Assessment of Polychlorinated Biphenyls and Organochlorine Pesticides in Water Samples from the Yamuna River
Abstract
Introduction
Materials and Methods
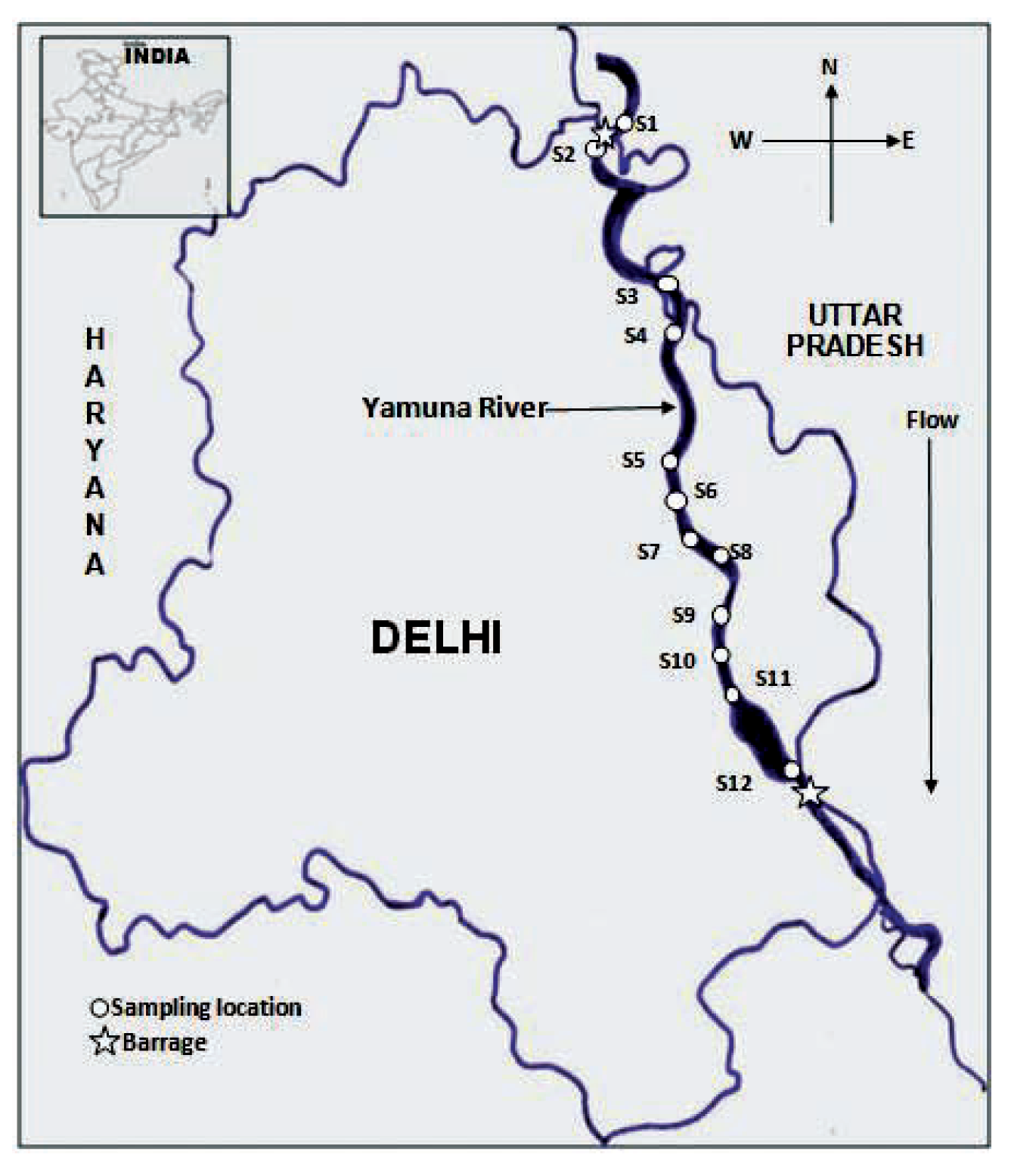
Description of study area
Sample collection
Chemicals and solvents
Sample extraction and cleanup
Instrumental analysis
Quality assurance/quality control
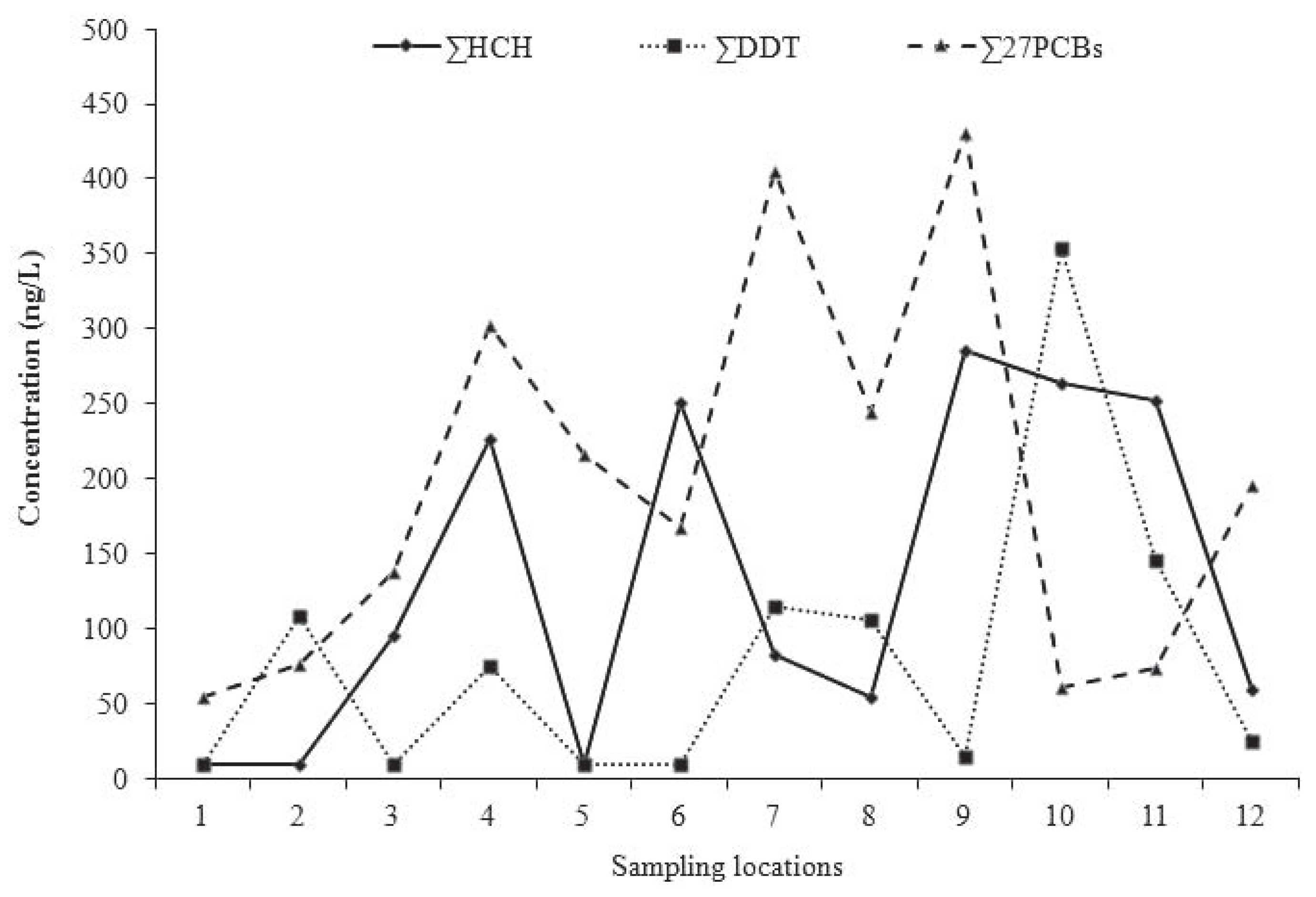
Results
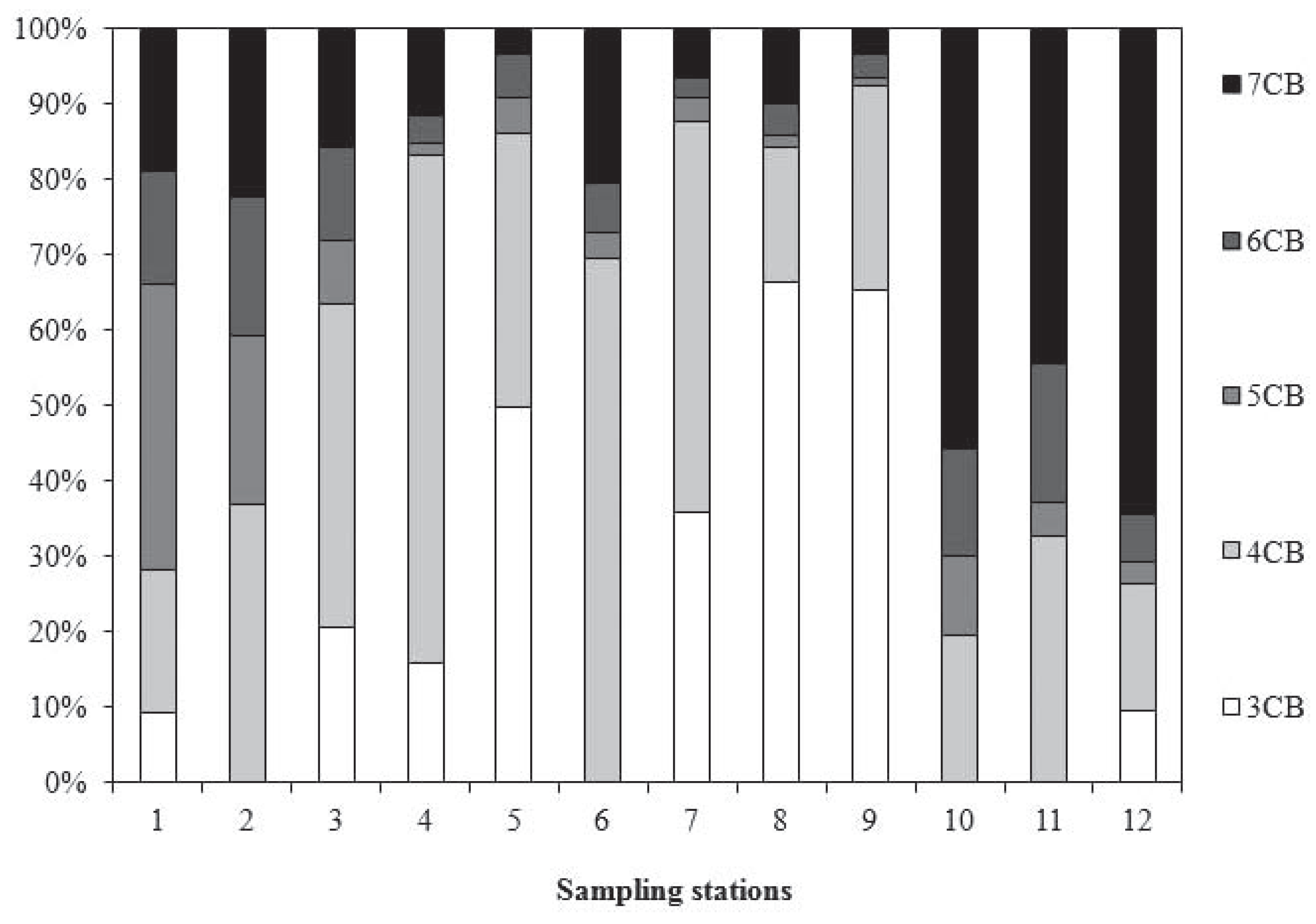
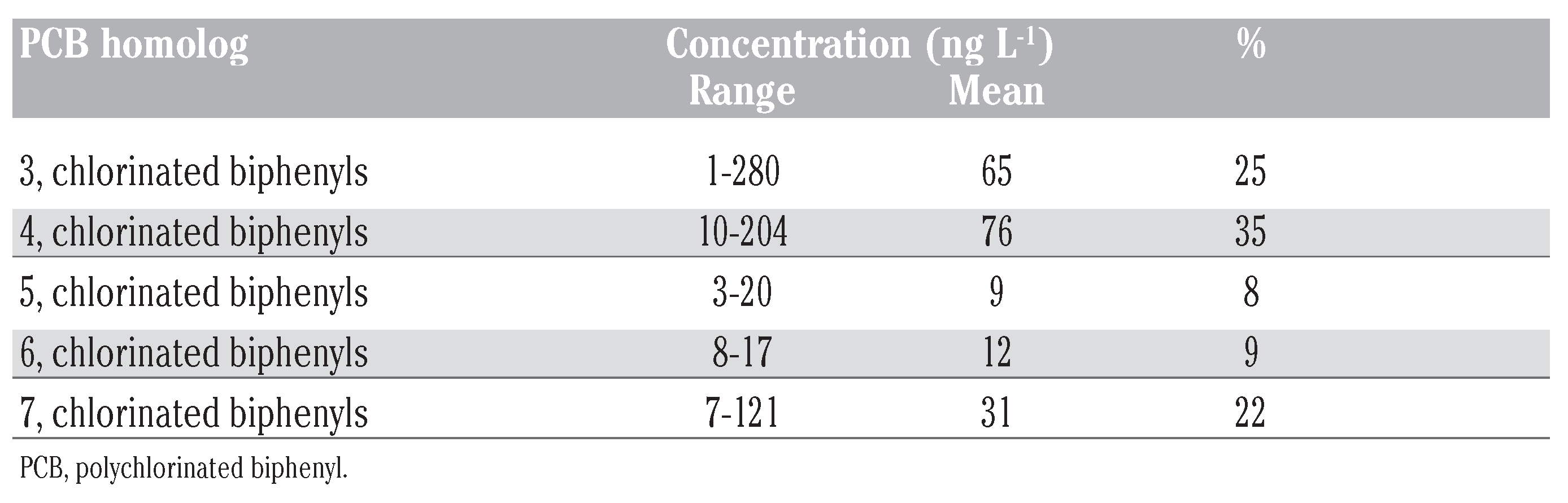
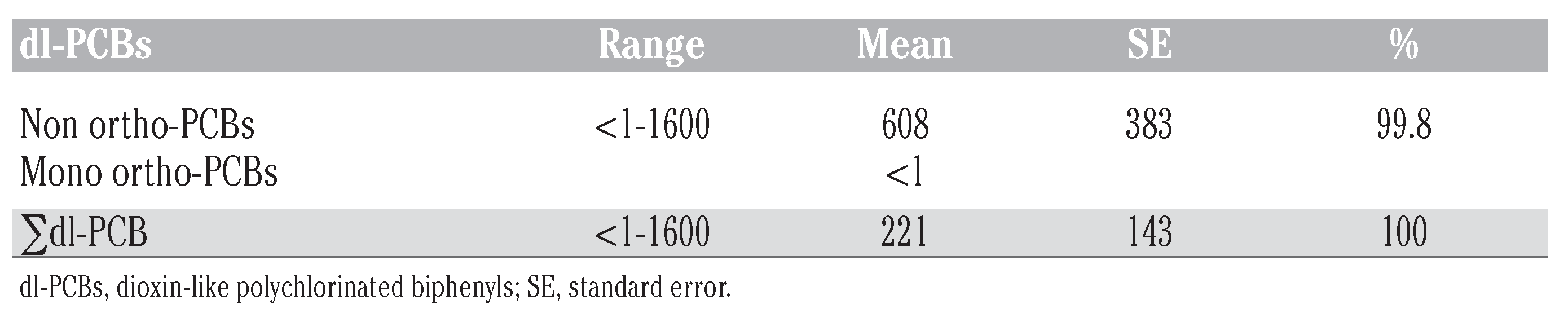
Polychlorinated biphenyls
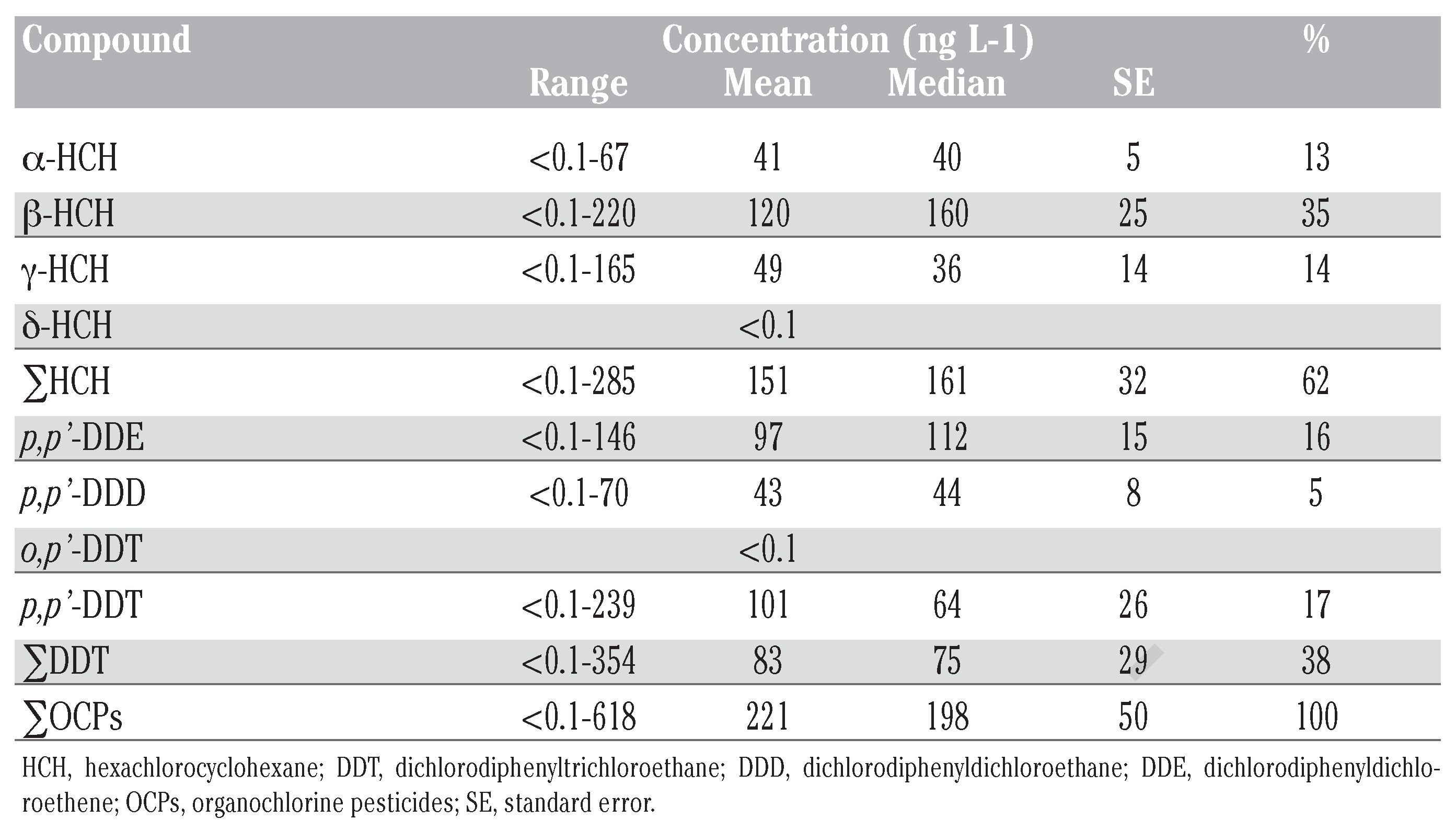
Organochlorine pesticides
Discussion
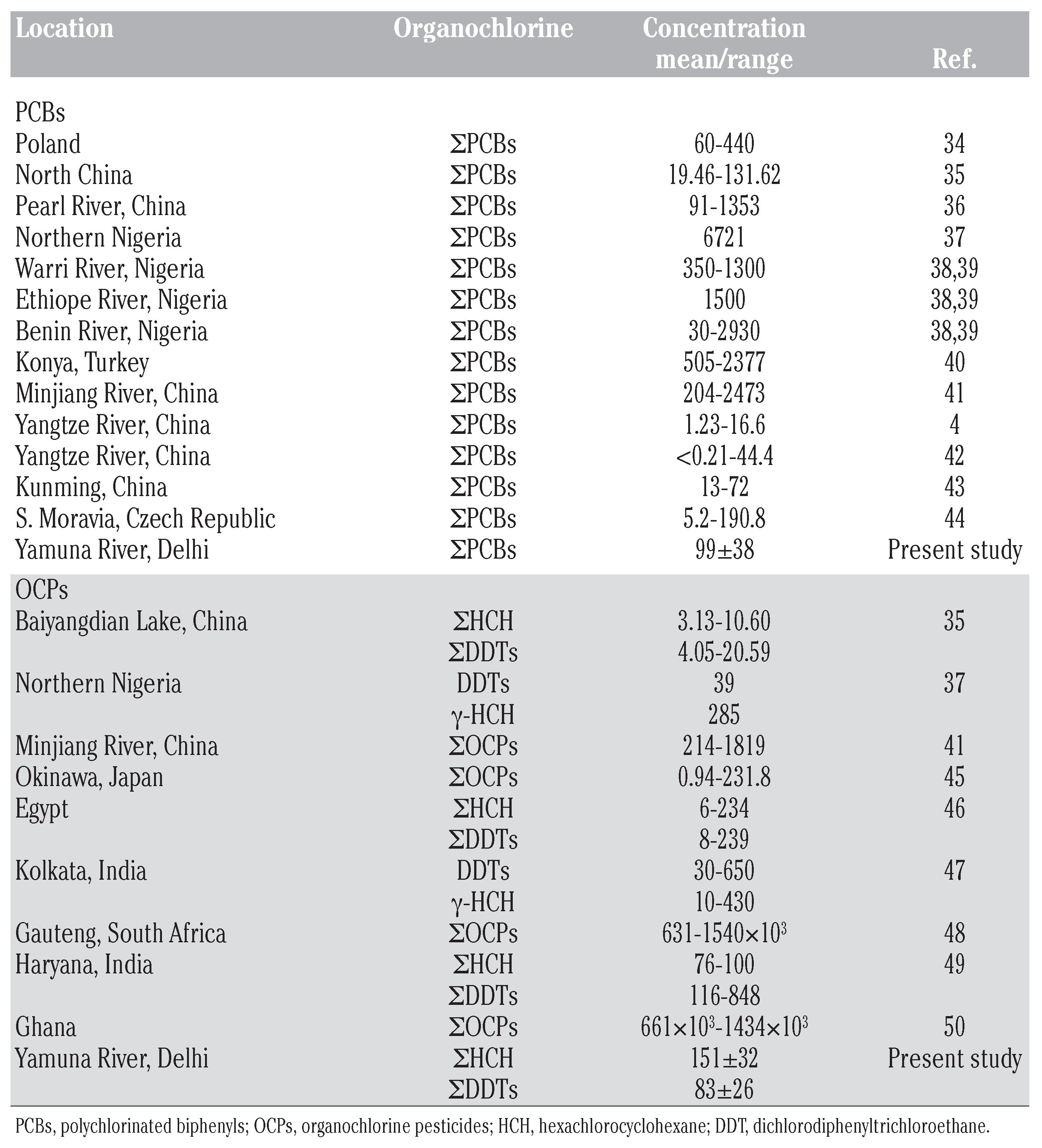
Polychlorinated biphenyls
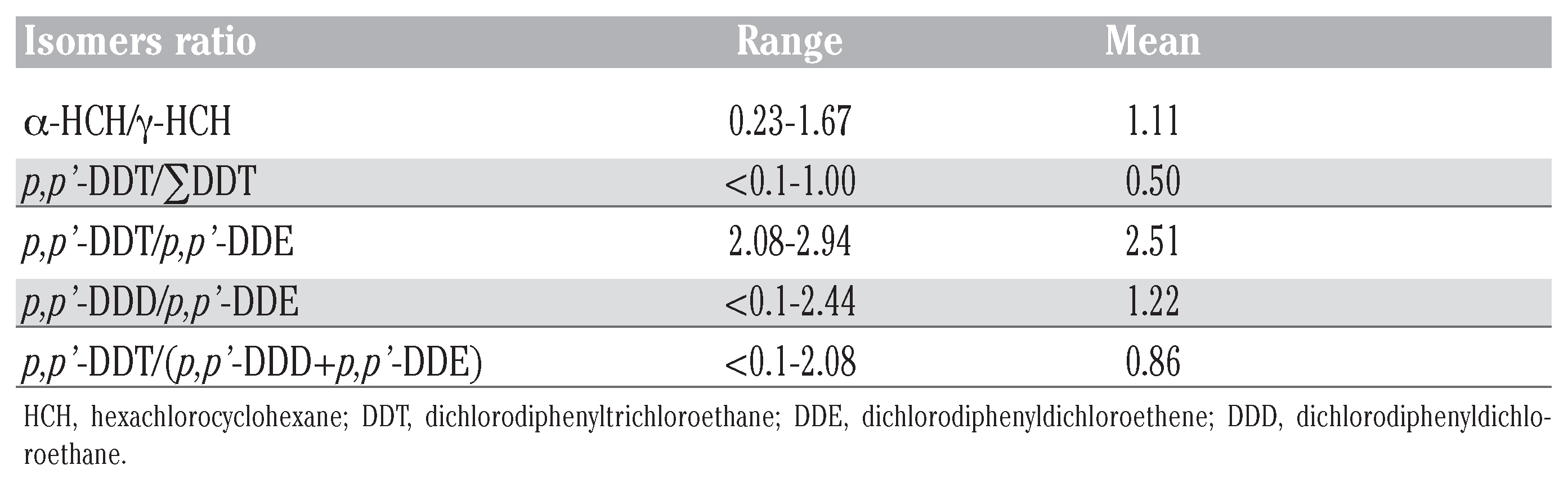
Organochlorine pesticides
Eco-toxicological risk assessment
Conclusions
Conflict of Interests
Author Contributions
Acknowledgments
References
- Atlas, E.; Giam, C.S. Ambient concentration and precipitation scavenging of atmospheric organic pollutants. Water Air Soil Pollut 1988, 38, 19–36. [Google Scholar] [CrossRef]
- Minh, N.H.; Minh, T.B.; Kajiwara, N.; Kunisue, T.; Subramanian, A.; Iwata, H.; et al. Contamination by persistent organic pollutants in dumping sites of Asian developing countries: implication of emerging pollution sources. Arch Environ Conta Toxicol 2006, 50, 474–81. [Google Scholar] [CrossRef]
- SC (Stockholm Convention). National Implementation Plan for POPs, India. www.pops.int/ Accessed: 30th January, 2012.
- Zhang, L.; Shi, S.; Dong, L.; Zhang, T.; Zhou, L.; Huang, Y. Concentrations and possible sources of polychlorinated biphenyls in the surface water of the Yangtze River Delta China. Chemosphere 2011, 85, 399–405. [Google Scholar] [CrossRef]
- Oehme, M. Further evidence for long-range air transport of polychlorinated aromates and pesticides: North America and Eurasia to the Arctic. Ambio 1991, 20, 293–7. [Google Scholar]
- George, J.L.; Frear, D.E.H. Pesticides in the Antarctic. J Appl Ecol 1966, 3, 155–67. [Google Scholar] [CrossRef]
- Hyun, P.; Lee, S.H.; Kim, M.; Kim, J.H.; Lim, H.S. Polychlorinated biphenyl congeners in soils and lichens from King George Island South Shetland Islands Antarctica. Antarctic Science 2010, 22, 31–8. [Google Scholar]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; et al. The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 2006, 93, 223–41. [Google Scholar] [CrossRef]
- Babu Rajendran, R.; Imagawa, T.; Taoa, H.; Ramesh, R. Distribution of PCBs HCHs and DDTs and their ecotoxicological implications in Bay of Bengal India. Environ International 2005, 31, 503–12. [Google Scholar] [CrossRef]
- Kumar, Sanjay; Kumar, B.; Sharma, C.S.; Makhijani, S.D.; Sengupta, B. Levels of polychlorinated biphenyl congeners in sea water and surface sediment of Alang ship breaking site Bhavnagar Gujrat India. Organohalogen Compounds 2008, 70, 19–22. [Google Scholar]
- Kumar, B.; Singh, S.K.; Kumar, S.; Sharma, C.S. Distribution of polychlorinated biphenyls in surface waters of various sources from national capital region Delhi India. J Nat Sci Res 2012, 2, 26–38. [Google Scholar]
- Kumar, B.; Gaur, R.; Goel, G.; Mishra, M.; Singh, S.K.; Prakash, D.; et al. Residues of pesticides and herbicides in soils from agriculture areas of Delhi Region India. J. Environ. Earth Sci 2011, 1, 1–8. [Google Scholar]
- Kumar, B.; Kumar, S.; Gaur, R.; Goel, G.; Mishra, M.; Singh, S.K.; et al. Persistent organochlorine pesticides and polychlorinated biphenyls in intensive agricultural soils from North India. Soil Water Res 2011, 6, 190–7. [Google Scholar] [CrossRef]
- Guzzella, L.; Roscioli, C.; Vigano, L.; Saha, M.; Sarkar, S.K.; Bhattacharyya, A. Evaluation of the concentration of HCH DDT HCB PCB and PAH in the sediments along the lower stretch of Hugli estuary West Bengal northeast India. Environ Int 2005, 31, 523–34. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, S.; Mishra, M.; Singh, S.K.; Sharma, C.S.; Makhijani, S.D.; et al. Distribution of pesticides herbicides synthetic pyrethroids and polychlorinated biphenyls in sediments from drains of Delhi India. Organohalogen Compounds 2008, 70, 1120–23. [Google Scholar]
- Kumar, B.; Mishra, M.; Goel, G.; et al. Distribution and ecotoxicological risk assessment of persistent organic pollutants (POPs) in river sediments from Delhi India. Adv Life Sci Technol 2011, 1, 1–8. [Google Scholar]
- Kumar, B.; Kumar, S.; Mishra, M.; Dev Prakash Singh, S.K.; Sharma, C.S.; et al. Persistent organochlorine pesticides in sediments from Haldi and Rupnarayan River in West Bengal India. Proc Indian Natl Sci Acad 2011, 77, 315–23. [Google Scholar]
- Zhang, G.; Chakraborty, P.; Li, J.; Sampathkumar, P.; Balasubramanian, T.; Kathiresan, K.; et al. Passive atmospheric sampling of organochlorine pesticides polychlorinated biphenyls and polybrominated diphenyl ethers in urban rural and wetland sites along the coastal length on India. Environ Sci Technol 2008, 42, 8218–23. [Google Scholar] [CrossRef]
- Chakraborty, P.; Zhang, G.; Li, J.; Xu, Y.; Liu, X.; Tanabe, S.; et al. Selected organochlorine pesticides in the atmosphere of major Indian cities: levels regional versus local variations and sources. Environ Sci Technol 2010, 44, 8038–43. [Google Scholar] [CrossRef]
- Pozo, K.; Harner, T.; Lee, S.C.; Sinha, R.K.; Sengupta, B.; Loewen, M.; et al. Assessing seasonal and spatial trends of persistent organic pollutants (POPs) in Indian agricultural regions using PUF disk passive air samplers. Environ Pollut 2011, 159, 646–53. [Google Scholar] [CrossRef]
- Devanathan, G.; Subramanian, A.; Someya, M.; Sundaryanto, A.; Isobe, T.; Takahashi, S.; et al. Persistent organochlorines in human breast milk from major metropolitan cities in India. Environ Pollut 2008, 157, 148–54. [Google Scholar] [CrossRef]
- Someya, M.; Ohtake, M.; Kunisue, T.; Subramanian, A.; Takahashi, S.; Chakraborty, P.; et al. Persistent organic pollutants in breast milk of mothers residing around an open dumpsite in Kolkata India: specific dioxin-like PCB levels and fish as a potential source. Environ Int 2009, 36, 27–35. [Google Scholar] [CrossRef]
- Sharma, M.P.; Singhal, S.K.; Patra, S. Water quality profile of Yamuna River India. Hydro Nepal 2008, 3, 1–6. [Google Scholar] [CrossRef]
- National Technical Information Service (NTIS) US Department of Commerce Port Royal Road Springfield VA 22161. Available from: http://wwwepagov/osw/hazard/testmethods/sw846/.
- You, H.; Ding, J.; Zhao, X.S.; Li, Y.F.; Liu, L.Y.; Ma, W.L.; et al. Spatial and seasonal variation of polychlorinated biphenyls in Songhua River China. Environ Geochem Health 2011, 33, 291–9. [Google Scholar] [CrossRef]
- Doong, R.A.; Peng, C.K.; Sun, Y.C.; Liao, P.L. Composition and distribution of organochlorine pesticide residues in surface sediments from the Wu-Shi River estuary Taiwan. Marine Pollut Bull 2002, 45, 246–53. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, L.; Yang, K.; Chen, Y. Distribution of organochlorine pesticides in surface water and sediments from Qiantang River East China. J Hazard Mater 2006, 137, 68–75. [Google Scholar] [CrossRef]
- Willet, K.L.; Ulrich, E.M.; Hites, H.A. Differential toxicity and environmental fates of Hexachlorocyclohexane isomers. Environ Sci Technol 1998, 32, 2197–207. [Google Scholar] [CrossRef]
- Guo, Y.; Yuh, Y.; Zheng, E.Y. Occurrence source diagnosis and biological effect assessment of DDT and its metabolites in various environmental compartments of the Pearl River Delta South China: A review. Environ Pollut 2009, 157, 1753–63. [Google Scholar] [CrossRef]
- Aislabie, J.M.; Richards, N.K.; Boul, H.L. Microbial degradation of DDT and its residues-a review. New Zeal J Agr Res 1997, 40, 269–82. [Google Scholar] [CrossRef]
- Tavares, T.M.; Beretta, M.; Costa, M.C. Ratio of DDT/DDE in the all Saints Bay Brazil and its use in environmental management. Chemosphere 1999, 38, 1445–52. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Ji, D.; Wang, T.; Wang, Y.; Wang, P.; et al. Levels and vertical distributions of PCBs PBDEs and OCPs in the atmosphere boundary layer: observation from the Beijing 325-m meteorological tower. Environ Sci Technol 2009, 43, 1030–5. [Google Scholar] [CrossRef]
- WHO (World Health Organization). DDT and its derivatives environmental aspects no. 83. Geneva: Environmental Health Criteria; 1989.
- Sulej, A.M.; Polkowska, Z.; Namiesnik, J. Contamination of runoff water at Gdansk airport (Poland) by polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs). Sensors 2011, 11, 11901–20. [Google Scholar] [CrossRef]
- Dai, G.; Liu, X.; Liang, G.; Han, X.; Shi, L.; Cheng, D.; et al. Distribution of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in surface water and sediments from Baiyangdian Lake in North China. J Environ Sci 2011, 23, 1640–9. [Google Scholar] [CrossRef]
- Chau, K.W. Characterization of transboundary POP contamination in aquatic ecosystems of Pearl River Delta. Marine Pollut Bulletin 2005, 51, 960–5. [Google Scholar] [CrossRef]
- Okeniyia, S.O.; Egwaikhideb, P.A.; Akporhonorc, E.E.; Obazed, I.E. Distribution of organochlorine and polychlorinated pesticide residue in water bodies of some rivers in Northern Nigeria. EJEAFChe 2009, 8, 1269–74. [Google Scholar]
- Ezemonye, L.I.N. Levels of polychlorinated biphenyls residues in Warri River Nigeria. Global J Environ Sci 2005, 4, 65–71. [Google Scholar] [CrossRef][Green Version]
- Ezemonye, L.I.N. Polychlorinated biphenyls (PCBs) levels and distribution in Ethiope and Benin Rivers of the Niger Delta Nigeria: surface water and sediments. Int J Environ Stud 2005, 62, 491–504. [Google Scholar] [CrossRef]
- Aydin, M.E.; Sari, S.; Özcan, S.; Wichmann, H.; Bahadir, M. Polychlorinated biphenyls in waste water of Konya-Turkey. Fresenius Environ Bull 2004, 13, 1090–93. [Google Scholar]
- Zhang, Z.L.; Hong, H.S.; Zhou, J.L.; Huang, J.; Yu, G. Fate and assessment of persistent organic pollutants in water and sediment from Minjiang River Estuary Southeast China. Chemosphere 2003, 52, 1423–30. [Google Scholar] [CrossRef]
- He, H.; Hu, G.J.; Sun, C.; Chen, S.; Yang, M.; Li, J.; et al. Trace analysis of persistent toxic substances in the main stream of Jiangsu section of the Yangtze River China. Environ Sci Pollut Res 2011, 18, 638–48. [Google Scholar] [CrossRef]
- Wan, X.; Pan, X.; Wang, B.; Zhao, S.; Hu, P.; Li, F.; et al. Distributions historical trends and source investigation of polychlorinated biphenyls in Dianchi Lake China. Chemosphere 2011, 85, 361–7. [Google Scholar] [CrossRef]
- Lana, R.; Vavrova, M.; Caslavsky, J.; Skoumalova, M.; Bilkova, A.; Sucman, E. PCBs in samples from the environment of the southern Moravia region Czech Republic. Bull Environ Contam Toxicol 2008, 81, 574–7. [Google Scholar] [CrossRef]
- Imo, S.T.; Sheikh, M.A.; Hirosawa, E.; Oomori, T.; Tamaki, F. Contamination by organochlorine pesticides from rivers. Int J Environ Sci Tech 2007, 4, 1–9. [Google Scholar] [CrossRef]
- Essumang, D.K.; Togoh, G.K.; Chokky, L. Pesticide residues in the water and fish (lagoon tilapia) samples from lagoons in Ghana. Bull Chem Soc Ethiop 2009, 23, 19–27. [Google Scholar] [CrossRef]
- Ghose, N.C.; Saha, D.; Gupta, A. Synthetic Detergents (surfactants) and organochlorine pesticide signatures in surface water and groundwater of greater Kolkata India. J Water Res Prot 2009, 4, 290–8. [Google Scholar] [CrossRef]
- Sibali, L.S.; Okwonkwo, J.O.; McCrindle, R.I. Determination of selected organochlorine pesticide (OCP) compounds from the Jukskei River catchment area in Gauteng South Africa. Water 2008, 34, 611–21. [Google Scholar] [CrossRef]
- Kaushik, C.P.; Sharma, H.R.; Kaushik, A. Organochlorine pesticide residues in drinking water in the rural areas of Haryana India. Environ Monit Assess 2012, 184, 103–12. [Google Scholar] [CrossRef]
- El Bouraie, M.; El Barbary, A.; Yehia, M. Monitoring of chlorinated hydrocarbon compounds residues in surface water and bed sediment samples from ElRahawy drain, Egypt. Int J Environ Sci 2011, 1, 1931–47. [Google Scholar]
- Mutiyar, P.K.; Mittal, A.K.; Pekdeger, A. Status of organochlorine pesticides in the drinking water well-field located in the Delhi region of the flood plains of river Yamuna. Drink Water Eng Sci Discuss 2011, 4, 85–115. [Google Scholar] [CrossRef]
- Pandey, P.; Khillare, P.S.; Kumar, K. Assessment of organochlorine pesticide residues in the surface sediments of river Yamuna in Delhi India. J Environ Protect 2011, 2, 511–24. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration (NOAA). NOAA screening quick reference tables (SQuiRTs). HAZMAT REPORT 1999-1. Seattle, Washington: National Oceanic and Atmospheric Administration; 2004.
- New Jersey Department of Environment. NJDEP Ecological Screening Criteria Table Updated 2009. Available from: http://www. nj.gov/dep/srp/guidance/ecoscreening.

© Copyright B. Kumar et al., 2012 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).
Share and Cite
Kumar, B.; Singh, S.K.; Mishra, M.; Kumar, S.; Sharma, C.S. Assessment of Polychlorinated Biphenyls and Organochlorine Pesticides in Water Samples from the Yamuna River. J. Xenobiot. 2012, 2, e6. https://doi.org/10.4081/xeno.2012.e6
Kumar B, Singh SK, Mishra M, Kumar S, Sharma CS. Assessment of Polychlorinated Biphenyls and Organochlorine Pesticides in Water Samples from the Yamuna River. Journal of Xenobiotics. 2012; 2(1):e6. https://doi.org/10.4081/xeno.2012.e6
Chicago/Turabian StyleKumar, Bhupander, Satish Kumar Singh, Meenu Mishra, Sanjay Kumar, and Chandra Shekhar Sharma. 2012. "Assessment of Polychlorinated Biphenyls and Organochlorine Pesticides in Water Samples from the Yamuna River" Journal of Xenobiotics 2, no. 1: e6. https://doi.org/10.4081/xeno.2012.e6
APA StyleKumar, B., Singh, S. K., Mishra, M., Kumar, S., & Sharma, C. S. (2012). Assessment of Polychlorinated Biphenyls and Organochlorine Pesticides in Water Samples from the Yamuna River. Journal of Xenobiotics, 2(1), e6. https://doi.org/10.4081/xeno.2012.e6




