Abstract
Untreated detergent bearing wastes discharged into the environment are sources of linear alkylbenzene sulfonate (LAS). Detergent wastes usually do not contain nitrogen or contain very low amounts. Biostimulation by introducing limiting nutrient element can be useful in biotreatment of such waste. The effect of inorganic and organic nitrogen supplements on aerobic degradation of LAS by LAS-utilizing bacteria was examined. Phosphate-buffered LAS mineral media were prepared and supplemented with different nitrogen sources: NPK fertilizer (inorganic) and urea fertilizer (organic). Individual and various consortia of pure cultures of Alcaligenes odorans, Citrobacter diversus, Micrococcus luteus and Pseudomonas putida, previously isolated from a detergent effluent polluted stream, were used. Biodegradation of LAS was monitored in terms of half-life (t½) of the surfactant. The rates of biodegradation by the consortia can be ranked as: 4-membered (t½ = 8–12 days) > 3-membered (t½ = 813 days) > 2-membered consortia (t½ = 1015 days) > individuals (t½ = 916 days). The inorganic nitrogen source enhanced utilization of the surfactant, while organic nitrogen supplementation generally slowed degradation of the surfactant. In undertaking biotreatment of detergent bearing effluent, inorganic nitrogen should be used as biostimulant.
Introduction
Detergents were first introduced in 1916 when the alkyl naphthalene sulfonate was developed in Germany as a replacement for soap to avoid soap-curd formation when soap reacted with mineral salts (Ca2+, Mg2+) in water.[1] By the early 1930s, fatty-alcohol based detergents were introduced; the first was finally introduced in the United States in 1946. However, it was after the World War II that detergents began to gain prominence.[2] Based on the Africa annual average per capita detergent consumption of 3 kg,[3] annual consumption of detergent in Nigeria (population 120 million people) may be in the order of approximately 360 million kg.
Environmental complications of detergents date back to the early 1960s when abnormal quantities of foam were noticed in streams in the United States. The branched chain alkyl benzene sulfonate was the surfactant then in use. It was consequently replaced by readily biodegradable linear alkyl benzene sulfonates (LAS).[4] LAS subsequently became the most widely used surfactants in detergents and household cleaning products.[5,6,7]
The fate and effect of a chemical compound released into the environment is largely dependent on its concentration which is influenced by the rate at which it degrades in the recipient environment and available dilution.[8] Degradation of surfactants through microbial activity is the primary transformation occurring in the environment.[8] Biodegradation also enhances the removal of these surfactants in the environment, thus reducing their impact on biota. During biodegradation, microorganisms can either utilize surfactants as substrates for energy and nutrients or cometabolize the surfactants by microbial metabolic reactions. The most important factors that affect biodegradation of a surfactant are its chemical structure and physiochemical conditions of the environmental media.[8]
In addition to carbon and oxygen, bacteria need nitrogen and phosphorus to survive. Nitrogen and phosphorus based fertilizers from farms and gardens on land also enter water bodies through stormwater runoff to encourage natural attenuation of the chemical compounds in the water. Bioremediation usually takes advantage of the metabolism (degradative ability) of organisms and speeds up the rate of degradation in order to remove the pollutant.[9] Bioremediation is often achieved by manipulating the environment; including addition of nutrients (biostimulation) to stimulate organisms associated with the material to degrade it at a faster rate. Walworth et al.[10] suggested that the addition of nitrogen can increase the rate of degradation of hydrocarbons. Khleifat[11] suggested that the nature of carbon and nitrogen source may affect the growth of biomass and enzyme involved in LAS degradation. He attributed the increased LAS degradation to increasing co-substrate diversity and complexity.
In most urban areas in Nigeria, detergent bearing wastes are regularly released into the environment without treatment. This results in varying amounts of surfactants persisting in different conditions: 180-240 mg/L in the River Asa; 180-440 mg/L in the Okun stream and 460-840 mg/L in the Osere stream. These surfactants persist for 12-15 days.[12,13,14] The banks of the streams and river are used as agricultural farms, for defeacation and as refuse dumps. Hence, various forms of nitrogen (inorganic and organic) are likely to be introduced in the water from washings/runoffs from on the banks.[12] Studies by Schoberl[15] and Tolls et al.[16] have shown that aquatic organisms and plants accumulate LAS within their tissues. LAS has a high bioconcentration factor (BCF) which is a ratio between the concentration in biota (CB) and the concentration in water (Cw), i.e. BCF=CB/Cw. Surfactant has to be taken up into an organism before it can elicit an effect. Therefore, if it can be removed by biotreatment before waste is discharged, its toxic effects can be reduced or eliminated.[16]
Biotreatment sometimes involves the addition of adapted microorganisms (bio-augmentation) or addition of micronutrients (biostimulation) or both. Jefferson et al. [17] reported that addition of nitrogen gray water resulted in stimulation of the biomass, increased chemical oxygen demand removal and oxygen uptake. The correct combination of bioaugmentation and biostimulation can be used to deal with large volumes and strengths of industrial wastewaters.[18] Hosseini and Borghei[19] found that biological treatment was very efficient for treatment of wastewater containing low concentration of detergent and that wastewater with detergent concentration of over 500 mg/L could not be treated by biological methods. They recommended physical and chemical treatment to reduce the concentration before biotreatment.
Failure of the biotreatment to deal with wastewater with high detergent concentration could be due to the toxicity of high concentrations of detergent to microorganisms.[14,20] Peressutti et al. [21] also observed that LAS levels of 50 and 100 mg/L led to a pronounced decrease in the extent of biodegradation and inhibited culture growth. They recommend the use of autochthonous consortium with high effectiveness for LAS degradation.
In this study, the effect of nitrogen supplementation on biodegradation of LAS by individual pure culture of LAS degrading bacteria and their consortia was examined. Nitrogen was supplied as inorganic nitrogen (NPK fertilizer) and organic nitrogen (urea). Rates of degradation of LAS by individual and consortia of bacteria were monitored as half-life (t½) which is the time it takes for microbes to break down half the amount of a chemical in water.
Materials and Methods
Test organisms
The organisms used in this study were: Alcaligenes odorans, Citrobacter diversus, Micrococcus luteus and Pseudomonas putida. These were selected from among surfactant utilizing bacteria previously isolated from a detergent effluent polluted steam.[22] Broth cultures of the organisms were prepared, standardized as 0.5 McFarland standard (108 cell/mL) and used as inocula in the study.
Aerobic biodegradation studies
LAS mineral medium (LMM) was prepared as described Eniola,[23] adjusted to pH 7 and buffered using phosphate buffer. Test media were prepared by supplementing the LMM with inorganic (NPK fertilizer) and organic (urea fertilizer) nitrogen sources. Different flasks containing 150 mL of different media (LMM and test media) were then inoculated with individual pure cultures and their various consortia; each setup was prepared in triplicate. The flasks were incubated at room temperature (28˚C±3) on an orbital shaker at 100 rpm. Aliquots (10 mL) of the resulting culture were withdrawn daily and the progress of degradation monitored as primary degradation (disappearance of surfactant) in terms of half-life (t½) using the hyamine titration method to measure surfactant concentration.[24] Growth of the organisms, separately and as various consortia, was monitored by measuring optical density of the resulting culture at interval of 12 h. Non-inoculated media treated in the same way served as controls. Data obtained were analyzed statistically using the ANOVA
Results
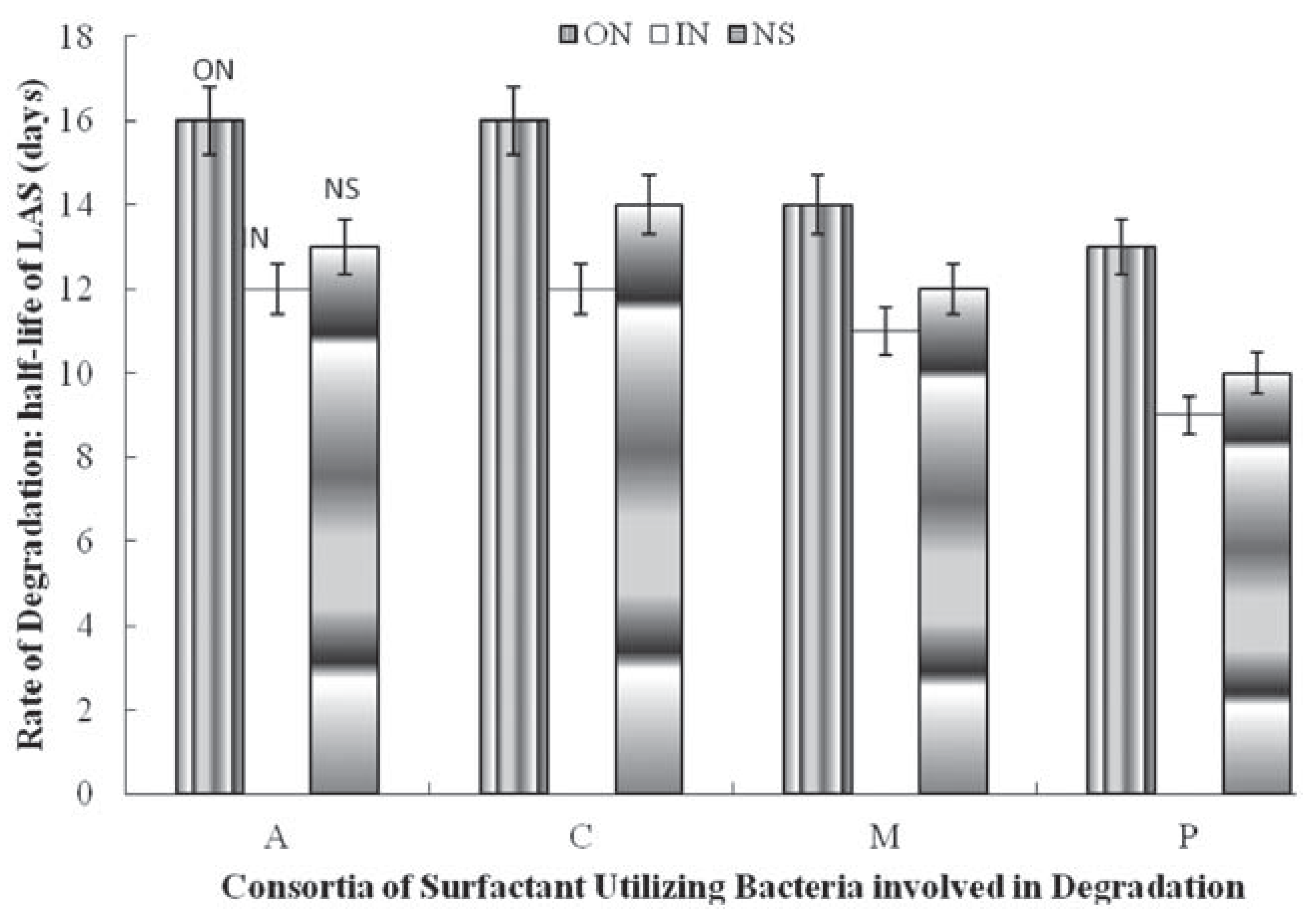
The effect of nitrogen sources on the utilization of the surfactant by pure cultures of surfactant utilizing bacteria is shown in Figure 1, Figure 2 and Figure 3. This shows that the presence of inorganic nitrogen source increased the rate of utilization of the surfactant in all cases while the organic nitrogen source was found to reduce the rate of degradation. Inorganic nitrogen source reduced the t½ by one day for Alcaligenes odorans, Pseudomonas putida and Micrococcus luteus, while organic nitrogen source increased t½: Pseudomonas putida and Alcaligenes odorans (3 days each) >Micrococcus luteus and Citrobacter diversus (2 days each) (Figure 1).
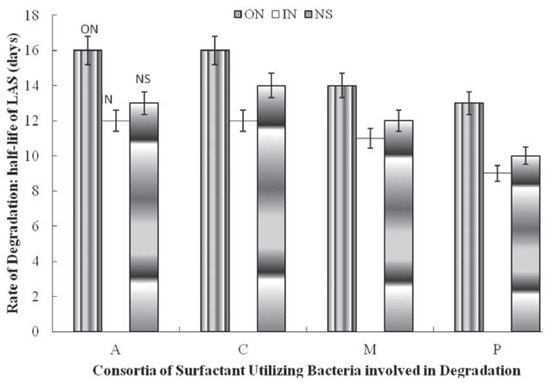
Figure 1.
Effect of nitrogen supplementation on biodegradation of linear alkylbenzene sulfonate (LAS) by individual LAS-utilizing bacteria. A, Alcaligenes odorans; C, Citrobacter diversus; M, Micrococcus luteus; P, Pseudomomas putida; ON, Organic nitrogen; IN, inorganic nitrogen; NS, not supplemented.
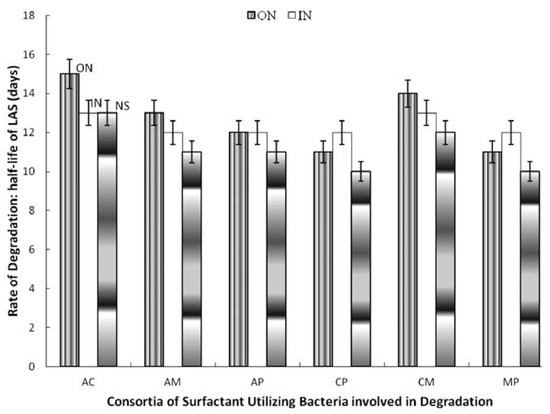
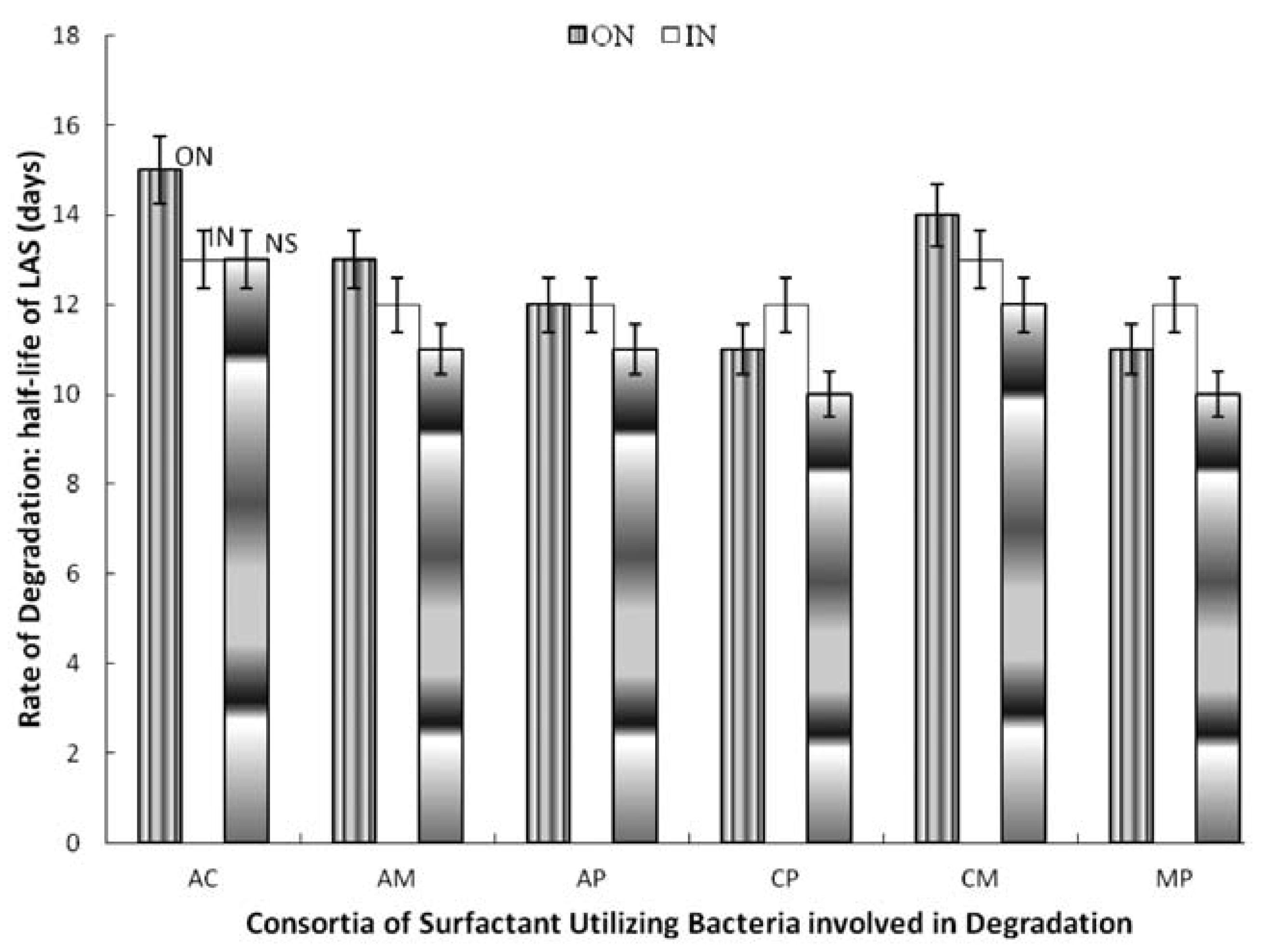
Figure 2.
Effect of nitrogen supplementation on biodegradation of linear alkylbenzene sulfonate (LAS) by 2-membered consortia of LAS-utilizing bacteria. AC, A. odorans and C. diversus; AM, A. odorans and M. luteus; AP, A. odorans and P. putida; CM, C. diversus and M. luteus; CP, C. diversus and P. putida; MP, M. luteus and P. putida; ON, organic nitrogen; IN, inorganic nitrogen; NS, not supplemented.
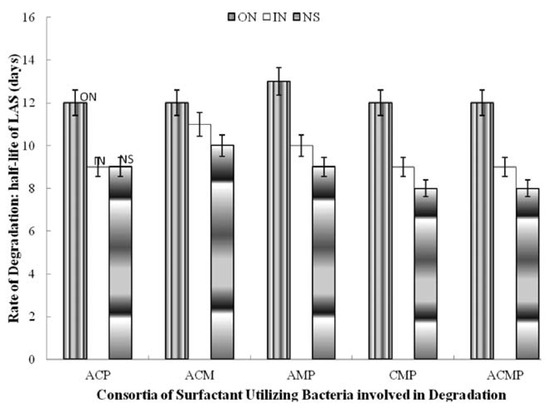
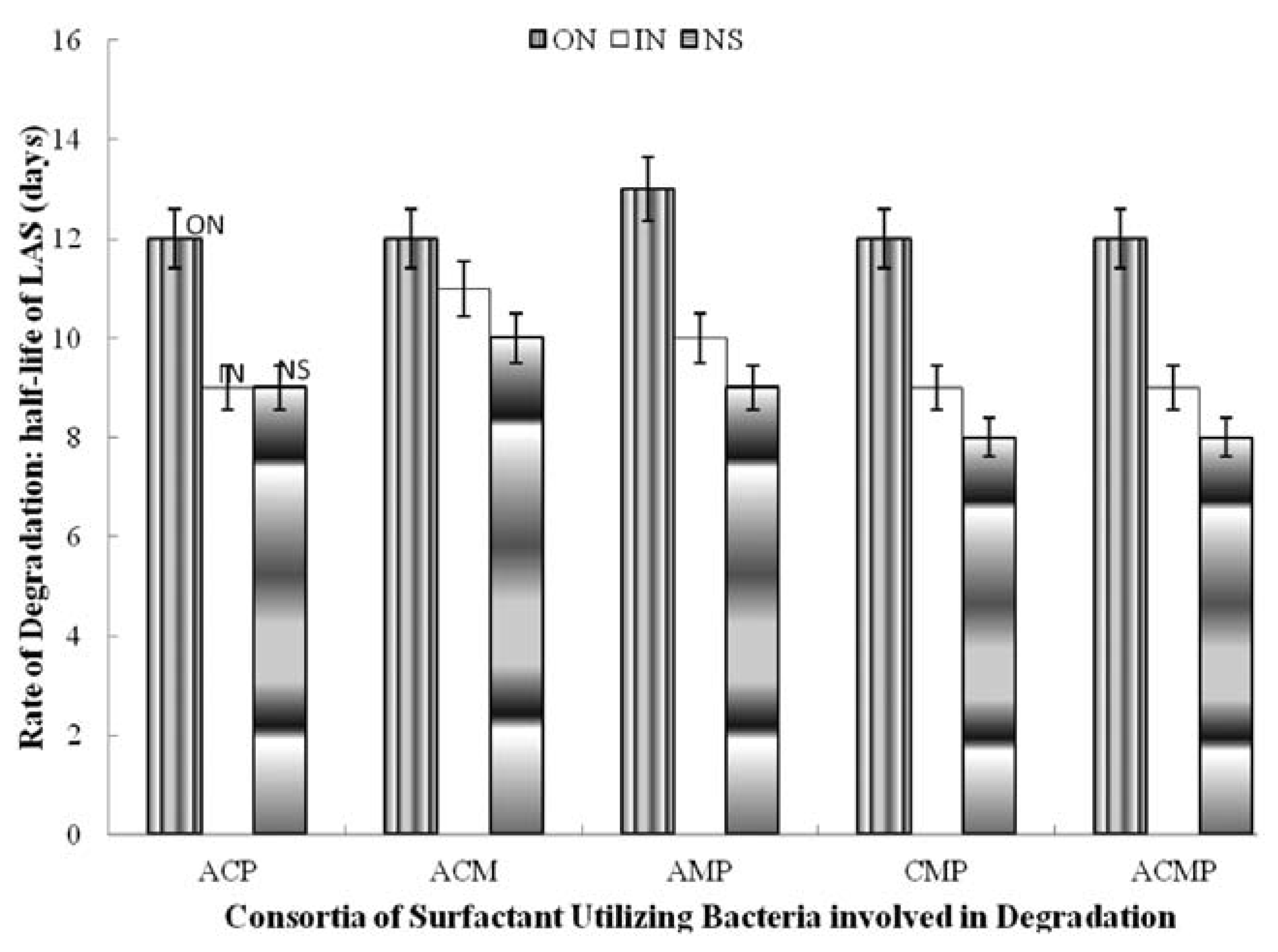
Figure 3.
Effect of nitrogen supplementation on biodegradation of linear alkylbenzene sulfonate (LAS) by 3- and 4-membered consortia of LAS-utilizing bacteria. ACM, A. odorans, C. diversus and M. luteus; ACP, A. odorans Citrobacter diversus and P. putida; AMP,A. odorans, M. luteus and P. putida; CMP, Citrobacter diversus M. luteus and P. putida; ACMP: A. odorans, C. diversus, M. luteus and P. putida; ON, organic nitrogen; IN, inorganic nitrogen; NS, not supplemented.
Among the two-membered consortia, supplementing with inorganic nitrogen increased t½ by two days (Citrobacter diversus and Pseudomonas putida; Micrococcus luteus and Pseudomonas putida) and by one day (Alcaligenes odorans and Micrococcus luteus; Alcaligenes odorans and Psudomonas putida as well as Citrobacter diversus and Micrococcus luteus. However, it did not affect degradation by the consortium of Alcaligenes odorans and Citrobacter diversus. Supplementing with organic nitrogen increased t½ by three days (Alcaligenes odorans and Citrobacter diversus), by two days (Alcaligenes odorans and Micrococcus luteus; Citrobacter diversus and Micrococcus luteus) and by one day (Alcaligenes odorans and Psudomonas putida; Citrobacter diversus and Pseudomonas putida as well as Micrococcus luteus and Pseudomonas putida) (Figure 2). Inorganic nitrogen increased t½ by one day for all the 3-membered consortia and the 4-membered consortium but did not affect degradation by Alcaligenes odorans, Citrobacter diversus and Pseudomonas putida. The organic nitrogen source increased t½ by 2-4 days compared to the rate of degradation (t½) in the unsupplemented media (Figure 3).
The rates of degradation of the surfactant by the organisms, both separately and as various consortia, obtained in this study indicated that inorganic nitrogen appears to stimulate degradation of the surfactant. On the other hand, the rates of degradation suggest supplementing with organic nitrogen source seems to inhibit degradation of the surfactant. The finding is similar to observations of Walworth et al.[10] and Deksissa and Vanrolleghem.[25] In their study of nitrogen requirement for petroleum bioremediation, Walworth et al.[10] observed that the addition of nitrogen can increase the rate of biodegradation of petroleum. Deksissa and Vanrolleghem,[25] in their study of LAS degradation in a river, found that it was influenced by the type of nitrogen available in the medium.
Deksissa and Vanrolleghem[25] found that addition of inorganic nitrogen (ammonia nitrogen: NH4-N) resulted in more rapid LAS degradation than when organic nitrogen source (acetate) was used. However, they observed that the high concentration of nitrate nitrogen, which can also be potentially used as source of nitrogen, in the river did not result in rapid degradation of the surfactant. They concluded that ammonia nitrogen (NH4-N) rather than total inorganic nitrogen limits the LAS degradation. They attributed the sensitivity of LAS degradation to the preferential use of ammonia nitrogen by heterotrophic bacteria under aerobic condition rather than nitrate. Similarly, Yadav et al.[26] reported that degradation of LAS by a fungus, Phanerochaete chrysosporium, which uses a pathway similar to that of bacteria, was inhibited by high concentrations of nitrogen. Malt extract (organic nitrogen source) resulted in production of sulfophenyl carboxylates (SPCs) of a longer alkyl chain length (1c SPCs), i.e. prolonged degradation. It is, therefore, likely that the high concentration of nitrogen supplements could have an inhibitory action on LAS degradation.
Khleifat[11] indicated that nitrogen increased degradation of LAS by increasing cosubstrate diversity and complexity. He observed that nitrogen sources were the most efficient cosubstrate for removal of LAS and that the nature of nitrogen source may affect growth biomass and enzyme involved in LAS degradation. Osmotic stress was identified as a factor that can reduce the population of hydrocarbonoclastic organisms. This will affect the rate of biodegradation because there is a direct correlation between rate of primary degradation of LAS and the initial bacterial population.[27,28]
The effect of organic nitrogen source could be due to the possible use of the substrate as an alternative carbon source in addition to nitrogen source. The presence of readily utilizable carbon sources has been found to have inhibitory effects on the degradation of compounds.[23,29,30] Eniola[23] found that the presence of additional carbon source which is easily degraded inhibited degradation of LAS. Organic nitrogen source can also serve as an alternative source of carbon and slow down the rate of degradation of LAS in the presence of organic carbon source. The water bodies that receive the detergent bearing effluent also receive various forms of organic matter from farmland and residential areas within their catchment. In addition, their banks are used for defaecation and the fecal materials (organic nitrogen) are often washed into the water bodies. Eniola and Olayemi13 identified nutrient inbalance as a factor in the persistence of LAS in the Okun stream and the River Asa; the inhibition of LAS degradation by organic nitrogen source shows that the presence of organic nitrogen could also contribute to persistence of the surfactant in the water bodies.
Ginkel[31] reported that the limited metabolic capacities of pure cultures of microorganisms utilizing surfactants indicate the need for consortia to degrade surfactants completely. He indicated complete degradation of surfactants is accomplished by mixed cultures of microorganisms on the basis of synergistic and commensalistic relationships. The results obtained in this study further demonstrate that the use of a consortium of organisms was better in degradation of the surfactant and supports reports by Ginkel.[31] Jimenez et al.[32] showed that four bacteria in a consortium synergistically mineralized LAS by catabolic cooperation among the four members. Cook and Hrsak[33] also indicated that catabolic cooperation among community members was necessary for complete degradation of LAS and proposed involvement of three tiers of microbial communities.
Similarly, Peressutti et al. [21] observed that a consortium of four organisms (2 strains of Aeromonas caviae, Pseudomonas alcaliphila and Vibrio species) produced higher biomass from LAS and CO2 release (mineralization) than individual cultures, and also degraded 86% of LAS (20 mg/L), whereas pure strains only degraded 21-60%. Khleifat11 reported that the two bacteria used as a consortium in their study complement each other in the degrading ability of LAS, and concluded there was catabolic cooperation between the two consortium members.
Conclusions
This study shows that the presence of inorganic nitrogen enhanced degradation of LAS while the organic nitrogen had an inhibitory effect. It also shows that consortia of organisms degraded the surfactant faster than individual pure cultures. In undertaking biotreatment of detergent bearing effluent, this study indicates that biostimulation should involve the use of inorganic nitrogen rather than organic nitrogen. Similarly, bioaugmentation should involve consortia of surfactant degrading organisms rather than individual pure cultures. This study provides information on the influence of nitrogen on degradation of LAS. However, development of effective and efficient biotreatment system to deal with detergent bearing effluent requires further investigation of the survival of LAS degrading organisms, the degradation intermediates, and optimization of the nutrient requirements and other conditions.
References
- Houston, C.A. Detergents: changing expectation. Content Inform 1997, 8, 928–38. [Google Scholar]
- Manaham, S.E. Eutrophication. Fundamentals of environmental chemistry. Boca raton, FL: Lewis Publishers; 1993. pp 373-475.
- Kertesz, M.A.; Kolbener, P.; Stockinger, H.; Bell, S.; Ook, A.M. Desulphonation of linear alkylbenezene sulfonate and related compounds by bacteria. Appl Environ Microbiol 1994, 60, 2296–303. [Google Scholar] [CrossRef] [PubMed]
- WHO. International Programme on Chemical Safety. Environmental health criteria 169. Linear alkylbenzene sulfonates and related compounds. Geneva, Switzerland: World Health Organization; 1996.
- Rapaport, R.A.; Larson, R.J.; McAvoy, D.C.; Nielsen, A.M.; Trehy, M. The fate of commercial LAS in the environment. CLER Rev 1995, 1, 20–31. [Google Scholar]
- Mcavoy, D.C.; Eckhoff, W.S.; Rapaport, R.A. The fate of linear alkylbenzene sulfonate in the environment. CLER Rev 1997, 3, 4–17. [Google Scholar] [CrossRef]
- Folker, J.; Landner, L. Risk assessment of LAS in sewage sludge and soil. Report to Kemisk-Tekniska Loverantorbundent (KTF). Stockholm: AFMiljoforskar Gruppen Stockholm- Sweden; 2000.
- Ying, G. Fate, behavior and effects of surfactants and their degradation products in the environment. Environ Int 2006, 32, 417–31. [Google Scholar] [CrossRef]
- Thomas, J.M.; Ward, C.H.; Raymond, R.L.; Wilson, J.T.; Lohehr, R.C. Bioremediation. In: Lederberg J, ed. Encyclopedia of microbiology I. London: Academic Press; 1992. pp 369-77.
- Walworth, J.; Pond, A.; Snape, I.; Rayner, J.; Ferguson, S.; Harvey, P. Nitrogen requirements for maximizing petroleum bioremediation in a sub-Antarctic soil. Cold Reg Sci Technol 2007, 48, 84–91. [Google Scholar] [CrossRef]
- Khleifat, K.M. Biodegradation of linear alkylbenzene sulfonate by a two-member facultative anaerobic bacterial consortium. Enzyme Microb Technol 2006, 39, 1030–5. [Google Scholar] [CrossRef]
- Eniola, K.I.T.; Olayemi, A.B. Impact of effluent from a detergent producing plant on some water bodies in Ilorin. Int J Environ Health Res 1999, 9, 335–40. [Google Scholar] [CrossRef]
- Eniola, K.I.T.; Olayemi, A.B. Utilization of detergent surfactant by bacteria isolated from freshwater bodies. Nig J Microbiol 2000, 14, 49–53. [Google Scholar]
- Eniola, K.I.T.; Olayemi, A.B. Some aspects of bacterial-detergent interaction in freshwater environment. Biosci Res Commun 2002, 14, 645–9. [Google Scholar]
- Schoberl, P. Basic principles of LAS biodegradation. Tenside Surfact Deterg 1989, 26, 86–94. [Google Scholar] [CrossRef]
- Tolls, J.; Haller, M.; Seinen, W.; Sijm, D.T.H.M. LAS bioconcentration: tissue distribution and effect of hardness-implications for processes. Environ Sci Technol 2000, 34, 304–10. [Google Scholar] [CrossRef]
- Jefferson, B.; Burgess, J.E.; Pichon, A.; Harkness, J.; Judd, S.J. Nutrient addition to enhance biological treatment of greywater. Water Res 2001, 35, 2702–10. [Google Scholar] [CrossRef]
- Burgess, J.E.; Quarmby, J.; Stephenson, T. Role of micronutrients in activated sludgebased biotreatment of industrial effluents. Biotechnol Adv 1999, 17, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.N.; Borghei, S.M. Removal of alkylbenzene sulfonate from detergent industries wastewater by activated sludge. B-343-7. Proceedings of the 9th International Conference on Environmental Science and Technology. Rhodes Island Greece, 1-3 September 2005.
- Flores, G.P.; Badillo, C.M.; Cortázar, M.H.; Hipólito, C.N.; Pérez, R.S.; Sánchez, I.G. Toxic effects of linear alkylbenzene sulfonate, anthracene and their mixture on growth of a microbial consortium isolated from polluted sediment. Rev Int Contam Ambient 2010, 26, 39–46. [Google Scholar]
- Peressutti, S.R.; Olivera, N.L.; Babay, P.A.; Costagliola, M.; Alvarez, H.M. Degradation of linear alkylbenzene sulfonate by a bacterial consortium isolated from the aquatic environment of Argentina. J Appl Microbiol 2008, 476–84. [Google Scholar]
- Eniola, K.I.T.; Olayemi, A.B. Linear alkylbenzene sulfonate tolerance in bacteria isolated from sediment of tropical water bodies polluted with detergents. Rev Biol Trop 2008, 56, 1595–601. [Google Scholar]
- Eniola, K.I.T. Effect of additional carbon source on biodegradation of linear alkylbenzene sulfonate by LAS-utilizing bacteria. J Xenobiotics 2011, 1, e2. [Google Scholar] [CrossRef]
- Mettler Toledo M603 Turbidimetric titration. Determination of anionic surfactant; 2011. Available from: http://www.mt.com.
- Deksissa, T.; Vanrolleghem, P.A. Effect of nutrient dynamics on organic contaminant fate in rivers: a microcosm study. Comm Appl Biol Sci 2003, 68, 111–4. [Google Scholar]
- Yadav, J.S.; Lawrence, D.L.; Nuck, B.A.; Federle, T.W.; Reddy, C.A. Biotransformation of linear alkylbenezene sulfonate by phanerochaete chrysosporium: oxidation of alkyl side chain. Biodegradation 2001, 12, 443–53. [Google Scholar] [CrossRef]
- Yediler, A.; Zhang, Y.; Cai, J.P.; Korte, F. Effect of the microbial population size on the degradation of linear alkylbenzene sulfonate in lake water. Chemosphere 1989, 18, 1589–97. [Google Scholar] [CrossRef]
- Dentel, S.K.; Allen, H.E.; Srinivasarao, C.; Divincenzo, J. Effects of surfactants on sludge dewatering and pollutant fate. Third year completion report project number 06. Water Resources Center. Delaware, USA: University of Delaware; 1993.
- Bajaj, M.; Callert, C.; Winter, J. Effect of cosubstrates on aerobic phenol degradation by acclimatized and non-acclimatized enrichment cultures. Eng Life Sci 2008, 8, 125–31. [Google Scholar] [CrossRef]
- Shimp RPfeander, F.K. Influence of easily degradable naturally occurring carbon substrate on biodegradation of monosubstituted phenols by aquatic bacteria. Appl Environ Microbiol 1985, 42, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ginkel, C.G. Complete degradation of xenobiotic surfactants by consortia of aerobic microorganisms. Biodegradation 1996, 7, 151–64. [Google Scholar] [CrossRef] [PubMed]
- Jim’enez, L.; Breen, A.; Thomas, N.; Federle, T.W.; Sayler, G.S. Mineralization of linear alkylbenzene sulfonate by a four-member aerobic bacterial consortium. Appl Environ Microbiol 1991, 57, 1566–9. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Hrsak, D. The complete degradation of LAS is becoming better understood with pure cultures of bacteria. CLER Rev 2000, 6, 46–53. [Google Scholar]
© Copyright K.I.T. Eniola, 2012 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).