Abstract
The sublethal toxicity of diazinon to the adult African toad, Bufo regularis was assessed using an integration of biomarkers. Changes in acetylcholinesterase (AChE), corticosterone and total protein levels were assessed in the serum, brain, liver, lungs and gastrointestinal tract (GIT) and the results supported by bioaccumulation data. The biomarkers were chosen as indicators of key physiological functions: AChE for neurotoxicity, corticosterone and total protein levels as indicators of oxidative stress. Toads were exposed to 0.01, 0.02, 0.03 and 0.04 g/L for 28 days. Brain AChE activity reduced by 96% in the highest concentration (0.04 g/L) compared to the control brain. Similarly, AChE activities in serum, liver, lungs and GIT tissues (88%, 88%, 87, 87% umg−1 protein respectively) were also inhibited in the toads. Corticosterone and total protein levels in the tissues decreased compared to the control. The accumulation results obtained showed accumulation in the tissues (liver>serum>brain>lung>GIT), with a direct relationship between tissue concentration and changes in the biochemical indices. The alterations in all the indices were significantly concentration dependent. The biomarkers described in this study could be useful complementary indices in the risk assessment of diazinon pesticide.
Introduction
Diazinon is one of the most widely used organophosphate insecticides throughout the world.[1] It is a non systemic organophosphate insecticide used to control a wide range of sucking and chewing insects and mites on a range of food crops. It is also used to control agricultural soil-dwelling insects, and is applied as a sheep dip to control ectoparasites.[1] However, like other organophosphate pesti-cides, they have been found to cause irrepara-ble damage to environmental health.[2] Historical pesticide use has been implicated asa potential factor for declines for multiple amphibian species. [3,4,5,6,7,8] Much of the interest on amphibian’s declines is currently focused on the role of pesticides on the observed global declines.[3,9,10,11,12] Concentrations of diazinon pesti-cide typically found in Nigerian waters range from 0.01-3.64, which are above the ecological benchmark (0.01 µg/L) recommended by Nigerian Environmental Protection agency.[13]
The effects of diazinon on adult amphibians are limited. Most studies are centered basically on tadpoles.[11,12,14] Diazinon destroys the delicate balance between species that characterize a functioning ecosystem. It has been reported to affect survival and growth in tadpoles,[11,14] depress cholinesterase (ChE) activity in tadpoles[12] and it also produces many biochemical changes in freshwater organisms especially amphibians by influencing the activities of several enzymes.[4] All biological responses are initially expressed at the molecular or sub cellular level[15] and the levels of response to chemical exposure are believed to be the most sensitive and earliest detectable sub organisimal response. The use of biomarkers, indices of biological stress is widespread within the field of ecotoxicology and they give information about the bioavailability (exposure), the mechanism of action of the pollutant (effects) and the susceptibility of the response to different contaminants.[16] The specific objective of this study was to integrate an array of biomarkers: acetylcholinesterase (AChE), corticosterone and total protein levels to assess the sublethal effect of diazinon a commonly used organophosphate pesticide on the dominant African toad, Bufo regularis, with a view of providing suitable biomarkers for the evaluation of the toxic effect of diazinon pesticide.
Materials and Methods
Collection of test organisms
Adult toads, Bufo regularis of both sexes were collected from their spawning ponds in unpolluted areas. It was ensured that that the toads were not collected from agricultural sites.[17] Toads samples were collected by hand net from their spawning ponds and transported in a covered basket to the Laboratory. Adult toads of the same size and almost the same weight (32.87g±0.03) were acclimatized in glass tanks (51×32×33 cm) containing 2 L of dechlorinated tap water, for 7 days prior to the experiments.[18] Tanks were placed on a slant to provide the option of both aqueous and dry environment.[17] Water was changed every 2 days and the tank cleaned thoroughly. Toads were fed earthworms twice weekly. Uneaten earthworms and fecal wastes were removed and water replenished regularly.[17]
Sublethal bioassay
The pesticide used for the sublethal toxicity tests was the organophosphate, Diazinon, commonly used in agricultural farms in Nigeria for the control of insects on food crops.[19] Water for toxicity testes was dechlorinated tap water. The water was dechlorinated by allowing it to stand exposed for 36 h[20] and used for acclimatization, control tests and for making the various concentrations of the test chemical. During the exposure periods for the bioassay, temperature, pH, dissolved oxygen, conductivity, turbidity and alkalinity were determined using standard methods.[21]
The bioassay procedures started with a range finding test.[22] This was used to determine the range of concentrations to be tested and the approximate range that would produce the desired effective concentrations for the definitive test. For the definitive test, stock solutions of the required concentrations were prepared. The stock was then diluted serially into environmental relevant treatment concentrations of 0.01, 0.02, 0.03 and 0.04 g/L. Two replicates per test concentration were used to avoid test repetition and to provide a stronger statistical baseline. Each test chamber contained an equal volume of test solution (2 L) and equal number of toads.[10] Replicate test chambers were physically separated.
Biochemical analysis
Two toads each for each biomarker analysiswere removed from the tanks after 28 days exposure. Brain, liver, lungs and gastrointestinal tract (GIT) were removed and weighed. Isolated tissues were frozen to -18°C for subsequent biochemical analysis. Blood samples were collected by cardiac puncture, using 2 mL hypodermic syringe. Serum was collected from whole blood samples by allowing the tubes containing whole blood samples stand at ambient temperature for 1-2 h in an upright position to let the clot begin to contract. The clot was then removed by decanting into fresh tubes and samples were centrifuged for 10 min and the clear serum removed. Two mL of serum was used.
Determination of acetylcholineste-raseactivity
AChE activity was assayed spectrophometrically according to Ellman et al.[23] Frozen samples of tissues were defrosted on ice. Tissues (serum, brain, liver, lungs and GIT) were homogenized in ice-cold phosphate buffer (0.1 M), pH 6.5 using a politron homogenizer (Ystral, D-79282, Ballrechten-Dottingen, Germany). Analysis was conducted using the supernatants following centrifugation of the homogenate at 9000 rpm for 30 min. Supernatants were stored at -80°C until analysis. Enzymatic assays were performed at 25°C. The substrate acetylthiocholine iodide was added to the reaction mixture containing the homogenate and 5, 5’, -dithio-bis-2-nitrobenzoic acid. Activity was expressed as mg−1 protein. Each unit of activity corresponds to 1 nmol of substrate hydrolyzed per minute.
Determination of corticosterone
Corticosterone levels in the tissues were estimated by the method as described by Barseghian et al.[24] Individual tissues were incubated in 2 M Soerensen buffer, pH 7.6, for 16 h at 40°C. Corticosterone-d3 was used as internal standard. Purification of the incubation medium was achieved on SPE C18 isolute extraction columns followed by an alkaline liquid-liquid extraction with diethylether. The eluate was evaporated to dryness and resuspended in 25 μL of acetonitrile/ammonium formiate. The chromatography was operated on a LC Packings Superba Nucleosil C18 column using a linear gradient of acetonitrile from 30% to 70% in 10 min. The corticosterone standard (corticosterone-d3) was then injected into the chromatograph to determine the retention time. A series of concentrations ranging from 0.025 mg/L to 25 mg/L were then injected. The resulting peak areas were plotted against concentrations to determine the linearity of detector response to the standard. The extract was then loaded and injected into the valve of the chromatography system. The resulting chromatogram for each sample was printed out. The retention times and concentrations were recorded.
Determination of total protein
Total protein was estimated according to the methods described by Lowry et al.[25] Two mL of homogenate was added to 2 mL of alkaline copper sulphate solution. The whole mixture was allowed to stand for 10 min and 0.5 mL of Folin-Wu reagent was added and mixed in a vortex mixture. The mixture was kept for 30 min and final optical density was measured using a spectrophotometer at 740 nm.
Bioaccumulation
Toads were weighed and killed by decapitation and the brain, liver, lungs and GIT were removed from the animal and weighed. Isolated tissues were frozen to -18°C.[26] Deep frozen tissues were ground while still partially frozen, as this makes the tissue more brittle. Extraction, purification of extracts and analysis were done according to the method described by Harri et al.,[8] and Steinwandter.[26] Serum was collected in the same way as for the biochemical analysis and 2 mL of serum was used.
Statistical analysis
The susceptibility of the adult toads to diazinon pesticide were determined using Students’t-test, Pearson correlation and one-way analysis of variance SPSS (14.0 version) (SPSS Inc, Chicago, IL, USA) to test the variable at P<0.05 level of significance. Multiple bar graphs and line graphs were also used in this study for the pictorial representation of assessment endpoints.
Results
Biochemical indices
The results of the biochemical indices (AChE, corticosterone and total proteins) are shown in Table 1, Table 2 and Table 3 and in Figure 1, Figure 2 and Figure 3.

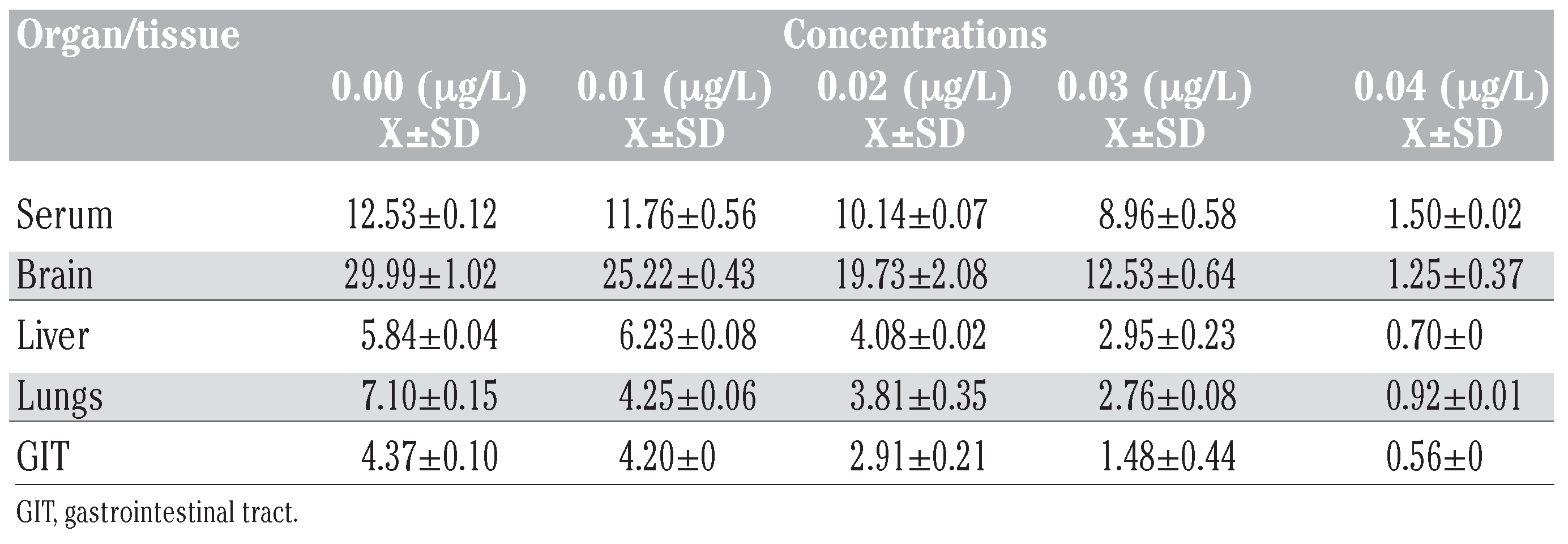
Table 1.
Acetylcholinesterase activities (umg–1 protein) in tissues of Bufo regularis exposed to diazinon for 28 days (mean±SD).

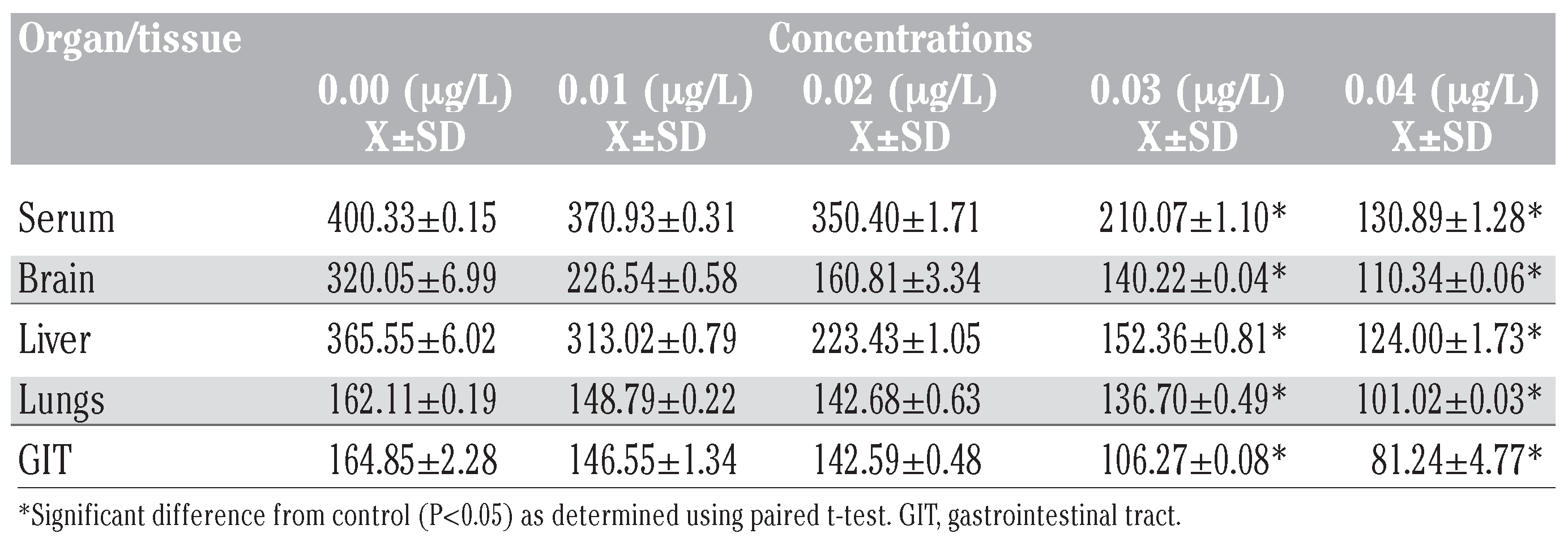
Table 2.
Corticosterone levels (ng/mL) in tissues of Bufo regularis exposed to diazinon for 28 days (mean±SD).

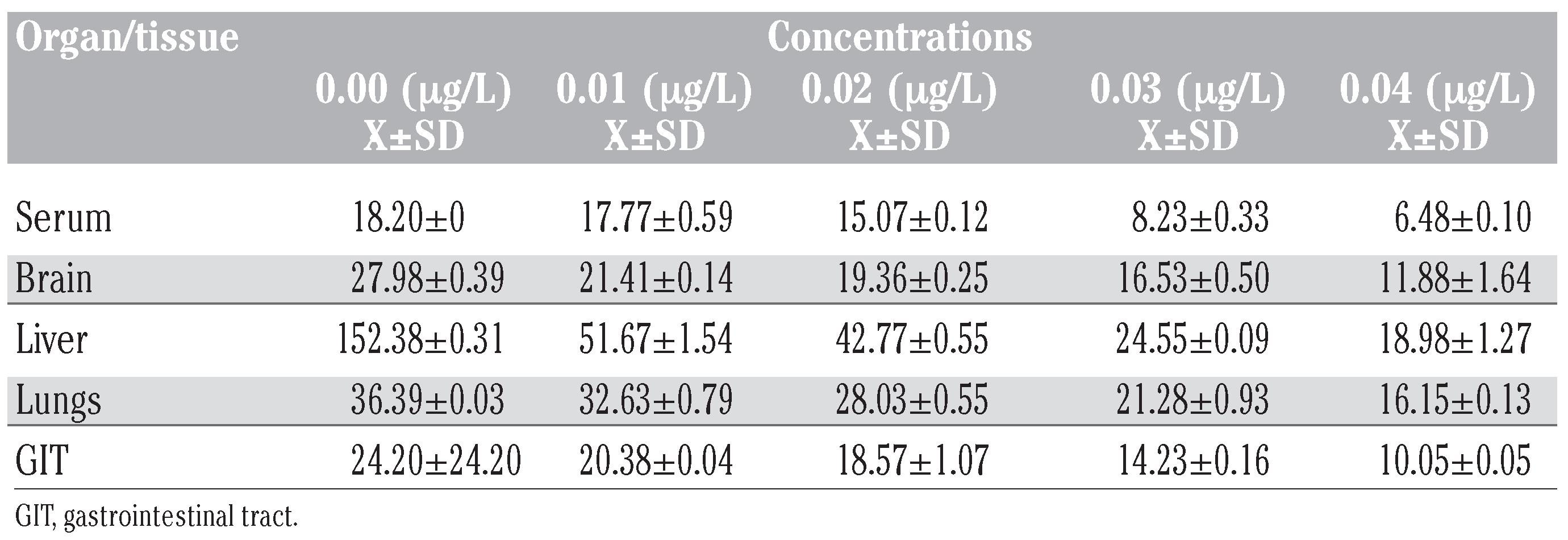
Table 3.
Total protein levels (mg/100 mL) in tissues of Bufo regularis exposed to diazinon for 28 days (mean±SD).
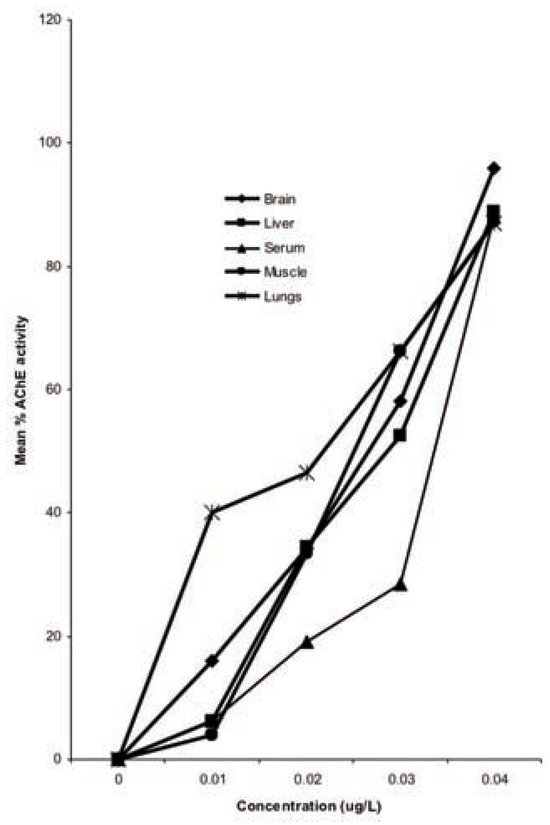
Figure 1.
Percentage reduction in acetyl-cholinesterase activity in tissues of Bufo regularis exposed to diazinon for 28 days.
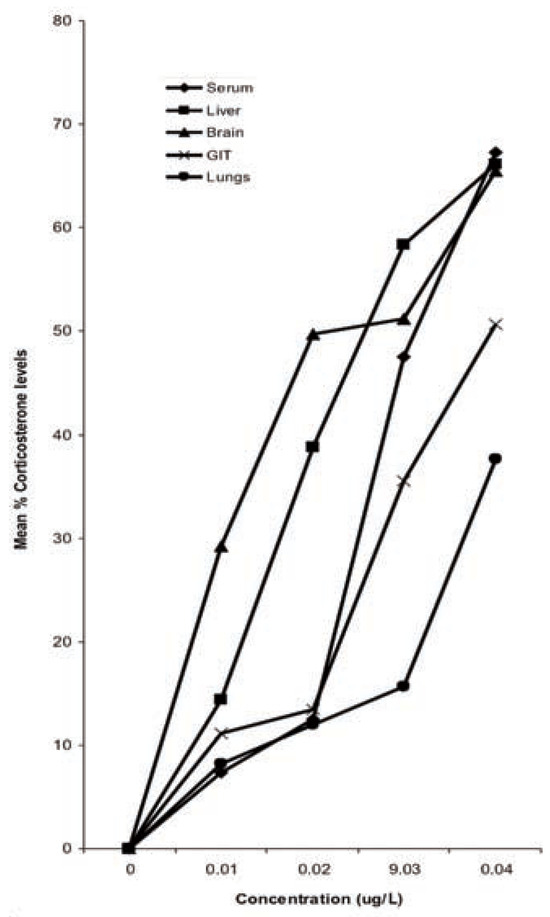
Figure 2.
Percentage reduction in corticos-terone levels in organ/tissues of Bufo regu-laris exposed to diazinon for 28 days.
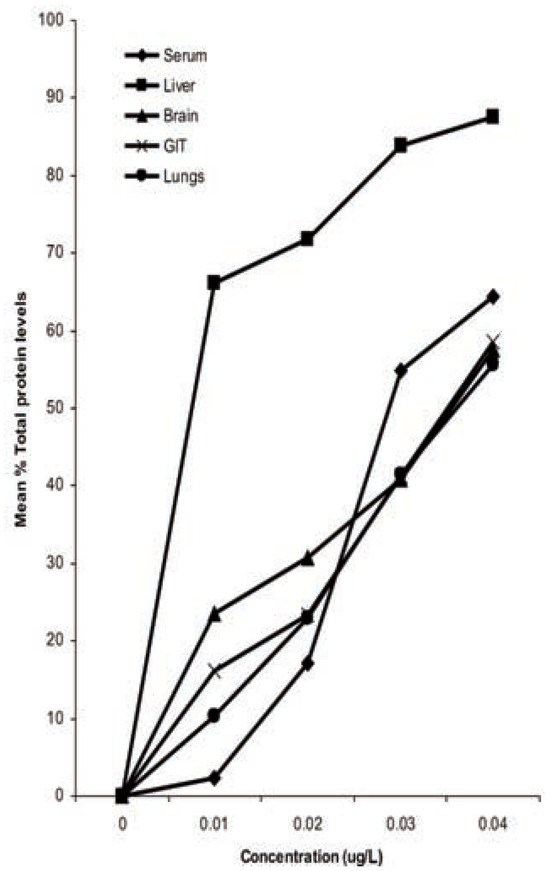
Figure 3.
Percentage reduction in total pro-tein levels in organ/tissues of Bufo regularis exposed to diazinon for 28 days.
The results of the effect of diazinon pesticide on the biochemical indices generally showed a reduction. The results showed an inhibition in AChE activities in the serum, brain, liver, lungs and GIT tissues (Table 1). AChE activity in exposed toads deceased with increase in concentration. The differential inhibition was in the sequence brain>serum>liver>GIT>lungs (Figure 1). There was a significant 96% reduction in brain AChE activity compared with the control in the highest concentration (0.04 g/L). Similar results were also observed for serum (88%), liver (89%), lungs (87%) and GIT (87%) tissues (Figure 1).
Results of corticosterone levels in toads exposed to diazinon showed a decrease compared to the control in all the tissues (Table 2). The percentage reduction was in the sequence serum>liver>brain>GIT>lungs (Figure 2). More pronounced effects were also observed at higher concentrations. The control values for corticosterone were significantly higher (P<0.05) than in the concentrations except in 0.01 g/L and 0.02 g/L (P>0.05) (Table 2). There was also significant difference in corticosterone levels between the tissues (P<0.05, F=3.74).
Total protein levels in toads exposed to diazinon showed that the administration of the pesticide caused reduction in total protein levels in all the tissues studied (Table 3). Total protein levels in toads exposed to diazinon pes-ticide decreased with increase in concentration. The differential reduction in total protein levels was in the sequence liver>serum>GIT> brain>lungs (Figure 3).
Bioaccumulation
Chromatographic analysis showed that diazinon pesticide accumulated in the serum, brain, liver lungs and GIT tissues examined (Figure 4). No pesticide residue was observed in the control. Differences in accumulation were found between the tissues, in the sequence liver>serum>brain>lungs>GIT. The accumulation clearly followed the fat concentration of the tissues. Diazinon levels in the various concentrations of 0.01, 0.02, 0.03 and 0.04 g/L in the serum, brain, liver, lungs and GIT tissues at 28 days were 0.014, 0.036, 0.052, 0.085 ppm (serum), 0.013, 0.034, 0.054, 0.080 mg/g-1 (brain), 0.017, 0.045, 0.071, 0.099 mg/g-1 (liver), 0.005, 0.020, 0.033, 0.057 mg/g-1 (lungs) and 0.004, 0.007, 0.018, 0.026 mg/g-1 (GIT). There was however no significant difference (P>0.05, F=1.10) in accumulation of diazinon in the different tissues.
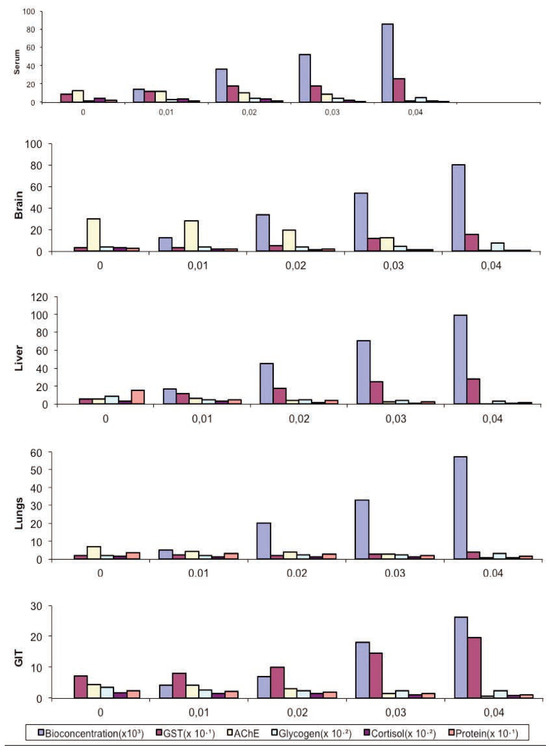
Figure 4.
Toxicity comparisons between bioaccumulation and alteration in biochemical indices of adult Bufo regularis exposed to diazinon pesticide.
Relative correlation between bioaccumulation and alteration in biochemical indices
There was a direct relationship between tissue concentration of the pesticide (diazinon) and changes in the biochemical indices (Figure 4). Significant negative correlations between mean tissue concentration and the indices were observed for all the tissues sampled; AChE (P=0.05, r=-0.9), corticosterone levels (P=0.01, r=-1).
Discussion
AChE is the target of many organophosphate and carbamate pesticides. They block the function of AChE and thus cause excessive acetylcholine to accumulate in the synaptic cleft.[27] The changes in AChE activity in the serum, brain, liver, lungs and GIT tissues of diazinon exposed toads decreased significantly. The differential inhibition of AChE activity in the five tissues (brain>serum>liver>GIT>lungs) may be due to the presence of isozymes with different affinities for the substrate and the inhibitor. Further more, the pesticide itself may bepresent in different amounts in the different tissues producing differential inhibition or the inhibitor may be metabolized at different rates.[28] It was observed that the AChE activity in all the tissue examined decreased after treatment. Amphibians are known to be vulnerable to pesticides that are cholinesterase inhibitors.[29] Pesticides, especially organophosphates have been reported to have reduced enzyme activity of ChE in amphibians.[30,31,32,33,34] Results showed that diazinon caused inhibition of brain ChE activity in adult B. regularis at sub-lethal and environmentally realistic concentrations. The brain response was more sensitive to diazinon than other tissues and may be useful as a diagnostic tool in detecting diazinon exposure in B. regularis. The AChE inhibition in this study correlated with tissue concentrations (Figure 4). Hence, it may be inferred that the higher the tissue concentration the higher the inhibition susceptibility.
Corticosterone levels maintain blood glucose levels, liver glycogen reserves and controls osmotic balance.[35,36] Stress response often has a hormonal basis and alterations in the levels of the hormones involved can be used as a biomarker.[37] Corticosterone is a steroid hormone associated with immunosuppression, growth retardation and susceptibility to disease.[38] Measurement of corticosterone levels is useful in diagnosing conditions related to functions of the adrenal cortex. There are however limited data on effects of pesticides on corticosterone levels of amphibians. However, studies by Hayes et al.,[3] and Hopkins et al.,[39] showed increased circulating levels of corticosterone which is consistent with this study.For fish studies, alterations in corticosterone levels in exposed organisms to pesticide have been observed.[36,38,40,41,42] In the present study, corticosterone levels in the five tissues of B. regularis exposed to diazinon pesticide were significantly lower than the control. The level of inhibition also positively correlated with the tissues residue. The reduction in corticosterone levels might have resulted from the impairment of corticosterone secretion by the adrenocortical cells. The results from this study corroborated previous works of Lacroix and Hontela,[43] Regoli and Principato,[44] and Bachowski et al.[45] who reported an inhibition of corticosterone secretion after in vitro exposure to pesticides.
The response of B. regularis to diazinon pesticide revealed a reduction in protein levels, indicating pesticide-induced changes in the biochemical systems. In various species, proteins are of importance as structural compounds, biocatalysts and hormones for control of growth and differentiations.[46] So the variation in amphibian proteins could be used as bioindicator for monitoring the physiological status of the treated toads. Proteins play an important role in the life of all living organisms. Pesticides disturb protein synthesis. Under stress many organisms will mobilize proteins as an energy source through the oxidation of amino acids.[46] Decreased protein level observed in this study may be attributed to stress mediated immobilization of these compounds to fulfill an increased element for energy by the organism to cope with environmental condition caused by the toxicant.[47] The depletion in total protein content may also be due to augmented proteolysis and possible utilization of their product for metabolic purposes as reported by Ravinder and Suryanarayana.[48] Neff [49] has opined that decline in protein content may be related to impaired food intake, increased energy cost of homeostasis, tissue repair and detoxification mechanism during stress. It is therefore logical to presume that in the case of prolonged and continued exposure to the pesticide, the deleterious effects of these pesticides on protein synthesis accounts for the progressive reduction in the concentration of total protein in the tissues.[50]
Conclusions
In conclusion, the importance of integrating biomarkers to assess the effect of diazinon on B. regularis has been confirmed by the results showing significant reduction in AChE, corticosterone and total protein levels. The study showed the significance of integrating biochemical indices in assessing diazinon toxicity to amphibians. It is therefore recommended that these biochemical endpoints using these biomarkers be used as complementary indices in assessing the toxicity of diazinon.
Author Contributions
the authors contributed equally.
Acknowledgments
the authors would like to thank the University of Benin, Benin City, Nigeria for its support.
References
- US Environmental Protection Agency US EPA. Office of Prevention, Pesticides and Toxic Substances. 2000. [Google Scholar]
- WHO. Environmental Health Criteria 198 - Diazinon. International Programme on Chemical Safety, World Health Organization: Geneva, 1998. [Google Scholar]
- Hayes, T.B.; Case, P.; Chui, S.; Chung, D.; Haeffele, C.; Haston, K.; et al. Pesticide mixtures, endocrinedisruption, and amphibian declines: are we underestimating the impact? Environ Health Perspect 2006, 114, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Law, F.C. Adverse effects of pesticides and related chemicals on enzyme and hormone systems of fish, amphibians and reptiles: a review. Proc Pakistan Acad Sci 2005, 42, 315–23. [Google Scholar]
- Sih, A.; Kerby, J.; Bell, A.; Relyea, R. Response to Schmidt. Pesticides, mortality and population growth rate. Trends Ecol Evol 2004, 19, 460–1. [Google Scholar] [CrossRef]
- Sparling, D.W.; Linder, G.; Bishop, C.A. Ecotoxicology of amphibians and reptiles. Pensocila, FL: Society of Environmental Toxicology and Chemistry SETAC; 2000.p. 904.
- Blaustein, A.R.; Wake, D.B.; Sousa, W.P. Amphibian declines: judging stability, persistence, and susceptibility of populations to local and global extinctions. Conserv Biol 1994, 8, 60–71. [Google Scholar] [CrossRef]
- Harri, M.N.E.; Laitinen, J.; Valkama, E.L. Toxicity and retention of DDT in adult frogs, Rana temporaria L. Envir Pollut 1979, 201, 45–55. [Google Scholar] [CrossRef]
- Sparling, D.W.; Cowman, D.F. Amphibians and pesticides in pristine areas. In Amphibiandecline: an integrated analysis of multiple stressor effects; Linder, G., Krest, S.K. and Sparling, D.W., Eds.; Society of Environmental Toxicology and Chemistry SETAC: Pensacola, FL, USA, 2003; pp. 257–264. [Google Scholar]
- Houlahan, J.E.; Fridlay, C.S.; Schmidt, B.R.; Mayers, A.H.; Kuzmin, S.L. Quantitative evidence for global amphibian population declines. Nature 2001, 404, 752–5. [Google Scholar] [CrossRef]
- Relyea, A.R. Growth and survival of five amphibian species exposed to combinations of pesticides. Envir Toxicol Chem 2004, 23, 1737–42. [Google Scholar] [CrossRef]
- Cowman, D.F.; Sparling, D.W.; Fellers, G.M.; Lacher, T.E. Cholinesterase inhibition in metamorphs and tadpoles in the Sierra Nevada Mountains,California. 26th Annual Meeting, SETAC, 13-17 November, Baltimore, MD. Platform. 2005.
- Ezemonye, L.I.N.; Ikepsu, T.O.; Ilechie, I. Distridution of diazinon in water, sediment and fish from Warri River, Niger Delta, Nigeria. Jordan J Biol Sci 2008, 1, 31–7. [Google Scholar]
- Sumanadasa, D.M.; Wijesinghe, M.R.; Ratnasooriya, W.D. Effects of diazinon on larvae of the Asian common toad (Bufo melanostictus, Schneider 1799). Envir Toxicol Chem 2008, 27, 2320–5. [Google Scholar] [CrossRef]
- Huggett, R.J.; Kimerle, R.A.; Mehrle, P.M.; Bergman, H.L. Biomarkers: biochemical, physiological and histological markers of anthropogenic stress. Lewis: Boca Raton, FL, USA, 2002. [Google Scholar]
- Clements, W.H. Integrating effects of contaminants of biological organization: an overview. J Aquat Ecol Stress Rec 2000, 7, 113–6. [Google Scholar] [CrossRef]
- Allran, J.W.; Karasov, W.H. Effects of atrazine on embryos, larvae and adults of anuran amphibians. Envir Toxicol Chem 2001, 20, 761–75. [Google Scholar] [CrossRef]
- Vogiatzis, A.K.; Loumbourdis, N.S. Uptake, tissue distribution and depuration of cadmium Cd in the frog, Rana ridibunda. Bull Environ Contam Toxicol 1997, 59, 770–6. [Google Scholar] [CrossRef] [PubMed]
- Badejo, M.A.; Sosan, M.B. Effects of pesticides on non-target organisms in Nigeria. Soc Envir Toxicol Chem 2005, 6, 2–24. [Google Scholar]
- Ezemonye, L.I.N.; Enuneku, A. Acute toxicity of cadmium to tadpoles of Bufo maculatus and Ptychedena bibroni. Pollut Health 2005, 41, 13–20. [Google Scholar]
- APHA. Standard methods for the examination of water and wastewater, 20th ed.; American Public Health Association: Washington, DC, 1998. [Google Scholar]
- American Society for Testing and Materials. Standard practices for conducting acute toxicity test with fishes, macro invertebrates, and amphibians. Annual Book of ASTM standards 1996, 11, 1–29. [Google Scholar]
- Ellman, L.G.; Courtney, K.D.; Andres, V., Jr; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholine -sterase activity. Biochem Pharma col 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Barseghian, G.; Rachmiel, L.; Epps, P. Direct effect of cortisol and cortisone on insulin and glucagon secretion. Endocrinology 1982, 111, 1648. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with folin phenol reagent. J Biol Chem 1951, 193, 265. [Google Scholar] [CrossRef]
- Steinwandter, H. Contributions to the online method for the extraction and isolating of pesticide residues and environmental chemicals II. Miniaturization of the online method. Fresenius. J Anal Chem 1990, 336, 8–11. [Google Scholar]
- Futerman, I.S. Modes of attachment of actylcholinesterase to surface membrane. Eur JBiochem 1988, 170, 11–22. [Google Scholar]
- Sahib, A.K.; Sailatha, D.; Ramana Rao, K.L. Impact of malathion on actylcholine-sterase in the tissues of the fish Tilapia mossambica Peters – a time course study.
- Wang, C.; Murphy, S.D. Kinetic analysis of species difference in acetylcholinesterase sensitivity to organophosphate insecticides. Toxicol Appl Pharmacol 1982, 66, 409–19. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Shah, E.Z.; Ahmed, I.; Fatima, F. Effects of agricultural pesticides permethrin pyrethroid on protein contents in kidney and liver of lizard species Calotes versicolor in comparison to that in frog Rana tigrina. Bull Pure Appl Sci 2002, 21A, 93–7. [Google Scholar]
- Khan, M.Z.; Fatima, F.; Ahmad, I. Effect of cypermethrin on protein contents in lizard calotes versicolor in comparison to that in frog Rana tigrina. J Biol Sci 2002, 2, 780–1. [Google Scholar]
- Khan, M.Z.; Maria, Z.; Fatima, F. Effect of lambdacyhalothrin Pyrethroid and Mono -crotophos organophosphate on cholineste -raseactivity in liver, kidney and brain of Rana cyanophlyctis. Korean J Biol Sci 2003, 72, 165–8. [Google Scholar] [CrossRef]
- Khan, M.Z.; Tabassum, R.; Shah, E.Z.; Tabassum, F.; Ahmad, I.; Fatima, F.; et al. Effect of cypermethrin and permethrin on cholinesterase activity and protein contents in Rana tigrina amphibia. Turk J Zool 2003, 27, 43–246. [Google Scholar]
- Khan, M.Z.; Ghazala, Y. Pesticides depend-ent cholinesterase activity in the brain tissue of Rana cyanophlyctis. J Exp Zool India 2005, 8, 135–40. [Google Scholar]
- Hontela, A. Endocrine and physiological responses of fish to xenobiotics. Role of glucocorticosteriod hormones. Rev Toxicol 1997, 1, 1–46. [Google Scholar]
- Hontela, A.; Gagnon, A.; Jumarie, C. Effects of Cu on plasma cortisol and cortisol secretion by adrenocortical cells of rainbow trout Oncorhynchus mykiss. Aquat Toxicol 2006, 78, 59–65. [Google Scholar]
- Bowman, W.C.; Rand, M.J. Textbook on pharmacology; Blackwell Scientific Publication: Melbourne, Australia, 1981. [Google Scholar]
- Parxton, R.; Gist, D.H.; Umminger, B.L. Serum coristol levels in thermally acclimated pathological investigations on monosex Tilapia following chronic exposure to pathology. In Handbook of Toxicologic Pathology; Haschek WM and Rousseaux, CG, Ed.; Academic Press Inc.: San Diego, CA, USA, 1984; pp. 279–314. [Google Scholar]
- Hopkins, W.A.; Mendonca, M.T.; Congdon, J.D. Increased circulating levels of testosterone and corticosterone in southern toads, Bufo terrestris, exposed to coal combustion waste. Gener Compar Endocrinol 1997, 108, 237–46. [Google Scholar] [CrossRef]
- Gendron, A.D.; Bishop, C.; Fortin, R.; Hontela, A. In vivo testing of the functional integrity of the corticosterone-producing axis in mudpuppy (amphibia) exposed to chlorinated hydrocarbons in the wild. Envir Toxicol Chem 1997, 16, 1694–706. [Google Scholar]
- Bisson, M.; Hontela, A. Cytotoxic and endocrine-disrupting potential of atrazine, diazinon, endosulfan, and mancozeb in adrenocortical steroidogenic cells of rainbow trout exposed in vitro. Toxicol Appl Pharmacol 2002, 180, 110–7. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F.; Wood, C.M. Kinetics of branchial calcium uptake in the rainbow trout: effects of acclimation to various external calcium levels. J Exp Biol 1985, 116, 411–33. [Google Scholar] [CrossRef]
- Lacroix, M.; Hontela, A. The organochlorine o,p9-DDD disrupts the adrenal steroidogenic signaling pathway in rainbow trout (Oncorhynchus mykiss). Toxicol Appl Pharmacol 2003, 190, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Regoli FPrincipato, G. Glutathione, glutathione-dependent, and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory condition, implications for the use of biochemical biomarkers. Aquat Toxicol 1995, 31, 143–64. [Google Scholar] [CrossRef]
- Bachowski, S.; Xu, Y.; Stevenson, D.E.; Walborg, E.F.; Klaunig, J.E. Role of oxidative stress in the selective toxicity of dieldrin in the mouse liver. Toxicol Appl Pharmacol 1998, 150, 301–9. [Google Scholar] [CrossRef]
- Begum, G.; Vijayaraghavan, S. Alterations in protein metabolism of muscle tissue in the fish Clarias batrachus Linn by commercial grade dimethoate. Bull Environ Contam Toxicol 1996, 57, 223–8. [Google Scholar] [CrossRef]
- Jenkins, F.; Smith, J. Effects of sublethal concentration of endosulfan on haematological and serum biochemical parameters in the carp, Cyprinus carpio. Bull Environ Contam Toxicol 2003, 70, 993–7. [Google Scholar] [CrossRef]
- Ravinder, V.; Suryanarayana, N. Pesticides induced biochemical alterations in a fresh water catfish, Clarias batrachus. Indian J Comp Animal Physiol 1988, 6, 5–12. [Google Scholar]
- Neff, J.M. Use of biochemical measurements to detect pollutant – mediated damage to fish. Aquatic toxicology and hazard assessment, Carwel, R.D., Purdy, R., Bahner, R.C., Eds.; America Society for Testing Material: Philadelphia, 1985; 155–181. [Google Scholar]
- Das, B.K.; Mukherjee, S.C. Sublethal effect of Quinalphos in selected blood parameters of labeo rohita (Ham.) fingerlings. Asian Fish Sci 2000, 13, 225–33. [Google Scholar] [CrossRef]
© Copyright I. Tongo et al., 2012 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).