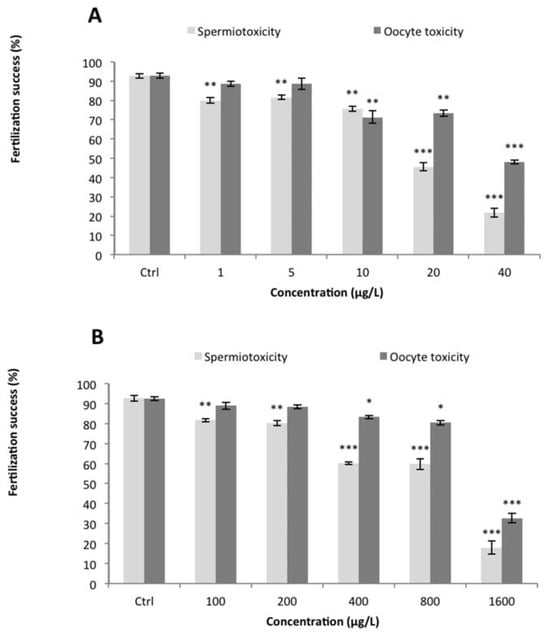
Figure 1.
Percentages (mean±SEM) of fertilization success after gamete exposure to (A) copper or (B) cadmium for 30 min. Asterisks indicate statistical differences between control and exposed treatments at *P<0.05, **P<0.01, ***P<0.001.

Table 2.
Mean effective concentrations (EC50) and their 95% coefficients of variation, and non-observed effect concentration (NOEC) values for different toxicity assays with C. gigas for cadmium and copper. Less than (<) values are given for cases where NOEC could not be calculated.
To date, most bioassays using bivalves have been performed using either embryos and larvae or adults, while the sensitivity of spermatozoa and oocytes to toxicants are less understood. Most of the existing data on the effects of metals on sperm and eggs have been obtained with the sea-urchin [11,12] and to a lesser extend with blue mussel [13].
Herein it is shown that Cu and Cd can induce deleterious effects in C. gigas gametes. Cu pre-exposure did significantly decrease fertilization capacity of oyster spermatozoa at very low concentrations (1 µg.L−1) consistent with those measured in harbor environment. Sperm cells of Pacific oyster seem to be more sensitive to Cu than spermatozoa from other marine invertebrates such as blue mussel [13] and sea squirt [14] with LOEC at 100 µg.L−1 and 1,024 µg.L−1, respectively. In the present study, the deduced EC50 of Cu for oyster sperm is 20 µg.L−1. This EC50 value is very close to that reported for spermatozoa of various sea urchins [15]. Indeed, they reported an EC50 values of 25 µg.L−1 Cu for purple sea urchin and 59 µg.L−1 Cu for green sea urchin. Cd was less toxic to Pacific oyster sperm cells than Cu with significant adverse effects observed after 30- min Cd exposure at 100 µg.L−1 Cd. The Cd con-centration causing 50% unsuccessful fertilization on spermatozoa was 830 µg.L−1 in this study (Table 2), whereas EC50 values for several sea urchin species [15] ranged from 8000 to 26,000 µg.L−1. Unlike spermiotoxicity, eggs fertilization was only altered after exposure to high exposure concentrations: 10 µg.L−1 for Cu and 400 µg.L−1 for Cd. This is consistent with previous studies in which acute exposure of oocytes had no, or limited, effect on fertility [10,13,14]. A likely explanation is that the complex envelop of oocyte may act as a protective barrier against metals or other contaminants accumulation into eggs [14] As previously reported [9] Pacific oyster embryos are also very sensitive to Cu and Cd inducing embryotoxicity at 0.1 µg.L−1 for Cu and 10 µg.L−1 for Cd. The EC50 values for embryotoxicity reached 12 µg.L−1 for Cu and 210 µg.L−1 for Cd (Table 2). The results are in good agreement with existing data on EC50 values of embryotoxicity assays of those metals in other marine invertebrate species [2,15]. The results confirm previous studies [11] demonstrating that Pacific oyster embryos were more sensitive than sperm or oocytes to pollutant exposure. Gametes and embryos were relatively resistant to Cd toxicity compare to Cu. The impact of Cd has apparently no ecological relevance as the effects on oyster early life stage only appear at environmentally unrealistic concentrations. We can, however, expect that Cu could represent a threat for the reproduction of wild or cultivated Pacific oysters in particular in the Arcachon Bay where concentrations exceeding 0.7 µg L−1 are currently measured [16].
Acknowledgments
the authors would like to thank the French and Vietnamese Governments for PhD fellowship.
References
- His, E.; Beiras, R.; Seaman, M.N.L.; Southward, P.A.T.; Young, C.M. The assessment of marine pollution-bioassays with bivalve embryos and larvae. Adv Mar Biol 1999, 37, 1–178. [Google Scholar]
- Nadella, S.R.; Fitzpatrick, J.L.; Franklin, N.; Bucking, C.; Smith, S.; Wood, C.M. Toxicity of dissolved Cu, Zn, Ni and Cd to developing embryos of the blue mussel (Mytilus trossolus) and the protective effect of dissolved organic carbon. Comp Biochem Physiol C 2009, 149, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Bambang, Y.; Charmantier, G.; Thuet, P.; Trilles, J.P. Effect of cadmium on survival and osmoregulation of various developmental stages of the shrimp Penaeus japonicus (Crustacea: Decapoda). Mar Biol 1995, 123, 443–450. [Google Scholar] [CrossRef]
- Larrain, A.; Riveros, A.; Silva, J.; Bay-Schmith, E. Toxicity of metals and pesticides using the sperm cell bioassay with the Sea Urchin Arbacia spatuligera. Bull Environ Contam Toxicol 1999, 62, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Bowen, H.J.M. The cycles of copper, silver and gold. In The handbook of environmental chemistry, The Natural Environment and the Biogeochemical Cycles; Hutzinger, O., Ed.; Springer-Verlag: Berlin, Germany, 1985. [Google Scholar]
- Solomon, F. Impacts of copper on aquatic ecosystems and human health. Environ Commun 2009, 25–28. [Google Scholar]
- Wright, D.A.; Welbourn, P.M. Cadmium in the aquatic environment: a review of ecological, physiological, and toxicological effects on biota. Environ Rev 1994, 2, 187–214. [Google Scholar] [CrossRef]
- Beiras, R.; Albentosa, M. Inhibition of embryo development of the commercial bivalves Ruditapes decussatus and Mytilus galloprovincialis by trace metals; implications for the implementation of seawater quality criteria. Aquaculture 2004, 230, 205–13. [Google Scholar] [CrossRef]
- Mai, H.; Cachot, J.; Brune, J.; Geffard, O.; Belles, A.; Budzinski, H.; et al. Embryotoxic and genotoxic effects of heavy metals and pesticides on early life stages of Pacific oyster(Crassostrea gigas). Mar Pollut Bull 2012, 64, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Budzinski, H.; Pardon, P.; Gonzalez, P.; Morin, B.; Cachot, J. Environmental concentrations of irgarol, diuron and S-metolachlor induce deleterious effects on gametes and embryos of the Pacific oyster, Crassostrea gigas. Mar Environ Res, 2013; 89, 1–8. [Google Scholar]
- Warnau, M.; Temara, A.; Jangoux, M.; Dubois, P.; Iaccarino, M.; De Biase, A.; et al. Spermiotoxicity and embryotoxicity of heavy metals in the echinoid Paracentrotus lividus. Environ Toxicol Chem 1996, 15, 1931–1936. [Google Scholar] [CrossRef]
- Moschino, V.; Marin, M.G. Spermiotpxicity and embryotoxicity of triphenyltin in the sea urchin Paracentrotus lividus. Appl Organometal Chemi 2002, 16, 175–181. [Google Scholar] [CrossRef]
- Fitzpatrick, J.L.; Nadella, S.; Bucking, C.; Balshine, S.; Wood, C.M. The relative sensitivity of sperm, eggs and embryos to copper in the blue mussel (Mytilus trossulus). Comp Biochem Physiol C 2008, 147, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Vázquez, E.; Beiras, R. Toxicity of Hg, Cu, Cd, and Cr on early developmental stages of Ciona intestinalis (Chordata, Ascidiacea) with potential application in marine water quality assessment. Water Res 2001, 35, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Dinnel, P.A.; Link, J.M.; Stober, Q.J.; Letourneau, M.W.; Roberts, W.E. Comparative sensitivity of sea urchin sperm bioassays to metals and pesticides. Arch Environ Contam Toxicol 1989, 18, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Geffard, O.; Budzinski, H.; His, E. The effects of elutriates from PAH and heavy metal polluted sediments on Crassostrea gigas (Thunberg) embryogenesis, larval growth and bio-accumulation by the larvae of pollutants from sedimentary origin. Ecotoxicology 2002, 11, 403–416. [Google Scholar] [CrossRef] [PubMed]
© Copyright H. Mai et al., 2013. Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BYNC 3.0).