Abstract
Pharmaceutical substances represent a risk for aquatic environments and their potential impacts on the receiving environment are poorly understood. Municipal effluents are important sources of contaminants including common pharmaceuticals like anti-inflammatory and anti-convulsive substances. The removal of pharmaceuticals, particularly those highly soluble can represent a great challenge to conventional wastewater treatment processes. Hydrophilic drugs (e.g., acidic drugs) have properties that can highly influence removal efficiencies of treatment plants. The performance of different wastewater treatment processes for the removal of specific pharmaceutical products that are expected to be poorly removed was investigated. The obtained results were compared to inherent properties of the studied substances. Clofibric acid, carbamazepine, diclofenac, ibuprofen and naproxen were largely found in physicochemical primary-treated effluents at concentrations ranging from 77 to 2384 ng/L. This treatment type showed removal yields lower than 30%. On the other hand, biological treatments with activated sludge under aerobic conditions resulted in much better removal rates (>50% for 5 of the 8 studied substances). Interestingly, this latter type of process showed evidence of selectivity with respect to the size (R2 = 0.7388), solubility (R2 = 0.6812), and partitioning (R2 = 0.9999) of the removed substances; the smallest and least sorbed substances seemed to be removed at better rates, while the persistent carbamazepine (392 ng/L) and diclofenac (66 ng/L) were poorly removed (<10%) after biological treatment. In the case of treatment by aerated lagoons, the most abundant substances were the highly soluble hydroxy-ibuprofen (350–3321 ng/L), followed by naproxen (42–413 n/L) and carbamazepine (254–386 ng/L). In order to assess the impacts of all these contaminants of various properties on the environment and human health, we need to better understand the chemical and physical transformations occurring at the treatment plant and in the receiving waters.
Introduction
Pharmaceutical and personal care products (PPCPs) are introduced into the environment via a number of routes, the primary one being the discharge of treated and poorly treated wastewater to surface water.[1] The presence of theses substances and their metabolites in municipal wastewaters and receiving aquatic ecosystems raises growing concerns about environmental and human health.[2,3]
Nowadays, certain major treatment plants are still using limited physicochemical processes that unfortunately generate low removal efficiencies for emergent contami-nants such as pharmaceutical substances. Physicochemical treatment processes are renowned for their higher values of water qual- ity parameters than are observed with biologi- cal treatments.[4] As a result, physicochemical treatments typically present poorly improved values for key parameters like total organic carbon (TOC), biological oxygen demand (BOD) and coliforms. Besides the improved biological quality of the treated wastewater, information on the removal of chemical con- taminants like the ubiquitous pharmaceutical products found especially in poorly treated wastewaters is required. The information could be used to evaluate the sources of phar- maceuticals into the receiving environment, and therefore contribute to global environmen- tal risk assessments of discharges of effluents treated with various wastewater treatment processes.
Recent studies have clearly shown that the elimination of PPCPs in municipal sewage treatment plants (STPs) is often incomplete with efficiencies averaging 75%, but in many cases less than 20% depending on the treat- ment process used, the environmental temper- ature, light and matrix effects, and substance’s properties as well.[5,6,7] Hence, the removal rate of acidic and hydrophilic drugs is expected to be low, due to their high water-solubility and rela- tively poor degradability. The group of acidic pharmaceuticals is mainly defined by the fact they possess a carboxylic acid moiety (pka ~ 4) and are extractable at acid pH.[8] Among acidic pharmaceuticals are listed the lipid regulator clofibric acid and the non-steroidal anti- inflammatory drug (NSAIDs) family.
An important consideration when assessing the environmental fate of PPCPs is that, as a specific class compounds, they generally pos- sess characteristics that make them different than conventional industrial chemical pollu- tants.[9] Owing to their hydrophilic properties and stability, PPCPs generally tend to remain in the aqueous phase and are not totally elimi- nated by STPs; as a consequence they and their metabolites are still frequently detected in surface waters.[10,11]
A major factor influencing the efficiency of pollutants removal from raw sewage water is their ability to interact with solid particles, either natural (clay, sediments, microorgan- isms) or chemical additive mixtures to the medium (e.g., active carbon, coagulants). This action tends to facilitate the removal or biodegradation of pollutants by physicochemi- cal (precipitation, flotation) or biological (acti- vated sludge) processes.[12] However, as report- ed by Carballa et al. [13] and Loffler et al.,14 com- pounds with low partitioning coefficient (Kd) or low Kow values tend to remain in the aque- ous phase, which favor their mobility through the STP and in the receiving environment.
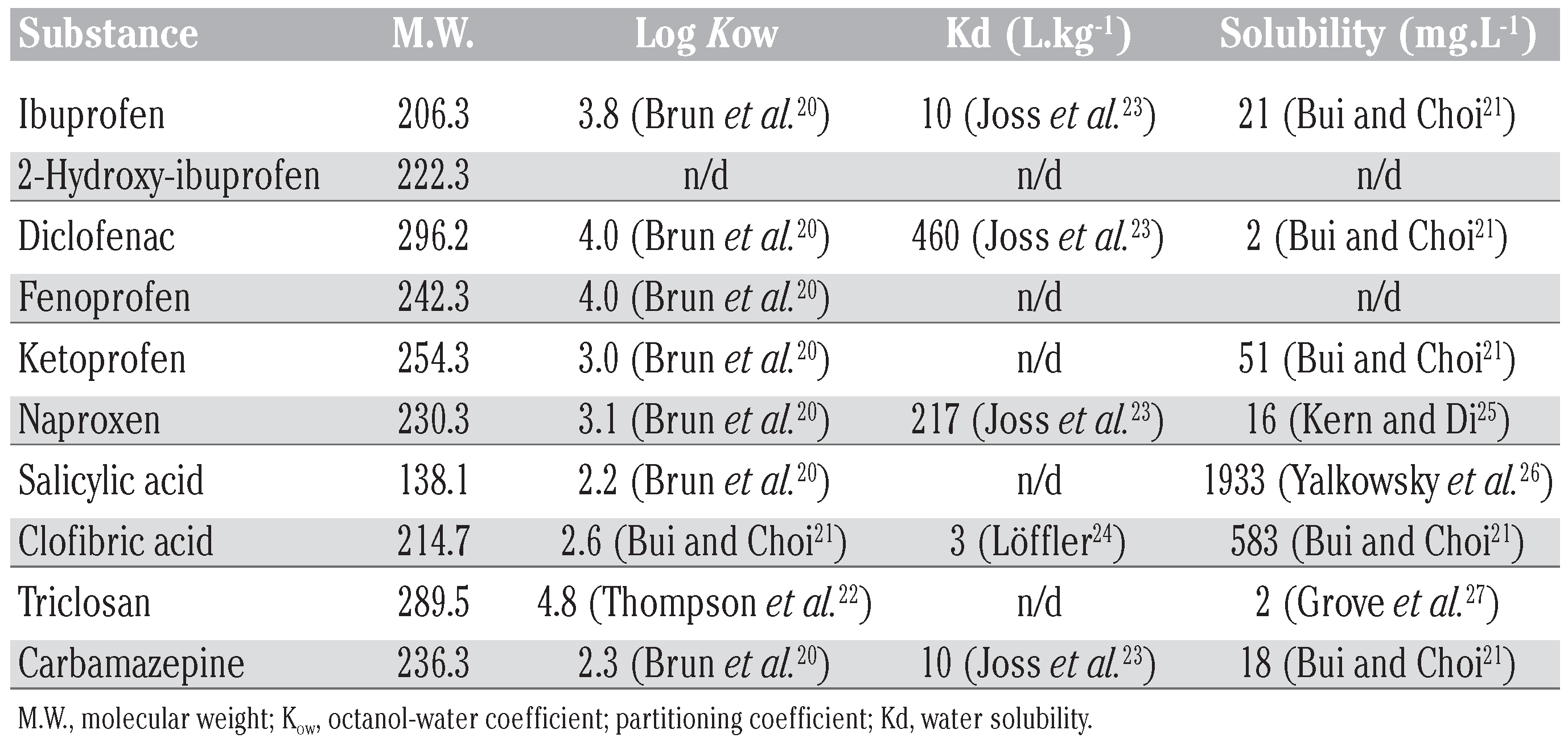
Among the studied substances (Figure 1), the heteroatom content and the chemical func- tionalities revealed by the hydroxyl and car- boxylic acid moieties make them polar, ionic molecules with physicochemical properties that could largely explain their occurrence in surface water samples taken from sewage treatment plants.[15,16,17] For practical reasons, acidic drugs are usually selected among phar- maceuticals on the basis of levels of use and the abundance in municipal effluents.[8] Acidic drugs, especially analgesic/anti-inflammatory drugs such ibuprofen, diclofenac, naproxen and ketoprofen are found to be the most detected pharmaceuticals in municipal waste- water effluents.[18] In addition to the previous list of substances, the persistent neutral anti- convulsive drug carbamazepine is also fre- quently detected in wastewater-impacted waters.[15,16,17,18,19] The reported properties (Table 1), coupled with trace quantities, create unique challenges for both removal processes and analytical detection. As such, the lack of infor- mation about removal efficiency of pharma- ceutical residues in municipal sewage has forced the scientific community in the last decade to rapidly investigate on the capacity of existing STPs to remove these emergent con- taminants. Therefore, more studies are need- ed to better understand the environmental fate of PPCPs following different STP processes.
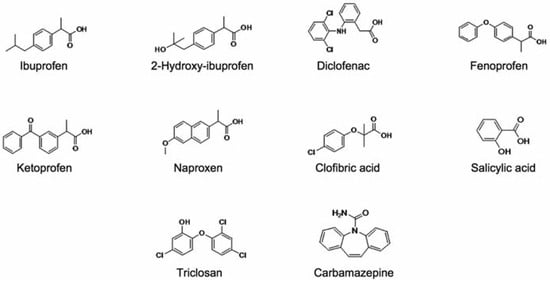
Figure 1.
Molecular structures of studied pharmaceutical substances.

Table 1.
Properties of the studied substances: molecular weight, octanol-water coefficient, partitioning coefficient and water solubility.
In this paper, the removal efficiency for tar- get pharmaceuticals by physicochemical and biological municipal wastewater treatment technologies is studied. The main objectives of this work were as follow: i) to report on the occurrence of selected acidic and neutral com- pounds detected in various treated effluent sources (aerated lagoons, physicochemical and biological plants), ii) to establish some possible correlations between their removal and key physicochemical parameters such Kd, log Kow, solubility, and molecular weight.
Material and Methods
Wastewater treatment
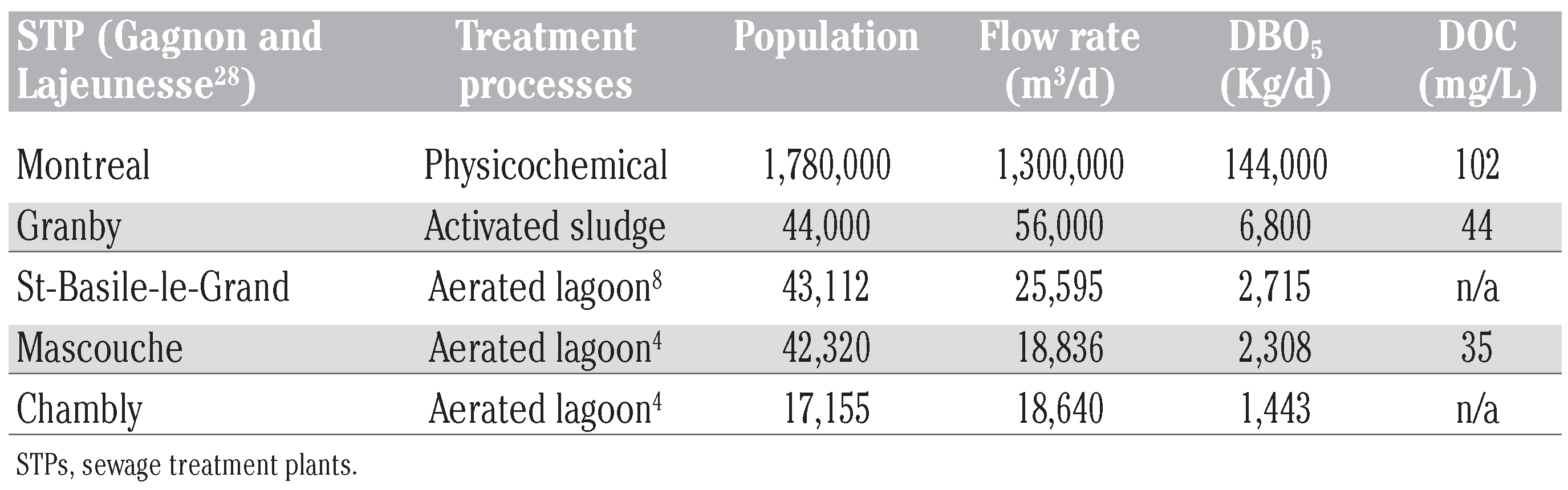
The treatment processes investigated were of various types, from physicochemical to bio- logical processes, as well as simple aerated lagoons. Information on visited treatment plants is given in Table 2. The investigated physicochemical wastewater treatment plant, located in Montreal, Canada, is the largest one in North America and processes 1.3 million m3 of raw sewage daily (Table 2). This primary- treated wastewater results from a physical and chemical treatment (screening and suspended matter removal by the addition of flocculants (alum 10 mg/L, FeCl3 10-20 mg/L) that removes suspended materials and associated contami- nants. The lightly treated effluent generally contains less than 5 mg/L of suspended solids but has relatively high coliform bacteria counts (concentrations greater than 1 million cells / 100 mL). Dissolved organic carbon (DOC) con- centrations and pH values ranged from 90 to 110 mg/L and 8.1 to 8.2, respectively.

Table 2.
Characteristics of the visited sewage treatment plants.
Municipal STP of Granby consists of mechanical pre-treatment (grid removal set-up and sand filtration), followed by a secondary treatment process involving the formation of aerobic activated sludge.
Regarding aerated lagoons, three municipal sewage treatment plants located in the cities of Chambly, St-Basile-le-Grand and Mascouche were each visited in triplicate. These STPs are connect- ed to a sewage system servicing about 17,000- 43,000 population equivalents with a mean flow rate of 21,000 m3/d. The Mascouche STP which receives mostly urban wastewaters from about 42,320 population equivalent is, in addition, directly connected to a hospital complex.
Pharmaceutical sampling and analysis
The PPCPs selected for the study are listed in Figure 1 alongside their respective chemical structures. Except the neutral carbamazepine, all investigated substances were acidic phar- maceuticals and their metabolites.
For the physicochemical and the biological sewage treatment plants (STPs), waters sam- ples were taken as 24 h flow-proportional com- posite samples from mechanical devices. Regarding the aerated lagoons, rapid snapshot samples were taken around noon at each STP. Mean pH values for all visited STP ranged from 8.1 to 8.3. Samples of treated and, in some cases, untreated effluents (or influents) were taken three times (from spring to fall) directly at the plant and transported to the laboratory in Spartanburg™ stainless steel containers and stored in the dark at 4ºC for less than 24 h until the extraction step. Prior to extraction, each wastewater sample was filtered under a nitrogen flow from the Spartanburg™ contain- er through a 142-mm glass fiber filter (0.7 μm) and then on a 90-mm GF/F glass microfiber fil- ter (0.7 μm) with a fritted, all-glass filtration device and Celite 545 under tab vacuum. Pharmaceutical residues were then extracted from wastewater samples following the method of Lajeunesse and Gagnon.[15] Briefly, solid-phase extractions were performed with polymeric cartridges (Strata-X™, Phenome- nex, Torrance, CA, USA). For the derivation step, the dried extract was reconstituted with 50 μL of acetonitrile and 100 μL of BSTFA + 10% TMCS. The substances under study were analysed by a GC-MS/MS system (Trace GC Ultra – PolarisQ, Thermo Electron Corpora- tion, Waltham, MA, USA).
The mean rate of recovery for 12 studied sub- stances in wastewater samples was 91.5% with values ranging from 72 (SALY) to 102% (TRI) where recoveries were similar (±5%) for influ- ent and effluent samples. Linearity tests were performed on extracted effluent samples by adding set amounts of analytes from 0 to 2000 ngL-1 prior to derivatisation and gave perfect linear trend with a mean correlation coefficient R2 >0.995 for all substances. Mean matrix effect was 105% with values varying between - 16% (CLO, suppression) and +22% (CAR, enhancement). Limit of detection (LOD) of the method was defined as the minimum detectable amount of analyte in effluent extract with a signal-to-noise ratio of 3:1 in SRM mode and values ranged from 1 to 18 ngL-1.
Results and Discussion
Occurrence of pharmaceutical products in treated wastewater
Physicochemical treatments
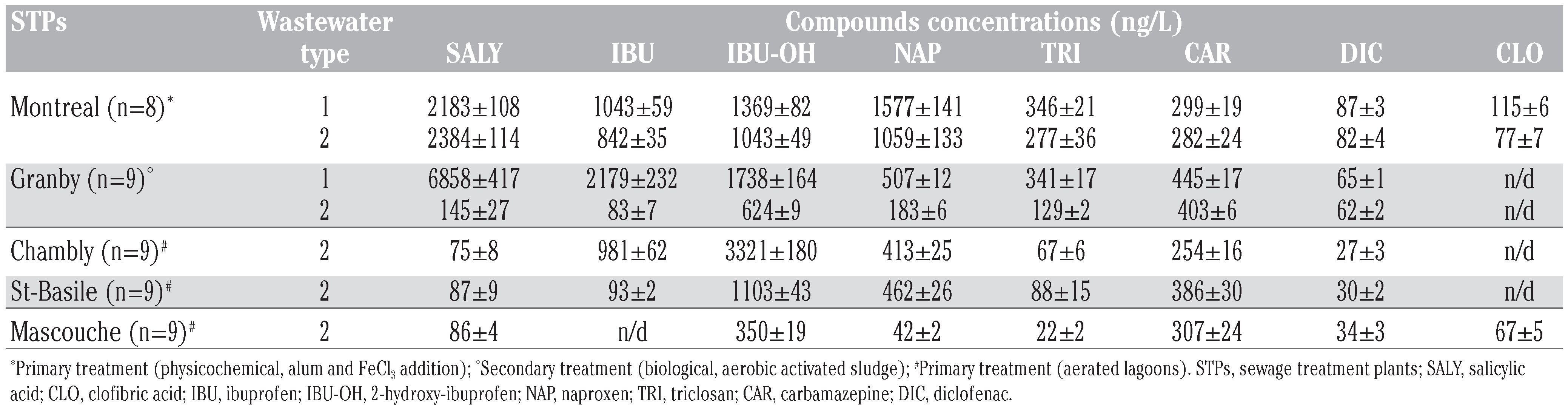
The most abundant pharmaceuticals were found in physicochemical-treated effluents. Concentrations of the target pharmaceutical products in the primary-treated Montreal efflu- ent ranged from 77 ng/L to 2384 ng/L (Table 3). Salicylic acid, 2-hydroxy-ibuprofen, ibuprofen, and naproxen were most abundant (>800 ng/L); indeed, these substances seem to resist physicochemical wastewater treatments, which are relatively ineffective in removing pharmaceuticals in general at the plant.[15,29,30,31]

Table 3.
Concentrations of pharmaceutical and personal care products in wastewater influent (1) and effluent (2) at different sewage treatment plants.
Biological treatments
Compared to physicochemical treatments, pharmaceutical substance concentrations were typically lower in biological-treated efflu- ents (Table 3). With the exception of the metabolite 2-hydroxy-ibuprofen, the highest concentration observed was for naproxen with a maximum concentration of 637 ng/L. Relatively high concentrations (<900 ng/L) of 2-hydroxy-ibuprofen could be explained by lower removal efficiency for the metabolite compare to its parent compounds.
Treatments using aerated lagoons
Concentrations of pharmaceuticals meas- ured in effluents from aerated lagoons were comparable, in several cases, to those from activated sludge (Table 3). The substances hydroxy-ibuprofen (350-3321 ng/L), ibuprofen (93-981 ng/L), naproxen (42-462 ng/L) and car- bamazepine (254-386 ng/L) were the most abundant in lagoon-treated wastewaters (Table 3). The metabolite 2-hydroxy-ibuprofen appeared in relatively high concentrations in comparison to its parent molecule ibuprofen. This observation could be explained by an extended aeration stage under bacterial activi- ty, as reported by Lishman et al.[8] This type of increase in metabolite forms was also observed with biological treatment processes using activated sludge (Table 3)
Removal of pharmaceutical substances from municipal wastewater
Physico-chemical treatments
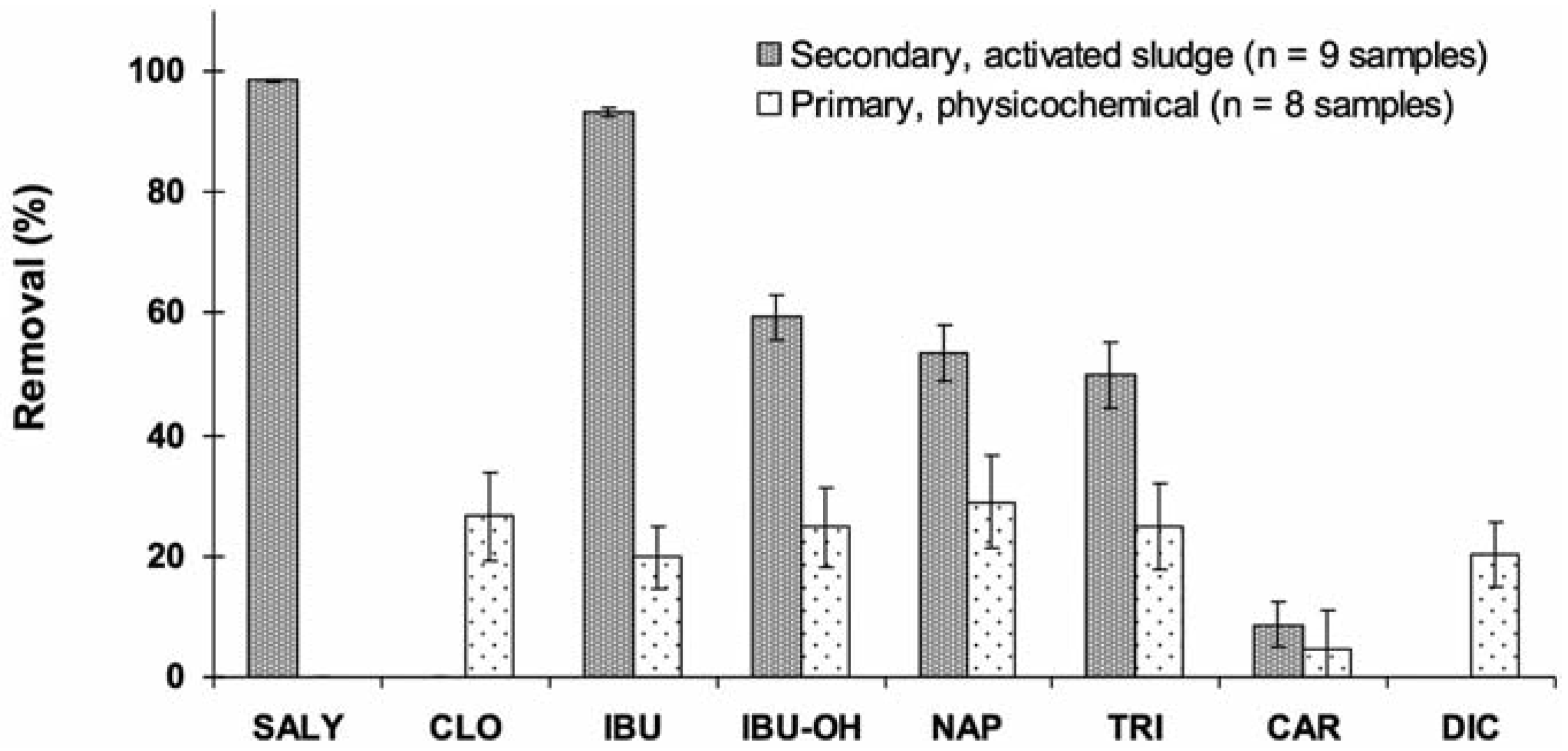
Removal of compounds from wastewaters was calculated as ([Influent] - [Effluent])/ [Influent]×100. Results in Figure 2 clearly depict low removal of pharmaceuticals in physicochemical-treated effluents. Best removal efficiencies were about 30% only. No significant removal was even observed for sali- cylic acid and carbamazepine. Based on a pub- lished database for hundreds substances, pri- mary treatments generally remove pharmaceu- ticals with low efficiency (0-40%) compared to biological treatments with removal efficiencies of 50-90%.[16] As this type of treatment is based on accelerated (forced) flocculation of matter, sorption onto suspended particles does not appear to be of relevance to these types of hydrophilic substances. Due to their polar struc- ture (Figure 1), most PPCPs are not removed in any significant way by treatment plants.[5,32] As an example, carbamazepine displays a moderat- ed affinity for solid phase,24 explaining the low removal efficiency observed at the physico- chemical plant (Figure 2). Another similar case was clofibric acid which also displays a low affinity for solid phase where the negligible sorption would be due to its dissociated form (pKa=2.84,).[24] An extreme case was ibuprofen and its metabolite hydroxyl-ibuprofen. Relatively low affinity for sorption onto particles for ibuprofen, and even practically no sorption for its metabolites, was reported for physico- chemical treatments.[33,34] However, among the acidic pharmaceuticals, naproxen was the most removed (29%) by this treatment type (Figure 2) and this could be explained by its sorption onto particles, a potential reduction process.[8]
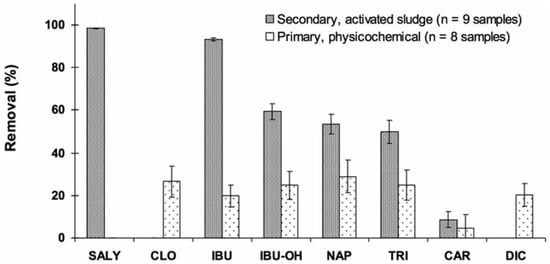
Figure 2.
Removal of pharmaceuticals from physicochemical and biologically treated wastewater effluents. SALY, salicylic acid; CLO, clofibric acid; IBU, ibuprofen; IBU-OH, 2-hydroxy-ibuprofen; NAP, naproxen; TRI, triclosan; CAR, carbamazepine; DIC, diclofenac.
Biological treatments
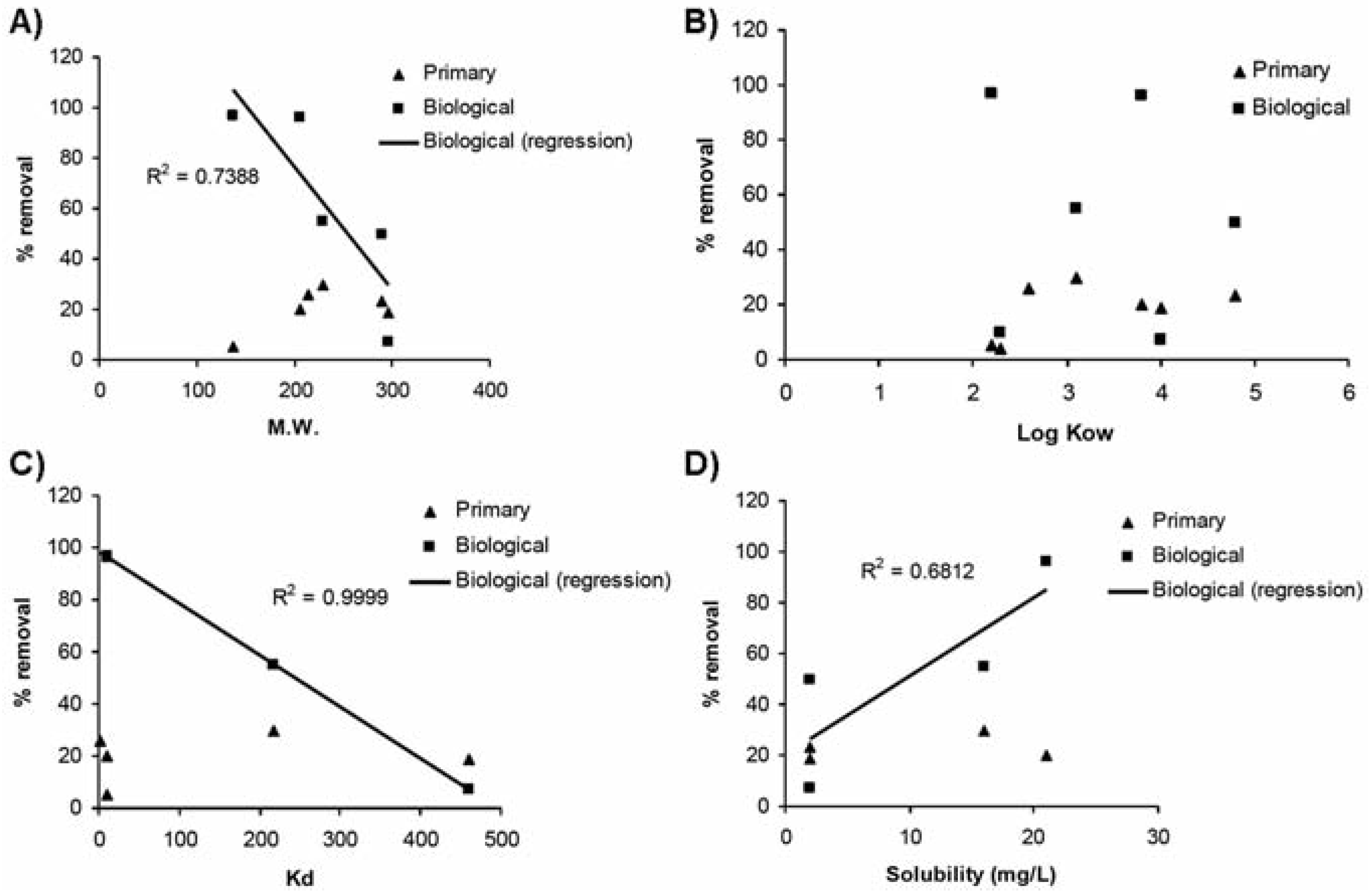
Biological treatment with activated sludge was found to be the most efficient (>50% for 5 of the 7 detected compounds) among all treat- ment types investigated (Figure 2). Salicylic acid and ibuprofen were practically eliminated (>93%). High removal efficiencies (>70%) were reported for these substances as the result of a rapid degradation.[17] Ibuprofen and naproxen as well were reported as pharmaceu- ticals that have high reduction (78-98%) in biological treatments.[8,35] At such high removal efficiency, treatment types were reported as of little importance despite we observed in this study quite low removal for the physicochemi- cal treatment. This non-biological treatment is more based on sorption process than degrada- tion. High removal efficiencies observed for the antibacterial triclosan (74-98%) by biologi- cal treatments were already reported by Lishman et al.[8] and Singer et al.[36] Removed tri- closan would be mostly (≈ 80%) biologically degraded while 15% of the removed fraction would be sorbed onto waste sludge.[36] Biodegradation was thus identified as the main removal mechanism for triclosan.[22] Despite triclosan is very hydrophilic, more than 95% of triclosan would be removed by activated sludge treatment.[22] While most sub- stances were highly affected by this type of treatment, carbamazepine and diclofenac remained slightly removed (4-9%). Similar removal efficiencies were also reported by Lee et al.[17] Extremely low degrability of carba- mazepine in biological treatment plants (<10%) is typically reported in the literature (e.g.,[23,24]). Interestingly, this treatment seemed to indicate selectivity with respect to the size and solubility of the removed substances (Table 1). This observation could point out cer- tain influence of the inherent properties of the studied substances on their fate in wastewater treatment plants. Despite the reported persist- ence of carbamazepine and diclofenac,[17,37] the smallest molecules were typically more removed than the largest ones. In this study, the size of the molecules was significantly cor-related (R2=0.7388) to its removal by biological treatments (Figure 3A). While the molecular weight of the substance seems to influence its removal at biological plants, no significant relationships were observed for physicochemi- cal plants (Figure 3A). With their low Kd values (Table 1), sorption onto sludge particles would not be significant.[23]
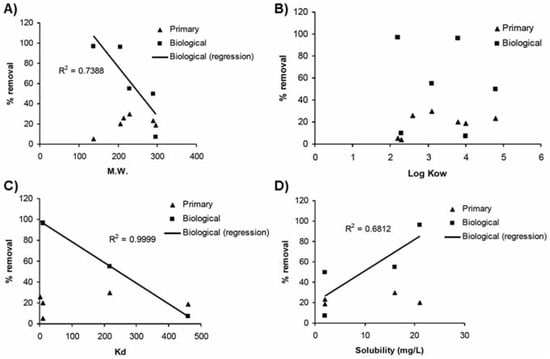
Figure 3.
Relationships between substance properties and their removal by wastewater treat- ment plants (open circle: physico-chemical treatments; solid circle: biological treatments). A) Molecular weight, B) octanol-water coefficient, C) partitioning coefficient, D) solubility.
The reported partitioning coefficients (Kd) were quite variable with values from less than 50 to 460 among the studied substances (Table 1). Great relationships (R2=0.9999), with the exception of the neutral carbamazepine, were observed between Kd values and removal effi- ciencies at biological treatment plants (Figure 3C). Pharmaceuticals having high affinity to particles were poorly (lower than 6%) removed by biological treatments. In the same way, the most soluble pharmaceuticals were the most degraded ones by biological treatments (R2=0.6812, Figure 3D). On the other hand, no relationships were observed in the case of physico-chemical treatments (Figure 3D). Removal at this type of treatment plants was typically low (<30%) for all studied substances, especially when compared to efficiency values at biological treatment plants (Figure 2). Their high solubility combined with their relatively low affinity for the particulate phase likely result in low removal, particularly by physico- chemical treatment plants.
Treatments by aerated lagoons typically seemed to result in mitigated rates of removal efficiency for several studied substances. Despite it is practically impossible to sample the exact water mass upstream the plant (due to variable flows over long residence period, 18 to 21 days) for purpose of comparison between concentrations after and before treatment, wastewater treatment using lagoons cannot be entirely considered with respect to the resilience of all substances studied here. Although no removal efficiency rates were therefore calculated for the long residence time treatment plants, the resulting concentrations after treatment could provide some insights on their removal efficiency. These final concen- trations, in some cases, were not significantly lower (Table 3) than ones in effluents of com- parable size and type of plant (e.g., Granby). Removal rates could be expected to be low for substances such as ibuprofen or carba- mazepine, which are either highly hydrophilic or biologically persistent. Better removal results seem to be observed for substances such as triclosan and diclofenac, which had low concentrations (<88 ng/L) in treated wastewater effluents. In fact, diclofenac was proved to be a light sensitive compound: rapid degradation of this molecule was reported in the literature after sunlight exposition in nat- ural environment.[38] As reported elsewhere, lagoon treatment was found as one of the best treatment process for the elimination of tri- closan, a well-known antibacterial substance used in many household products.[8]
Conclusions
The results of the present study clearly point out quite low removal efficiency of the hydrophilic pharmaceuticals from physico- chemical treatments. Much higher removal efficiencies were observed at aerated lagoons, and even better with biological processes like activated sludge. The removal efficiency was significantly influenced by the molecular size and partitioning of the substances. Certain substances such as carbamazepine, diclofenac and hydroxy-ibuprofen typically remained per- sistent in the investigated treatment plants.
Acknowledgments
the authors are indebted to the staff of the Montreal, Chambly, Granby, St-Basile-le-Grand and Mascouche STPs for their technical assistance. This work was funded by the St. Lawrence Action Plan.
References
- Halling-Sørensen, B.; Nors Nielsen, S.; Lanzky, P.F.; Ingerslev, F.; Holten Lutzhoft, H.C.; Jorgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the envi- ronment- A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environ- ment: agents of subtle changes? Environ. Health Perspect 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; et al. Behavior of pharmaceuticals, cosmetics and hor- mones in a sewage treatment plant. Water Res 2004, 38, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Gehr, R.; Nicell, J. Pilot studies and assess- ment of downstream effects of UV and ozone disinfection of a physicochemical wastewater. Water Qual Res J Canada 1996, 31, 263–281. [Google Scholar] [CrossRef]
- Heberer, T.; Dünnbier, U.; Reilich, C.; Stan, H.J. Detection of drugs and drug metabolites in ground water samples of a drinking water treatment plant? Fresenius. Environ Bull 1997, 6, 438–443. [Google Scholar]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- O’Brien, E.; Dietrich, D.R. Insight rather than foresight : reality versus the EU draft guide- line on pharmaceuticals in the environ- ment. Trends Biotechnol 2004, 22, 326–30. [Google Scholar] [CrossRef][Green Version]
- Lishman, L.; Smyth, S.A.; Sarafin, K.; Kleywegt, S.; Toito, J.; Peart, T.; et al. Occurrence and reductions of pharmaceuticals and personal care products and estrogens by municipal wastewater treatment plants in Ontario, Canada. Sci Total Environ 2006, 367, 544–58. [Google Scholar] [CrossRef]
- Kummerer, K. (Ed.) Pharmaceuticals in the environment, 2nd ed.; Springer: Berlin Heidelberg New-York, 2004. [Google Scholar]
- Gentili, A. Determination of non-steroidal anti-inflammatory drugs in environmental samples by chromatographic and elec- trophoretic techniques. Anal Bioanal Chem 2007, 387, 1185–1202. [Google Scholar] [CrossRef]
- Togola, A.; Budzinski, H. Analytical develop- ment of pharmaceuticals in water samples by SPE and GC-MS. Anal Bioanal Chem 2007, 388, 627–635. [Google Scholar] [CrossRef]
- Oulton, R.L.; Kohn, T.; Cwiertny, D.M. Pharma- ceuticals and personal care products in effluent matrices: a survey of transforma- tion and removal during wastewater treat- ment and implications for wastewater man- agement. J Environ Monit 2010, 12, 1956–1978. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; et al. Behavior of pharmaceuticals, cosmetics and hor- mones in sewage treatment plants. Water Res 2004, 38, 2918–2926. [Google Scholar] [CrossRef]
- Löffler, D.; Römbke, J.; Meller, M.; Ternes, T.A. Environmental fate of pharmaceuticals in water/sediment systems. Environ Sci Tech- nol 2005, 39, 5209–5218. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Gagnon, C. Determination of acidic pharmaceuticals and carbamazepine in roughly treated sewage by solid phase extraction and gas chromatography-tandem mass spectrometry. Intern J Environ Anal Chem 2007, 87, 565–578. [Google Scholar] [CrossRef]
- Miao, X.S.; Koenig, B.G.; Metcalfe, C.D. Ana- lysis of acidic drugs in the effluents of sewage treatment plants using liquid chro- matography - electrospray ionization tan- dem mass spectrometry. J Chromatogr A 2002, 952, 139–47. [Google Scholar] [CrossRef]
- Lee, R.B.; Safarin, K.; Peart, T.E.; Svoboda, M.L. Acidic pharmaceuticals in sewage – Methodology, stability test, occurrence and removal from Ontario Samples. Water Qual Res J Can 2003, 38, 667–682. [Google Scholar] [CrossRef]
- Miège, C.; Choubert, J.M.; Ribeiro, L.; Eusène, M.; Coquery, M. Fate of pharmaceuticals and personal care products in wastewater treat- ment plants – Conception of a database and first results. Environ Pollut 2009, 157, 1721–1726. [Google Scholar] [CrossRef]
- Miao, X.S.; Metcalfe, C.D. Determination of carbamazepine and its metabolites in aque- ous samples using liquid chromatography- electrospray tandem mass spectrometry. Anal Chem 2003, 75, 3731–3738. [Google Scholar] [CrossRef]
- Brun, G.L.; Bernier, M.; Losier, R.; Doe, K.; Jackman, P.; Lee, H.B. Pharmaceutically active compounds in Atlantic Canadian sewage treatment plant effluents and receiving waters, and potential for environmental effects as measured by acute and chronic aquatic toxicity. Environ Toxicol Chem 2006, 25, 2163–2176. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J Hazard Mater 2009, 168, 602–608. [Google Scholar] [CrossRef]
- Thompson, A.; Griffin, P.; Stuetz, R.; Cartmell, E. The fate and removal of triclosan during wastewater treatment. Water Environ Res 2005, 77, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Joss, A.; Keller, E.; Alder, A.C.; Göbel, A.; McArdell, C.S.; Ternes, T.; et al. Removal of pharmaceuti- cals and fragrances in biological wastewater treatment. Water Res 2005, 39, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Löffler, D.; Römbke, J.; Meller, M.; Ternes, T.A. Environmental fate of pharmaceuticals in water/sediment systems. Environ Sci Tech- nol 2005, 39, 5209–5218. [Google Scholar] [CrossRef] [PubMed]
- Kern, E.H.; Di, L. Chap. 7 Solubility. In Drug- like properties: Concepts, structure design and methods: from ADME to toxicity opti- mization; Academic Press Elsevier Ed.: Burlington, MA, 2008. [Google Scholar]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of aqueous solubility data, 2nd edition; CRC Press/Taylors & Francis Group: Boca Raton, FL, 2010. [Google Scholar]
- Grove, C.; Liebenberg, W.; Du Preez, J.L.; Yan, W.; De Villiers, M.M. Improving the aqueous sol- ubility of triclosan by solubilization, com- plexation, and in situ salt formation. J Cosmet Sci 2003, 54, 537–550. [Google Scholar] [PubMed]
- Gagnon, C.; Lajeunesse, A. Persistence and fate of highly soluble pharmaceutical prod- ucts in various types of municipal waste- water treatment plants. In Waste management and the environ- ment IV. International Conference on Waste Management and the Environment, Granada, Spain, 2008; Zamorano, M., Brebbia, C.A., Kungolos, A.G., Popov, V., Itoh, H., Eds.; WIT Press: Ashurst Lodge, UK, 2008; pp. 799–808. [Google Scholar]
- Lajeunesse, A.; Gagnon, C.; Sauvé, S. Determination of antidepressants and their N-desmethyl metabolites in raw sewage and wastewater using solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal Chem 2008, 80, 5325–5333. [Google Scholar] [CrossRef]
- Segura, P.A.; Garcia-Ac, A.; Lajeunesse, A.; Ghosh, D.; Gagnon, C.; Sauvé, S. Deter-mina- tion of six anti-infectives in wastewater using tandem solid phase extraction and liq- uid chromatography-tandem mass spec- troscopy. J Environ Monit 2007, 9, 307–313. [Google Scholar] [CrossRef]
- Snyder, S.A.; Westerhoff, P.; Yoon, Y.; Sedlak, D.L. Pharmaceuticals, personal care products, and endocrine disruptors in water: Implication for the water industry. Environ Eng Sci 2003, 20, 449–469. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol Lett 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Winkler, M.; Lawrence, J.R.; Neu, T.R. Selective degradation of ibuprofen and clofibric acid in two model river biofilm systems. Water Res 2001, 35, 3197–3205. [Google Scholar] [CrossRef]
- Buser, H.R.; Poigner, T.; Muller, M.D. Occurrence and environmental behavior of chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ Sci Technol 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Stumpf, M.; Ternes, T.A.; Wilken, R.D.; Rodrigues, S.V.; Bauman, W. Polar drug residues in sewage and natural waters in the state of Rio de Janeiro, Brazil. Sci Total Environ 1999, 225, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.; Muller, S.; Tixer, C.; Pillonel, l. Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treat- ment plants, surface waters, and lake sed- iments. Environ Sci Technol 2002, 36, 4998–5004. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Marotta, R.; Pinto, G.; Pollio, A. Carbamazepine in water: Persistence in the environment, ozonation treatment and pre- liminary assessment on algal toxicity. Water Res 2002, 36, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Poiger, T.; Buser, H.R.; Müller, M.D. Photo- degradation of the pharmaceutical drug diclo-fenac in a lake: pathway, field meas- urements, and mathematical modeling. Environ Toxicol Chem 2001, 20, 256–263. [Google Scholar] [CrossRef]
© Copyright C. Gagnon, A. Lajeunesse, 2012. Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 3.0 License (CC BY-NC 3.0).