Bisphenol A (BPA) Modifies Cancer Signaling Pathways: A Neglected Global Health Threat
Abstract
1. Introduction
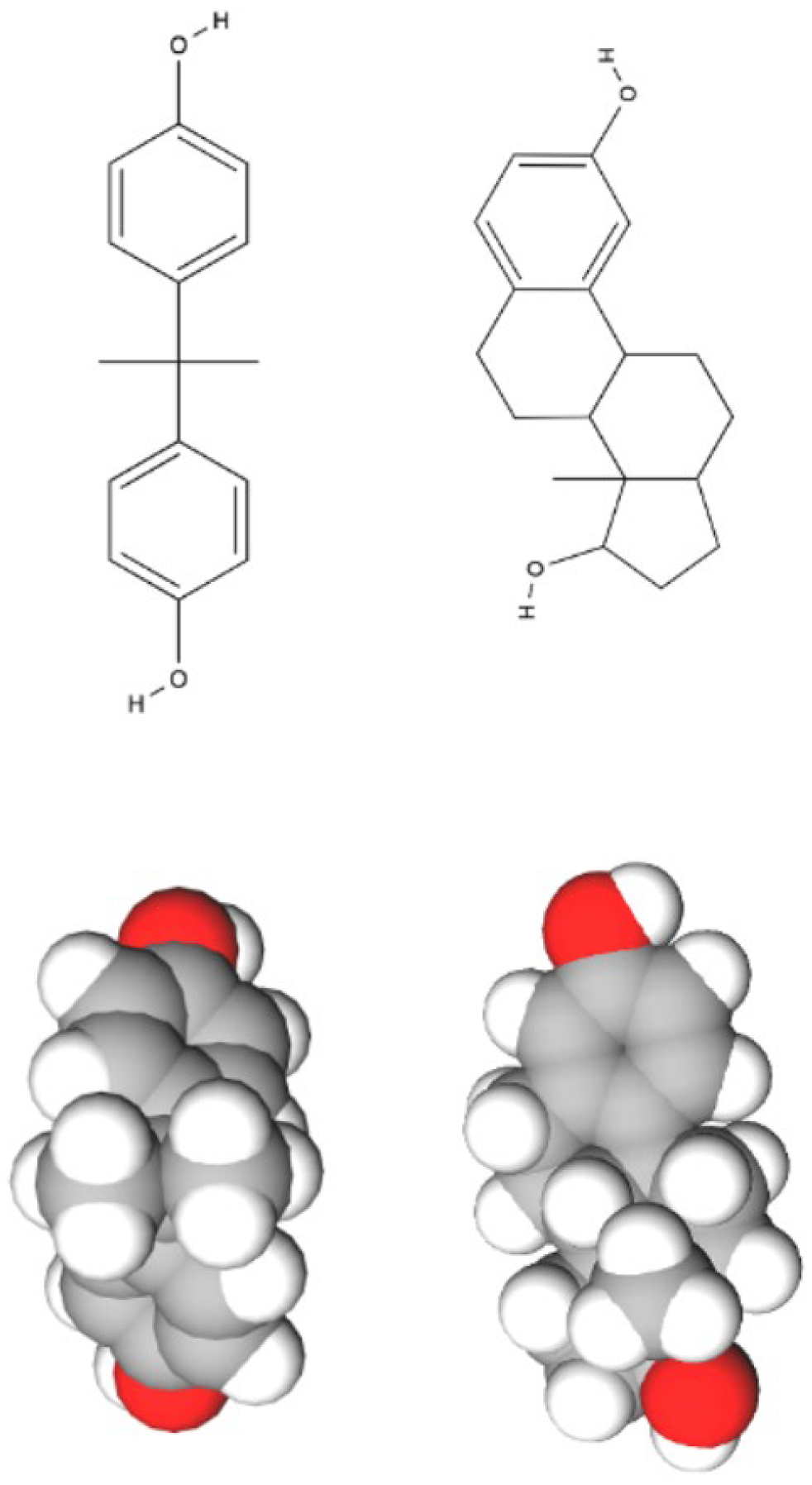
1.1. Plastics and BPA
1.2. BPA’s Routes of Exposure
1.3. BPA’s Mechanism of Action
1.4. Prevalence of Human Exposure to BPA
1.5. Economic Burden on Healthcare
2. Materials and Methods
3. Results
3.1. Breast Cancer
| Subtype | Cell Line | Dose of BPA and Exposure Time | Signaling Pathway and Target Gene | Results | Effect of Agonist/Antagonist | Reference |
|---|---|---|---|---|---|---|
| Estrogen receptor positive | MCF-7 | 0.01–1 μM of BPF and 0.001–1 μM of BPAF | ↑ERα and GPER1-mediated signaling pathways ↑MAPK ↑PI3K/Akt | ↑cell viability at lower doses ↓cell viability at higher doses ↑ROS ↑intracellular calcium ↑Cyclin D ↑c-myc | ↑Effect with ERα agonist (PPT); no effect with ERβ agonist (DPN) | [46] |
| MCF-7 | 10−7 to 10−5 M for 6 days | ↑ERα | ↑cell proliferation ↑progesterone receptor ↑cyclin D1 ↑G1/S transition ↓p21 | [36] | ||
| MCF-7 | 10−9 M (1 nM) for 24–48 h | ↑Erα ↑HOXB9 ↑phosphorylated ERK1/2 ↓p21 | ↑cell proliferation | HOXB9 silencing via siRNA ↓BPA-induced proliferation | [47] | |
| MCF-7 | 10−9 M BPA for every 24 h (up to 200 days) | ↑TNFα/NF-κB signaling | ↑epithelial–mesenchymal transition ↑cell proliferation ↑cell viability ↓E-cadherin expression ↑ATP concentration ↑migration ↑invasion | [33] | ||
| Triple-Negative Breast Cancer (TNBC) | TNBC 4T1 | 0.4, 1 and 2 μM BPA for 24 h and 1 μM BPA for 12, 18 and 24 h | ↑GPER | ↑MMP-2 ↑MMP-9 ↑cell migration ↑cell invasion | [40] | |
| TNBC MDA-MB-231 | 1 μM BPA for up to 48 h of treatment | ↑FAK ↑Src ↑ERK2 via GPER ↑EGFR transactivation pathway | ↑cell migration ↑focal adhesions assembly via GPER/EFGR | GPER knockdown with siRNA EGFR inhibitor (AG1478): Inhibit BPA-induced effects | [43] | |
| 7 | MDA-MB-231 MCF10A MCF12A | 0.1, 0.8, 1, 2, and 3 μM for up to 48 h of treatment | ↑GPER ↑p-FAK ↑Src ↑Ras/MEK/ERK1/2 | ↑cell migration ↑cell invasion ↑cell proliferation ↑AP-1-DNA binding ↑NFκB-DNA binding ↑p50 ↓IκBα | GPER siRNA FAK (FRNK), Src (PP2), and ERK2 inhibitors block BPA-induced effects | [39] |
| HER2-Positive ER-negative | SKBR3 CAF | 1 μM for up to 48 h of treatment | ↑ER ↑GPER/EGFR/ERK ↑c-FOS ↑CTGF | ↑cell proliferation ↑cell migration ↑ERK1/2 phosphorylation | GPER silencing (shGPER) EGFR and MEK inhibitors blocked BPA induced effects | [42] |
| MMTV-erbB2 | 0, 50, 500 ng/kg and 250 µg/kg | ↑ER and erbB2 ↑ERα, cyclin D1, and c-myc ↑EGFR, erbB-3, Erk1/2, and Akt ↑ERα ↑Bcl-2 ↑RTK signaling | ↑cell proliferation ↑ER-EGFR/erbB2 crosstalk | [48] | ||
| Multiple Subtypes (IBC), ER-negative | SUM149 SUM190 | 1 nM, 10 nM, 40 nM, 10 μM for up to 3 weeks | ↑EGFR/HER2 ↑NF- κB | ↑cell proliferation ↑tumor growth ↑SOD1 ↑Bcl-2 ↓apoptosis | EGFR inhibitor (GW583340/lapatinib analog) | [38] |
| MCF-7 SK-BR3 MDA-MB-231 | 10−8 M for 30 days | ↑VEGF/VEGFR ↑HIF signaling pathways | ↑cell viability ↑cell proliferation ↑cell migration ↑IL19, CA9 and SPARC ↑NKT, NK and T cell | [34] | ||
| BVEC SkBr-3 MDA-MB-231 | 150 mg/kg | ↑HIF-1α/VEGF ↑GPER | ↑cell migration ↑cell proliferation ↑cell viability ↑tumor growth | [35] |
3.2. Ovarian Cancer
| Subtype | Cell Line | Dose of BPA and Exposure Time | Signaling Pathway and Target Gene | Results | Reference |
|---|---|---|---|---|---|
| Serous Carcinoma | SKOV3 cells A2780 cells | 10 or 100 nM for 24 h | ↑Canonical Wnt Pathway ↑PI3K/Akt | ↑migration ↑invasion ↑β-catenin translocation to nucleus ↑miR-21 ↑miR-222 ↑EMT ↑MMP9 ↓SERPINB5 (maspin) ↓TIMP3 ↓ZO-1 | [49] |
| OVCAR-3 | 0.1, 10, 40, and 100 nM for 3, 6, 24, and 48 h | ↑MAPK ↑PI3K/Akt | ↑cell migration ↑MMP-2 ↑MMP-9 ↑N-cadherin | [50] | |
| OVCAR-3 | 0.2, 2, 8 and 20 ng/mL for 24 h | ↑Leptin ↑p-Stat3 ↑ERK1/2 ↑p-Akt. | ↑cell proliferation | [53] | |
| SKOV3 A2780 | 10 nM/100 nM/1 μM/10 μM/100 μM/1 mM for 24 h | ↑PINK1 ↓p53 | ↑OCT4 ↑NANOG ↑SOX2 ↓TOM20 and TIM23 ↑mitophagy | [19] | |
| OVCAR-3 | 10−5, 10−6, 10−7, 10−8, 10−9, 10−10 M for 24 h | ↑WNT/β-catenin pathway | ↑ALDH1A1 ↑CD133 ↑SOX2 ↑NANOG ↑OCT4 ↑CD44+CD24− | [56] | |
| ES-2 OVCAR-3 | 0.1, 1, 10, and 100 μM | ↑ERα/AKT/mTOR/HIF-1α ↑PI3K-AKT | ↑proliferation ↑migration ↑invasion ↑HIF-1α ↑GLUT3 ↑LDHA ↑lactate release ↑ATP production ↑Glycolysis | [51] | |
| OVCAR-3 | 1, 10 or 100 nM for 24 h or 5 days | ↑ERα | ↑Proliferation ↑Migration ↑Invasion ↑adhesion ↑MMP-2 andMMP-9 | [52] | |
| Endometroid Carcinoma | BG-1 | 10−6 M | ↑ER pathway ↑c-Fos gene ↓TGF-β | ↑cell proliferation ↑tumor volume ↑BrdU-positive nuclei ↑SnoN ↓p- Smad3 | [57] |
| BG-1 | 10−6 M for 1–48 h | ↑ER pathway ↓TGF-β | ↑cell migration ↑SnoN ↑vimentin ↓metastasis ↓E-cadherin ↓p- Smad3 | [54] | |
| BG-1 A2780 | 10−9 to 10−5 M for 24 h | ↑p38 MAPK ↑ERK 1/2 | ↑cell proliferation ↑tumor growth | [58] | |
| BG-1 | 10−5 M for 5 days | ↑ERα ↑IGF-1R | ↑cell proliferation ↑cell viability ↑p-IRS-1 ↑p-Akt 1/2/3 ↑cyclin D1 | [55] | |
| Multiple | OVCAR-3 SKOV-3 OV434 KGN | 1–20 nM in human serum | ↑ER signaling pathways | ↑cell proliferation ↑leptin mRNA ↑PPARγ ↓adiponectin ↓chemerin ↑PPARγ | [59] |
| 100 fM to 100 mM for 24 to 72 h | ↓PTEN ↑PI3K/Akt | ↑p-AKT(Thr308) ↑p-AKT(Ser473) ↑apoptosis ↑follicular atresia ↓cell viability ↓primordial follicle pool (Ser473) | [60] |
3.3. Other Cancers
| Cancer Type | Cell Line | Dose of BPA and Exposure Time | Signaling Pathway and Target Gene | Results | Reference |
|---|---|---|---|---|---|
| Uterine leiomyoma | UL | 10 μmol/L | ↑ERα ↑IGF-1 ↑VEGF | ↑cell proliferation ↑SnoN ↓p- Smad3 ↓c-fos protein | [72] |
| Uterine leiomyoma | Human UL cells | 100 μL of medium with 103 μmol/L of E2 10 μmol/L of BPA 32 μmol/L of NP 8 μmol/L of octylphenol for 24–72 h | ↑ERα signaling ↑IGF-1 ↑VEGF ↑Akt | ↑cell proliferation ↑SnoN protein ↓p-Smad3 protein ↓c-fos protein | [73] |
| Hepatocarcinoma | Hep3B MCF-7 LM8 | 50–200 µM of BPA or BPAF under normoxia or hypoxia for 6 h | ↑Lysosomal and Proteasomal Pathway | ↑cell proliferation ↑HSC70 ↓HIF-1alpha ↓EPO mRNA | [69] |
| Adrenal cortical | H295A NCI-H295 | 1–1000 nM for 72 h | ↑Shh pathway ↑ERβ-mediated activation | ↑adrenal gland weight ↑cell proliferation ↑nuclear translocation of Erβ ↑cyclin D1 ↑cyclin D2 | [63] |
| Lung | Lung tissue of adult female Wistar rats | 150 mg/kg body weight/day for 6 weeks | ↑EGFR ↑KRAS ↑ERK1/2 | ↑MMP-2 ↑MMP-9 ↑TNF-α, IL-6, IL-1β ↑GRP | [74] |
| Colon | HT-29 | 4.4 µM | ↑Erα ↑miR-200c and miR-141 | ↓PTEN ↓ATR | [64] |
| Colon | HCoEpiC HCT116 | 0.0043 nM for 2 months | ↑Wnt/β-catenin pathway | ↑Invasion ↑Proliferation ↑Migration | [66] |
| Colon | HT-29 | 4.4, 6.6, 8.8 µM | ↓FADD, FAS ↓APAF-1 ↓CASP2 ↓CASP9 ↓FOXO3 ↓P53 | ↑ESR1 ↑proliferation | [65] |
| Thyroid | BCPAP Nthy-ori3-1 Thyroid tissue of 3–4 weeks-old female SD rats | 10–10 −5 × 10−5 mol/L and 50 μmol/L for 24 h and 48 h | ↑ERK | ↑β-catenin ↑HDAC6 ↓PTEN ↑c-MYC ↑proliferation ↑AKT signaling ↑Migration ↑phospho-Akt | [75] |
| Endometrial | HEC265 | 1 nM to 1 μM for 0.5, 1, 3, and 6 h and 24 h | ↑activation of the EGFR/ERK pathway | ↑cell proliferation ↑nuclear translocation of ERRγ ↑influx of Ca2+ ↑EGF secretion | [62] |
| Osteosarcoma | SaOs-2 | 0.1, 1, 10 μM | ↑IL6/JAK2/STAT3 | ↑proliferation ↑migration ↑invasion ↑DLGAP5 | [76] |
| Neuroblastoma | IMR-32 SK-N-SH cells | 0, 1, 10, and 100 nM, and 1, 10, and 100 μM for 24 h | ↑NLRP3/caspase-1/GSDMD | ↑cell apoptosis ↑inflammation ↑IL-18, ASC, GSDMD ↑NLRP3, caspase-1 and GSDMD ↑ROS ↑LDH ↓mitochondrial membrane potential | [71] |
| Myeloblastic | Peripheral blood cells, kidney, liver and spleen tissue of embryos, larvae and adult zebrafish | 100, 500 and 2500 μg/L for 96 hpf (larvae) 5 to 30 mg/L concentrations for 96 h. (adult) | ↑EGFR/ERK signaling | ↑cell apoptosis ↑binding affinity to zebrafish EGFR ↑EGFR | [77] |
| Lymphoma | TK6 SUP-B15 | 10, 103, 105 nM of BPA for 24, 48, and 72 h | ↑CTNNB1 | ↑Survival of TK6 lymphoblastoid cells ↓TP53 ↓CDKN1A ↑NFKB1 ↑AR ↑IGF1 ↑TWIST1 | [70] |
| Nasopharyngeal | NPC CNE2 CNE1 5–8F | 10 nM BPA for 12 h, 24 h, 48 h | ↑Wnt/β-catenin pathway | ↑mRNA stability of β-catenin via miR-214-3 ↓pSer45 of β-catenin via CK1α ↑proliferation ↑migration ↑mRNA stability of β-catenin ↓miR-214-3p ↓phosphorylation of β-catenin ↓CK1α ↓CTNNB1 | [61] |
| Bronchial and epithelial | BEAS-2B | 12.5–200 μM for 24 h | ↑ER/GPR30-ERK signaling pathway | ↑ROS ↑DNA strand break ↑DNA tail formation ↑DNA histone damage ↑p-ATM/ATR complex ↑p-γH2AX | [68] |
| Reproductive Tract | SKOV3 BG-1 A2780 LNCaP | 100 μM BPA 10, 50, or 100 μM NP for 24 h | ↑ADAM17 ↑ERK pathway | ↑apoptosis at higher doses ↑migration ↑intracellular calcium ↑cell proliferation | [78] |
3.4. BPA Derivatives
| Cancer Type | Cell Line | BPA derivative and Concentration | Pathway | Results | Reference |
|---|---|---|---|---|---|
| Breast | Human breast cancer cells 4T1, 4T1-Luc and MDA-MB-231 | 5 mg/kg body mass via intraperitoneal administration of BPM, BPP, and BPA. | AKT | ↑Invasion, ↑Migration, ↑EMT expression, ↑AKT phosphorylation | [89] |
| Breast | MCF-7 cells of the breast cancer cell line | Final concentrations of 1 μM, 100 nM, and 10 nM of BPS were used. Following a 48-h incubation to allow cell attachment, the medium was changed to include various concentrations of BPS. | N/A a | ↑Methylation in transposons of MCF-7 cells and most breast cancer-related genes, ↑Gene expression of breast cancer cells | [92] |
| Breast | MDA-MB-231 human breast cancer cells | BPS-10 (10 μg/kg body weight/day) and BPS-100 (100 μg/kg body weight/day) | N/A a | ↑Proliferation, ↑Deterioration, ↑Intratumor heterogeneity of lipid and protein distribution | [93] |
| Breast | MCF-7 cells of breast cancer cell line | Ranged from 0.00001 to 100 μM | ERα and GPER1 | ↑Cell proliferation, ↑Cell viability, ↑Intracellular ROS and Ca2+, ↑Protein expressions of ERα, GPER1, c-myc, and cyclin D, ↑Phosphorylation of PKB and ERK1/2 | [46] |
| Breast | Human TNBC MDA-MB-231 and BT-549 breast cancer cell cultures | Nanomole (10−9 M) to millimole (10−3 M) | GPER/Hippo-YAP | ↑Migration, ↓YAP and TAZ phosphorylation levels, ↑CTGF and ANKRD1, ↓Phosphorylation levels of LATS1/2 | [88] |
| Includes breast | Human primary term cytotrophoblast cells (hCTBs) and MDA-MD-231 cells | 0.0001 to 10 μg/mL | N/A a | ↓EGF binding, ↓EGF-mediated phosphorylated EGFR, ↓EGF internalization, ↓EGF-mediated hCTB syncytialization at 200 ng/mL of BPS | [90] |
| Lung | Human NSCLC cell A549, H1299 and H358 cell cultures | 1, 10 and 100 nM of BPS, | TGF-β | ↑Migration of NSCLC cells, ↑Vimentin, and MMP-2, ↑TGF-β expression and transcription, ↑Smad2/3 activation, ↑IL-8 expression in A549 cell, ↑IL-10 expression in H1299 cell | [91] |
| Bladder | RT4 Non-Invasive Bladder Cancer Cells, T24 Invasive Bladder Cancer Cells and normal Urothelial Cells | 10−8 M BPA-gluc and 10−8 M BPS-gluc | N/A a | ↓Basal Glycolytic Capacity and migration of UCs, ↑Basal and maximal glycolytic capacity, mitochondrial respiration, and proliferation of RT4 cells, ↑Maximal glycolytic capacity and migration of T24 cells, ↑Proliferation of UCs | [94] |
| Type of Cancer | Pathway | Cell Line | Results | BPA/Derivatives Concentrations | Reference |
|---|---|---|---|---|---|
| Breast | AKT | Human breast cancer cells 4T1, 4T1-Luc and MDA-MB-231 | ↑Invasion, ↑Migration, ↑EMT expression, ↑AKT phosphorylation | 5 mg/kg body mass via intraperitoneal administration of BPM, BPP, and BPA. | [89] |
| Breast | N/A a | MCF-7 cells of the breast cancer cell line | ↑Methylation in transposons of MCF-7 cells and most breast cancer-related genes, ↑Gene expression of breast cancer cells | 1 μM, 100 nM, and 10 nM final concentrations of BPS were used. Following a 48-h incubation to allow cell attachment, the medium was changed to include various concentrations of BPS. | [92] |
| Breast | N/A a | MDA-MB-231 human breast cancer cells | ↑Proliferation, ↑Deterioration, ↑Intratumor heterogeneity of lipid and protein distribution | BPS-10 (10 μg/kg body weight/day) and BPS-100 (100 μg/kg body weight/day) | [95] |
| Breast | ERα and GPER1 | MCF-7 cells of the breast cancer cell line | ↑Cell proliferation, ↑Cell viability, ↑Intracellular ROS and Ca2+, ↑Protein expressions of ERα, GPER1, c-myc, and cyclin D, ↑Phosphorylation of PKB and ERK1/2 | Ranged from 0.00001 to 100 μM | [46] |
| Breast | GPER/Hippo-YAP | Human TNBC MDA-MB-231 and BT-549 breast cancer cell cultures | ↑Migration, ↓YAP and TAZ phosphorylation levels, ↑CTGF and ANKRD1, ↓Phosphorylation levels of LATS1/2 | Nanomole (10−9 M) to millimole (10−3 M) | [88] |
| Includes breast | N/A a | Human primary term cytotrophoblast cells (hCTBs) and MDA-MD-231 cells | ↓EGF binding, ↓EGF-mediated phosphorylated EGFR, ↓EGF internalization, ↓EGF-mediated hCTB syncytialization at 200 ng/mL of BPS | 0.0001 to 10 μg/mL | [90] |
| Lung | TGF-β | Human NSCLC cell A549, H1299 and H358 cell cultures | ↑Migration of NSCLC cells, ↑Vimentin, and MMP-2, ↑TGF-β expression and transcription, ↑Smad2/3 activation, ↑IL-8 expression in A549 cell, ↑IL-10 expression in H1299 cell | 1, 10, and 100 nM of BPS, | [91] |
| Bladder | N/A a | RT4 Non-Invasive Bladder Cancer Cells, T24 Invasive Bladder Cancer Cells and normal Urothelial Cells | ↓Basal Glycolytic Capacity and migration of UCs, ↑Basal and maximal glycolytic capacity, mitochondrial respiration and proliferation of RT4 cells, ↑Maximal glycolytic capacity and migration of T24 cells, ↑Proliferation of UCs | 10−8 M BPA-gluc and 10−8 M BPS-gluc | [94] |
4. Discussion
4.1. BPA and Cancer Signaling Pathways
4.2. Health Risks Associated with BPA
4.3. Clinical Relevance of BPA Exposure
4.3.1. BPA’s Role in Chemoresistance
4.3.2. Possible Treatments for BPA-Induced Carcinogenesis
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein Kinase B |
| AR | Androgen Receptor |
| ATM | Ataxia-Telangiectasia Mutated |
| ATP | Adenosine Triphosphate |
| ATR | ATM and Rad3-Related |
| BG-1 | Human Ovarian Adenocarcinoma Cell Line |
| BPA | Bisphenol A |
| BPAF | Bisphenol AF |
| BPF | Bisphenol F |
| BPM | Bisphenol M |
| BPP | Bisphenol P |
| BPS | Bisphenol S |
| CAGR | Compound Annual Growth Rate |
| DNA | Deoxyribonucleic Acid |
| ECHA | European Chemicals Agency |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial-to-Mesenchymal Transition |
| ER | Estrogen Receptor |
| ERK | Extracellular Signal-Regulated Kinase |
| ERRγ | Estrogen-Related Receptor Gamma |
| GPR30 | G Protein-Coupled Receptor 30 |
| GPCR | G Protein-Coupled Receptor |
| GPER | G Protein-Coupled Estrogen Receptor |
| HIF | Hypoxia-Inducible Factor |
| HT-29 | Human Colorectal Adenocarcinoma Cell Line |
| ICI | Imperial Chemical Industries |
| IGF-1R | Insulin-Like Growth Factor 1 Receptor |
| II | Grade II |
| III | Grade III |
| IMR-32 | Human Neuroblastoma Cell Line |
| JCP | Journal of Cancer Prevention |
| MBP | 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene |
| MCF-7 | Michigan Cancer Foundation-7 |
| MD | Medicine |
| MDA-MB | MD Anderson–Metastatic Breast Cancer |
| MMP | Matrix Metalloproteinase |
| MMP-2 | Matrix Metalloproteinase-2 |
| MMP-9 | Matrix Metalloproteinase-9 |
| MAPK | Mitogen-Activated Protein Kinase |
| MYC | c-Myc Oncogene |
| NSCLC | Non-Small Cell Lung Cancer |
| NPC | Nasopharyngeal Carcinoma |
| OVCAR-3 | Human Ovarian Cancer Cell Line |
| PCNA | Proliferating Cell Nuclear Antigen |
| PKB | Protein Kinase B |
| PKC | Protein Kinase C |
| PTEN | Phosphatase and Tensin Homolog |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| SH | Sonic Hedgehog |
| SHH | Sonic Hedgehog |
| SMA | Smooth Muscle Actin |
| SK-BR3 | HER2-Positive Breast Cancer Cell Line |
| SK-N | Neuroblastoma Cell Line Family |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| SUP-B15 | Lymphoblastic Leukemia Cell Line |
| SVHC | Substance of Very High Concern |
| TDI | Tolerable Daily Intake |
| TGF | Transforming Growth Factor |
| TIMP | Tissue Inhibitors of Metalloproteinases |
| TNBC | Triple-Negative Breast Cancer |
| USA | United States of America |
| US | United States |
| UV | Ultraviolet |
| VGEF | Vascular Endothelial Growth Factor |
| YAP | Yes-Associated Protein |
| ERα | Estrogen Receptor Alpha |
| ERβ | Estrogen Receptor Beta |
| PI3K | Phosphoinositide 3-Kinase |
| mTOR | Mechanistic Target of Rapamycin |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| NF-κB | Nuclear Factor Kappa B |
| JAK | Janus Kinase |
| HIF-1α | Hypoxia-Inducible Factor-1 Alpha |
| VEGF | Vascular Endothelial Growth Factor |
| TGF-β | Transforming Growth Factor Beta |
| FOXO3 | Forkhead Box O3 |
| PINK1 | PTEN-Induced Kinase 1 |
| SnoN | Ski-Related Novel Protein N |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene |
References
- Subramanian, M.N. Plastics Processing Technology. In Plastics Waste Management; Wiley: Hoboken, NJ, USA, 2019; pp. 53–72. [Google Scholar] [CrossRef]
- Yang, Y.J.; Hong, Y.-C.; Oh, S.-Y.; Park, M.-S.; Kim, H.; Leem, J.-H.; Ha, E.-H. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ. Res. 2009, 109, 797–801. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; An, L.; Liu, Q.; Ding, J. The Value of China’s Legislation on Plastic Pollution Prevention in 2020. Bull. Environ. Contam. Toxicol. 2022, 108, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Gil-Solsona, R.; Castaño-Ortiz, J.M.; Muñoz-Mas, R.; Insa, S.; Farré, M.; Ospina-Alvarez, N.; Santos, L.H.M.L.M.; García-Pimentel, M.; Barceló, D.; Rodríguez-Mozaz, S. A holistic assessment of the sources, prevalence, and distribution of bisphenol A and analogues in water, sediments, biota and plastic litter of the Ebro Delta (Spain). Environ. Pollut. 2022, 314, 120310. [Google Scholar] [CrossRef] [PubMed]
- Geueke, B.; Groh, K.J.; Maffini, M.V.; Martin, O.V.; Boucher, J.M.; Chiang, Y.-T.; Gwosdz, F.; Jieh, P.; Kassotis, C.D.; Łańska, P.; et al. Systematic evidence on migrating and extractable food contact chemicals: Most chemicals detected in food contact materials are not listed for use. Crit. Rev. Food Sci. Nutr. 2023, 63, 9425–9435. [Google Scholar] [CrossRef]
- Trivedi, J.; Chhaya, U. Bioremediation of bisphenol A found in industrial wastewater using Trametes versicolor (TV) laccase nanoemulsion-based bead organogel in packed bed reactor. Water Environ. Res. 2022, 94, e10786. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.-P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.-M.; Pussemier, L.; Scippo, M.-L.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Morris, B. The components of the Wired Spanning Forest are recurrent. Probab. Theory Relat. Fields 2003, 125, 259–265. [Google Scholar] [CrossRef]
- Gibson, R.; Durán-Álvarez, J.C.; Estrada, K.L.; Chávez, A.; Jiménez Cisneros, B. Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 2010, 81, 1437–1445. [Google Scholar] [CrossRef]
- Zhang, C.; Li, M.; Chen, X.; Li, M. Edible fungus degrade bisphenol A with no harmful effect on its fatty acid composition. Ecotoxicol. Environ. Saf. 2015, 118, 126–132. [Google Scholar] [CrossRef]
- Steinmetz, R.; Mitchner, N.A.; Grant, A.; Allen, D.L.; Bigsby, R.M.; Ben-Jonathan, N. The Xenoestrogen Bisphenol A Induces Growth, Differentiation, and c-fos Gene Expression in the Female Reproductive Tract*. Endocrinology 1998, 139, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Winz, C.; Suh, N. Understanding the Mechanistic Link between Bisphenol A and Cancer Stem Cells: A Cancer Prevention Perspective. J. Cancer Prev. 2021, 26, 18–24. [Google Scholar] [CrossRef]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef]
- Gao, H.; Yang, B.-J.; Li, N.; Feng, L.-M.; Shi, X.-Y.; Zhao, W.-H.; Liu, S.-J. Bisphenol A and Hormone-Associated Cancers: Current Progress and Perspectives. Medicine 2015, 94, e211. Available online: https://journals.lww.com/md-journal/fulltext/2015/01010/bisphenol_a_and_hormone_associated_cancers_.6.aspx (accessed on 17 July 2025). [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef]
- MacKay, H.; Abizaid, A. A plurality of molecular targets: The receptor ecosystem for bisphenol-A (BPA). Endocr. Disrupting Chem. Behav. 2018, 101, 59–67. [Google Scholar] [CrossRef]
- Gronemeyer, H.; Gustafsson, J.-Å.; Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004, 3, 950–964. [Google Scholar] [CrossRef]
- Batista-Silva, H.; Rodrigues, K.; De Moura, K.R.S.; Elie, N.; Van Der Kraak, G.; Delalande, C.; Silva, F.R.M.B. In vivo and in vitro short-term bisphenol A exposures disrupt testicular energy metabolism and negatively impact spermatogenesis in zebrafish. Reprod. Toxicol. 2022, 107, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, K.; Zheng, F.; Xu, S.; Li, Y.; Wang, Y.; Ni, H.; Wang, F.; Cui, Z.; Qin, Y.; et al. Low-dose BPA and its substitute BPS promote ovarian cancer cell stemness via a non-canonical PINK1/p53 mitophagic signaling. J. Hazard. Mater. 2023, 452, 131288. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Liu, P.; Phong, K.; Hinrichs, J.H.; Ataii, N.; Williams, K.; Hadler-Olsen, E.; Samson, S.; Gartner, Z.J.; Fisher, S.; et al. Bisphenol A replacement chemicals, BPF and BPS, induce protumorigenic changes in human mammary gland organoid morphology and proteome. Proc. Natl. Acad. Sci. USA 2022, 119, e2115308119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Liu, S. Low-Dose Bisphenol A Exposure: A Seemingly Instigating Carcinogenic Effect on Breast Cancer. Adv. Sci. 2017, 4, 1600248. [Google Scholar] [CrossRef]
- Seachrist, D.D.; Bonk, K.W.; Ho, S.-M.; Prins, G.S.; Soto, A.M.; Keri, R.A. A review of the carcinogenic potential of bisphenol A. Reprod. Toxicol. 2016, 59, 167–182. [Google Scholar] [CrossRef]
- Cathey, A.L.; Nguyen, V.K.; Colacino, J.A.; Woodruff, T.J.; Reynolds, P.; Aung, M.T. Exploratory profiles of phenols, parabens, and per- and poly-fluoroalkyl substances among NHANES study participants in association with previous cancer diagnoses. J. Expo. Sci. Environ. 2023, 33, 687–698. [Google Scholar] [CrossRef]
- Ho, S.-M.; Cheong, A.; Adgent, M.A.; Veevers, J.; Suen, A.A.; Tam, N.N.C.; Leung, Y.-K.; Jefferson, W.N.; Williams, C.J. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Dev. Orig. Dis. 2017, 68, 85–104. [Google Scholar] [CrossRef]
- Wormsbaecher, C.; Hindman, A.R.; Avendano, A.; Cortes-Medina, M.; Jones, C.E.; Bushman, A.; Onua, L.; Kovalchin, C.E.; Murphy, A.R.; Helber, H.L.; et al. In utero estrogenic endocrine disruption alters the stroma to increase extracellular matrix density and mammary gland stiffness. Breast Cancer Res. 2020, 22, 41. [Google Scholar] [CrossRef]
- Chevalier, N.; Fénichel, P. Endocrine disruptors: New players in the pathophysiology of type 2 diabetes? Diabetes Metab. 2015, 41, 107–115. [Google Scholar] [CrossRef]
- van Woerden, I.; Bruening, M.; Montresor-López, J.; Payne-Sturges, D.C. Trends and disparities in urinary BPA concentrations among U.S. emerging adults. Environ. Res. 2019, 176, 108515. [Google Scholar] [CrossRef]
- LaKind, J.S.; Pollock, T.; Naiman, D.Q.; Kim, S.; Nagasawa, A.; Clarke, J. Factors affecting interpretation of national biomonitoring data from multiple countries: BPA as a case study. Environ. Res. 2019, 173, 318–329. [Google Scholar] [CrossRef]
- Nelson, J.W.; Scammell, M.K.; Hatch, E.E.; Webster, T.F. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: A cross-sectional study within NHANES 2003–2006. Environ. Health 2012, 11, 10. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann. Glob. Health 2023, 89, 23. [Google Scholar] [CrossRef]
- Werkneh, A.A.; Gebru, S.B.; Redae, G.H.; Tsige, A.G. Removal of endocrine disrupters from the contaminated environment: Public health concerns, treatment strategies and future perspectives—A review. Heliyon 2022, 8, e09206. [Google Scholar] [CrossRef]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef]
- Ansari, M.I.; Bano, N.; Kainat, K.; Singh, V.K.; Sharma, P.K. Bisphenol A exposure induces metastatic aggression in low metastatic MCF-7 cells via PGC-1α mediated mitochondrial biogenesis and epithelial-mesenchymal plasticity. Life Sci. 2022, 302, 120649. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.S.; Moon, W.K. Comparison of transcriptome expression alterations by chronic exposure to low-dose bisphenol A in different subtypes of breast cancer cells. Toxicol. Appl. Pharmacol. 2019, 385, 114814. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, X.; Wu, N.; He, S.; Yi, W.; Xiang, S.; Zhang, P.; Xie, X.; Ying, C. Bisphenol A induces proliferative effects on both breast cancer cells and vascular endothelial cells through a shared GPER-dependent pathway in hypoxia. Environ. Pollut. 2017, 231, 1609–1620. [Google Scholar] [CrossRef]
- Lee, H.-R.; Hwang, K.-A.; Park, M.-A.; Yi, B.-R.; Jeung, E.-B.; Choi, K.-C. Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway. Int. J. Mol. Med. 2012, 29, 883–890. [Google Scholar] [CrossRef][Green Version]
- Katchy, A.; Pinto, C.; Jonsson, P.; Nguyen-Vu, T.; Pandelova, M.; Riu, A.; Schramm, K.-W.; Samarov, D.; Gustafsson, J.-Å.; Bondesson, M.; et al. Coexposure to Phytoestrogens and Bisphenol A Mimics Estrogenic Effects in an Additive Manner. Toxicol. Sci. 2014, 138, 21–35. [Google Scholar] [CrossRef]
- Sauer, S.J.; Tarpley, M.; Shah, I.; Save, A.V.; Lyerly, H.K.; Patierno, S.R.; Williams, K.P.; Devi, G.R. Bisphenol A activates EGFR and ERK promoting proliferation, tumor spheroid formation and resistance to EGFR pathway inhibition in estrogen receptor-negative inflammatory breast cancer cells. Carcinogenesis 2017, 38, bgx003. [Google Scholar] [CrossRef]
- Castillo Sanchez, R.; Gomez, R.; Perez Salazar, E. Bisphenol A Induces Migration through a GPER-, FAK-, Src-, and ERK2-Dependent Pathway in MDA-MB-231 Breast Cancer Cells. Chem. Res. Toxicol. 2016, 29, 285–295. [Google Scholar] [CrossRef]
- Torres-Alamilla, P.; Castillo-Sanchez, R.; Cortes-Reynosa, P.; Gomez, R.; Perez Salazar, E. Bisphenol A increases the size of primary mammary tumors and promotes metastasis in a murine model of breast cancer. Mol. Cell. Endocrinol. 2023, 575, 111998. [Google Scholar] [CrossRef]
- Chevalier, N.; Hinault, C.; Clavel, S.; Paul-Bellon, R.; Fenichel, P. GPER and Testicular Germ Cell Cancer. Front. Endocrinol. 2021, 11, 600404. [Google Scholar] [CrossRef]
- Marco, P.; Assunta, P.; Rosamaria, L.; Francesca, S.M.; Marianna, D.F.E.; Sergio, A.; Camillo, R.; Marcello, M. Bisphenol A Induces Gene Expression Changes and Proliferative Effects through GPER in Breast Cancer Cells and Cancer-Associated Fibroblasts. Environ. Health Perspect. 2012, 120, 1177–1182. [Google Scholar] [CrossRef]
- Castillo-Sanchez, R.; Ramirez-Ricardo, J.; Martinez-Baeza, E.; Cortes-Reynosa, P.; Candanedo-Gonzales, F.; Gomez, R.; Salazar, E.P. Bisphenol A induces focal adhesions assembly and activation of FAK, Src and ERK2 via GPER in MDA-MB-231 breast cancer cells. Toxicol. Vitr. 2020, 66, 104871. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, Y.; Shi, X.; Zhang, M.; Wang, X.; Tan, Y. Effect of bisphenol A on the EGFR-STAT3 pathway in MCF-7 breast cancer cells. Mol. Med. Rep. 2012, 5, 41–47. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, C.; Zhang, G.; Xiao, M.; Li, L.; Wu, S.; Lu, X. Co-exposure to BPA and DEHP enhances susceptibility of mammary tumors via up-regulating Esr1/HDAC6 pathway in female rats. Ecotoxicol. Environ. Saf. 2021, 221, 112453. [Google Scholar] [CrossRef]
- Lei, B.; Huang, Y.; Liu, Y.; Xu, J.; Sun, S.; Zhang, X.; Xu, G.; Wu, M.; Yu, Y.; Feng, C. Low-concentration BPF induced cell biological responses by the ERα and GPER1-mediated signaling pathways in MCF-7 breast cancer cells. Ecotoxicol. Environ. Saf. 2018, 165, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Hong, T.; Li, L.; Chen, L.; Luo, X.; Wu, Q.; Liu, Z. Isobaric tags for relative and absolute quantitation-based proteomics analysis of the effect of ginger oil on bisphenol A-induced breast cancer cell proliferation. Oncol. Lett. 2021, 21, 101. [Google Scholar] [CrossRef]
- Ma, Z.; Parris, A.B.; Howard, E.W.; Davis, M.; Cao, X.; Woods, C.; Yang, X. In Utero Exposure to Bisphenol a Promotes Mammary Tumor Risk in MMTV-Erbb2 Transgenic Mice Through the Induction of ER-erbB2 Crosstalk. Int. J. Mol. Sci. 2020, 21, 3095. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, H.; Lu, G.; Chen, Z.; Sun, W.; Shi, Y.; Fu, Z.; Huang, B.; Zhu, X.; Lu, W.; et al. Low Dose of Bisphenol A Modulates Ovarian Cancer Gene Expression Profile and Promotes Epithelial to Mesenchymal Transition Via Canonical Wnt Pathway. Toxicol. Sci. 2018, 164, 527–538. [Google Scholar] [CrossRef]
- Ptak, A.; Hoffmann, M.; Gruca, I.; Barć, J. Bisphenol A induce ovarian cancer cell migration via the MAPK and PI3K/Akt signalling pathways. Toxicol. Lett. 2014, 229, 357–365. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, Y.; Cheng, H.; Li, H.; Zhang, Y.; Wang, R.; Li, W.; Wu, F. BPA exposure enhances the metastatic aggression of ovarian cancer through the ERα/AKT/mTOR/HIF-1α signaling axis. Food Chem. Toxicol. 2023, 176, 113792. [Google Scholar] [CrossRef]
- Sang, C.; Song, Y.; Jin, T.; Zhang, S.; Fu, L.; Zhao, Y.; Zou, X.; Wang, Z.; Gao, H.; Liu, S. Bisphenol A induces ovarian cancer cell proliferation and metastasis through estrogen receptor-α pathways. Environ. Sci. Pollut. Res. 2021, 28, 36060–36068. [Google Scholar] [CrossRef]
- Ptak, A.; Gregoraszczuk, E.L. Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat, MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol. Lett. 2012, 210, 332–337. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Choi, K.-C.; Hwang, K.-A. Genistein suppressed epithelial–mesenchymal transition and migration efficacies of BG-1 ovarian cancer cells activated by estrogenic chemicals via estrogen receptor pathway and downregulation of TGF-β signaling pathway. Phytomedicine 2015, 22, 993–999. [Google Scholar] [CrossRef]
- Kang, N.-H.; Hwang, K.-A.; Lee, H.-R.; Choi, D.-W.; Choi, K.-C. Resveratrol regulates the cell viability promoted by 17β-estradiol or bisphenol A via down-regulation of the cross-talk between estrogen receptor α and insulin growth factor-1 receptor in BG-1 ovarian cancer cells. Food Chem. Toxicol. 2013, 59, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, B.; Yan, X.; Shen, X.; Ma, J.; Liu, S.; Zhang, D. Bisphenol A and polychlorinated biphenyls enhance the cancer stem cell properties of human ovarian cancer cells by activating the WNT signaling pathway. Chemosphere 2020, 246, 125775. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-A.; Choi, K.-C. Effects of 4-Nonylphenol and Bisphenol A on Stimulation of Cell Growth via Disruption of the Transforming Growth Factor-β Signaling Pathway in Ovarian Cancer Models. Chem. Res. Toxicol. 2014, 27, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, K.Y.; An, B.S.; Choi, J.H.; Jeung, E.B.; Leung, P.C.; Choi, K.C. Cell Growth of Ovarian Cancer Cells is Stimulated by Xenoestrogens through an Estrogen-Dependent Pathway, but Their Stimulation of Cell Growth Appears not to be Involved in the Activation of the Mitogen-Activated Protein Kinases ERK-1 and p38. J. Reprod. Dev. 2009, 55, 23–29. [Google Scholar] [CrossRef]
- Hoffmann, M.; Rak, A.; Ptak, A. Bisphenol A and its derivatives decrease expression of chemerin, which reverses its stimulatory action in ovarian cancer cells. Toxicol. Lett. 2018, 291, 61–69. [Google Scholar] [CrossRef]
- Hu, Y.; Yuan, D.; Wu, Y.; Yu, L.; Xu, L.; Yue, L.; Liu, L.; Xu, W.; Qiao, X.; Zeng, R.; et al. Bisphenol A Initiates Excessive Premature Activation of Primordial Follicles in Mouse Ovaries via the PTEN Signaling Pathway. Reprod. Sci. 2018, 25, 609–620. [Google Scholar] [CrossRef]
- Yaguchi, T. The endocrine disruptor bisphenol A promotes nuclear ERRγ translocation, facilitating cell proliferation of Grade I endometrial cancer cells via EGF-dependent and EGF-independent pathways. Mol. Cell. Biochem. 2019, 452, 41–50. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Zhang, J.; Gao, X.; Jin, H.; Liu, R.; Zhang, Z.; Zhang, X.; Wang, X.; Qu, P.; et al. Bisphenol A at a human exposed level can promote epithelial-mesenchymal transition in papillary thyroid carcinoma harbouring BRAFV600E mutation. J. Cell. Mol. Med. 2021, 25, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Medwid, S.; Guan, H.; Yang, K. Bisphenol A stimulates adrenal cortical cell proliferation via ERβ-mediated activation of the sonic hedgehog signalling pathway. J. Steroid Biochem. Mol. Biol. 2018, 178, 254–262. [Google Scholar] [CrossRef]
- Lozano-Herrera, S.J.; Luna-Bárcenas, G.; Guevara-González, R.G.; Campos-Vega, R.; Solís-Sáinz, J.C.; Hernández-Puga, A.G.; Vergara-Castañeda, H.A. Fermentation Extract of Naringenin Increases the Expression of Estrogenic Receptor β and Modulates Genes Related to the p53 Signalling Pathway, miR-200c and miR-141 in Human Colon Cancer Cells Exposed to BPA. Molecules 2022, 27, 6588. [Google Scholar] [CrossRef]
- Jun, J.H.; Oh, J.E.; Shim, J.-K.; Kwak, Y.-L.; Cho, J.S. Effects of bisphenol A on the proliferation, migration, and tumor growth of colon cancer cells: In vitro and in vivo evaluation with mechanistic insights related to ERK and 5-HT3. Food Chem. Toxicol. 2021, 158, 112662. [Google Scholar] [CrossRef]
- Nair, V.A.; Malhab, L.J.; Abdel-Rahman, W.M. Characterization of the Molecular Alterations Induced by the Prolonged Exposure of Normal Colon Mucosa and Colon Cancer Cells to Low-Dose Bisphenol A. Int. J. Mol. Sci. 2022, 23, 11620. [Google Scholar] [CrossRef]
- Zeng, W. Bisphenol A triggers the malignancy of nasopharyngeal carcinoma cells via activation of Wnt/β-catenin pathway. Toxicol. Vitr. 2020, 66, 104881. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Rupasinghe, H.P.V. DNA damaging and apoptotic potentials of Bisphenol A and Bisphenol S in human bronchial epithelial cells. Environ. Toxicol. Pharmacol. 2018, 60, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Oguro, A.; Imaoka, S. Bisphenol A and Its Derivatives Induce Degradation of HIF-1alpha via the Lysosomal Pathway in Human Hepatocarcinoma Cell Line, Hep3B. Biol. Pharm. Bull. 2018, 41, 374–382. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Tan, Y.-Y.; Yao, M.; Lin, H.-C.; Tsai, M.-H.; Li, Y.-Y.; Hsu, Y.-J.; Huang, T.-T.; Chang, C.-W.; Chuang, C.-Y.; et al. Bisphenol A-induced DNA damages promote to lymphoma progression in human lymphoblastoid cells through aberrant CTNNB1 signaling pathway. iScience 2021, 24, 102888. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Huang, C.; Liu, Y.; Liu, J.; Kuang, H.; Pang, Q.; Han, H.; Fan, R. Involvement of NLRP3/Caspase-1/GSDMD-Dependent pyroptosis in BPA-Induced apoptosis of human neuroblastoma cells. Biochem. Pharmacol. 2022, 200, 115042. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Lu, Q.; Zhang, P.; Ren, M. Transforming growth factor-β signaling pathway cross-talking with ERα signaling pathway on regulating the growth of uterine leiomyoma activated by phenolic environmental estrogens in vitro. Tumor Biol. 2016, 37, 455–462. [Google Scholar] [CrossRef]
- Shen, Y.; Lu, Q.; Zhang, P.; Wu, Y.; Ren, M. The effect of TGF-β signaling on regulating proliferation of uterine leiomyoma cell via ERα signaling activated by bisphenol A, octylphenol and nonylphenol in vitro. J. Cancer Res. Ther. 2018, 14 (Suppl. S2), S276–S281. Available online: https://journals.lww.com/cancerjournal/fulltext/2018/14002/the_effect_of_tgf___signaling_on_regulating.2.aspx (accessed on 17 July 2025). [CrossRef]
- Abo-Zaid, O.A.; Moawed, F.S.; Hassan, H.A.; Moustafa, E.M. Bisphenol-A/Radiation mediated inflammatory response activates EGFR/KRAS/ERK1/2 signaling pathway leads to lung carcinogenesis incidence. Int. J. Immunopathol. Pharmacol. 2022, 36, 03946320221092918. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, N.; Jin, H.; Liu, R.; Zhang, Z.; Cheng, C.; Fan, Z.; Zhang, G.; Xiao, M.; Wu, S.; et al. Bisphenol A drives di(2-ethylhexyl) phthalate promoting thyroid tumorigenesis via regulating HDAC6/PTEN and c-MYC signaling. J. Hazard. Mater. 2022, 425, 127911. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, J.; Wang, R.; Ramezani, K.; Bonakdar, M.; Moghimi, N.; Salimi, M.; Yao, Y.; Wang, K. Bisphenol A interacts with DLGAP5 and regulates IL-6/JAK2/STAT3 signaling pathway to promote tumorigenesis and progression of osteosarcoma. Chemosphere 2023, 312, 136545. [Google Scholar] [CrossRef] [PubMed]
- Sundarraj, S.; Sujitha, M.V.; Alphonse, C.R.W.; Kalaiarasan, R.; Kannan, R.R. Bisphenol-A alters hematopoiesis through EGFR/ERK signaling to induce myeloblastic condition in zebrafish model. Sci. Total Environ. 2021, 787, 147530. [Google Scholar] [CrossRef]
- Urriola-Muñoz, P.; Lagos-Cabré, R.; Patiño-García, D.; Reyes, J.G.; Moreno, R.D. Bisphenol-A and Nonylphenol Induce Apoptosis in Reproductive Tract Cancer Cell Lines by the Activation of ADAM17. Int. J. Mol. Sci. 2018, 19, 2238. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, J.; Chen, M.; Peng, D.; Liang, Y.; Song, M.; Zhang, J.; Jiang, G. Exposure to Bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ. Toxicol. 2016, 31, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, M.; Ozden, S. Potential impacts of bisphenols on prostate cells: An overview of cytotoxicity, proliferation, oxidative stress, apoptosis, and ER-stress response activation. Food Chem. Toxicol. 2024, 184, 114416. [Google Scholar] [CrossRef]
- Li Yin Burns Katherine, A.; Arao Yukitomo Luh Colin, J.; Korach Kenneth, S. Differential Estrogenic Actions of Endocrine-Disrupting Chemicals Bisphenol A, Bisphenol AF, and Zearalenone through Estrogen Receptor α and β in Vitro. Environ. Health Perspect. 2012, 120, 1029–1035. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Y.; Lv, L.; Li, X.; Qin, Z. Effects of low-dose bisphenol AF on mammal testis development via complex mechanisms: Alterations are detectable in both infancy and adulthood. Arch. Toxicol. 2022, 96, 3373–3383. [Google Scholar] [CrossRef]
- Shamhari, A.A.; Abd Hamid, Z.; Budin, S.B.; Shamsudin, N.J.; Taib, I.S. Bisphenol A and Its Analogues Deteriorate the Hormones Physiological Function of the Male Reproductive System: A Mini-Review. Biomedicines 2021, 9, 1744. [Google Scholar] [CrossRef]
- Matteo, G.; Leingartner, K.; Rowan-Carroll, A.; Meier, M.; Williams, A.; Beal, M.A.; Gagné, M.; Farmahin, R.; Wickramasuriya, S.; Reardon, A.J.F.; et al. In vitro transcriptomic analyses reveal pathway perturbations, estrogenic activities, and potencies of data-poor BPA alternative chemicals. Toxicol. Sci. 2023, 191, 266–275. [Google Scholar] [CrossRef]
- Kolatorova Sosvorova, L.; Chlupacova, T.; Vitku, J.; Vlk, M.; Heracek, J.; Starka, L.; Saman, D.; Simkova, M.; Hampl, R. Determination of selected bisphenols, parabens and estrogens in human plasma using LC-MS/MS. Talanta 2017, 174, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Analysis of environmental pollutant Bisphenol F elicited prostate injury targets and underlying mechanisms through network toxicology, molecular docking, and multi-level bioinformatics data integration. Toxicology 2024, 506, 153847. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-T.; Hur, S. Substrate recognition by TRIM and TRIM-like proteins in innate immunity; 1. Brain Organoids by Madeline Lancaster2. RNA Biology in Viral Infection by Holly Ramage and Sara Cherry. Semin. Cell Dev. Biol. 2021, 111, 76–85. [Google Scholar] [CrossRef]
- Deng, Q.; Jiang, G.; Wu, Y.; Li, J.; Liang, W.; Chen, L.; Su, Q.; Li, W.; Du, J.; Wong, C.K.C.; et al. GPER/Hippo-YAP signal is involved in Bisphenol S induced migration of triple negative breast cancer (TNBC) cells. J. Hazard. Mater. 2018, 355, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Hou, X.; Fan, L.; Yang, F.; Dai, Y.; Deng, Y.; Fu, Z.; Shu, X.; Sun, B.; et al. Bisphenol P and bisphenol M promote triple-negative breast cancer metastasis through activation of AKT pathways. Sci. Total Environ. 2023, 892, 164748. [Google Scholar] [CrossRef] [PubMed]
- Ticiani, E.; Gingrich, J.; Pu, Y.; Vettathu, M.; Davis, J.; Martin, D.; Veiga-Lopez, A. Bisphenol S and Epidermal Growth Factor Receptor Signaling in Human Placental Cytotrophoblasts. Environ. Health Perspect. 2021, 129, 027005. [Google Scholar] [CrossRef]
- Song, P.; Fan, K.; Tian, X.; Wen, J. Bisphenol S (BPS) triggers the migration of human non-small cell lung cancer cells via upregulation of TGF-β. Toxicol. Vitr. 2019, 54, 224–231. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, C.; Zhong, H.; Zhang, S.; Xia, Y.; Cai, Z. Bisphenol S induced epigenetic and transcriptional changes in human breast cancer cell line MCF-7. Environ. Pollut. 2019, 246, 697–703. [Google Scholar] [CrossRef]
- Miziak, P.; Baran, M.; Błaszczak, E.; Przybyszewska-Podstawka, A.; Kałafut, J.; Smok-Kalwat, J.; Dmoszyńska-Graniczka, M.; Kiełbus, M.; Stepulak, A. Estrogen Receptor Signaling in Breast Cancer. Cancers 2023, 15, 4689. [Google Scholar] [CrossRef]
- Pellerin, È.; Pellerin, F.-A.; Chabaud, S.; Pouliot, F.; Pelletier, M.; Bolduc, S. Glucuronidated Metabolites of Bisphenols A and S Alter the Properties of Normal Urothelial and Bladder Cancer Cells. Int. J. Mol. Sci. 2022, 23, 12859. [Google Scholar] [CrossRef]
- Zhao, C.; Yong, T.; Zhang, Y.; Xiao, Y.; Jin, Y.; Zheng, C.; Nirasawa, T.; Cai, Z. Breast cancer proliferation and deterioration-associated metabolic heterogeneity changes induced by exposure of bisphenol S, a widespread replacement of bisphenol A. J. Hazard. Mater. 2021, 414, 125391. [Google Scholar] [CrossRef] [PubMed]
- Wormsbaecher, C.; Cumbia, B.M.; Amurgis, E.G.; Poska, J.M.; Price, M.R.; Mo, X.M.; Knoblaugh, S.E.; Kurita, T.; Burd, C.J. Mammary gland development and EDC-driven cancer susceptibility in mesenchymal ERα-knockout mice. Endocr.-Relat. Cancer 2023, 30, e230062. [Google Scholar] [CrossRef]
- Oh, J.; Choi, J.W.; Ahn, Y.-A.; Kim, S. Pharmacokinetics of bisphenol S in humans after single oral administration. Environ. Int. 2018, 112, 127–133. [Google Scholar] [CrossRef]
- Okuda, K.; Takiguchi, M.; Yoshihara, S. In vivo estrogenic potential of 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, an active metabolite of bisphenol A, in uterus of ovariectomized rat. Toxicol. Lett. 2010, 197, 7–11. [Google Scholar] [CrossRef]
- Valentino, R.; D’Esposito, V.; Ariemma, F.; Cimmino, I.; Beguinot, F.; Formisano, P. Bisphenol A environ-mental exposure and the detrimental effects on human metabolic health: Is it necessary to revise the risk assessment in vulnerable population? J. Endocrinol. Investig. 2016, 39, 259–263. [Google Scholar] [CrossRef]
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S.; et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013, 42, 256–268. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Borgert, C.J.; Dietrich, D.; Rozman, K.K. Endocrine disruption: Fact or urban legend? Risk Assess. Endocr. Disrupting Chem. 2013, 223, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Bujalance-Reyes, F.; Molina-López, A.M.; Ayala-Soldado, N.; Lora-Benitez, A.; Mora-Medina, R.; Moyano-Salvago, R. Analysis of Indirect Biomarkers of Effect after Exposure to Low Doses of Bisphenol A in a Study of Successive Generations of Mice. Animals 2022, 12, 300. [Google Scholar] [CrossRef]
- Le Corre, L.; Besnard, P.; Chagnon, M.-C. BPA, an Energy Balance Disruptor. Crit. Rev. Food Sci. Nutr. 2015, 55, 769–777. [Google Scholar] [CrossRef]
- Geens, T.; Apelbaum, T.Z.; Goeyens, L.; Neels, H.; Covaci, A. Intake of bisphenol A from canned beverages and foods on the Belgian market. Food Addit. Contam. Part A 2010, 27, 1627–1637. [Google Scholar] [CrossRef]
- Catenza, C.J.; Farooq, A.; Shubear, N.S.; Donkor, K.K. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues. Chemosphere 2021, 268, 129273. [Google Scholar] [CrossRef]
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Liu, C.; Zhen, Y.; Ma, J. Fast adsorption of BPA with high capacity based on π-π electron donor-acceptor and hydrophobicity mechanism using an in-situ sp2 C dominant N-doped carbon. Chem. Eng. J. 2020, 381, 122510. [Google Scholar] [CrossRef]
- Bu, L.; Ding, J.; Zhu, N.; Kong, M.; Wu, Y.; Shi, Z.; Zhou, S.; Dionysiou, D.D. Unraveling different mechanisms of persulfate activation by graphite felt anode and cathode to destruct contaminants of emerging concern. Appl. Catal. B Environ. 2019, 253, 140–148. [Google Scholar] [CrossRef]
- Sorokhaibam, L.G.; Ahmaruzzaman, M. Chapter 8—Phenolic Wastewater Treatment: Development and Applications of New Adsorbent Materials. In Industrial Wastewater Treatment, Recycling and Reuse; Ranade, V.V., Bhandari, V.M., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 323–368. [Google Scholar] [CrossRef]
- Eltoukhy, A.; Jia, Y.; Nahurira, R.; Abo-Kadoum, M.A.; Khokhar, I.; Wang, J.; Yan, Y. Biodegradation of endocrine disruptor Bisphenol A by Pseudomonas putida strain YC-AE1 isolated from polluted soil, Guangdong, China. BMC Microbiol. 2020, 20, 11. [Google Scholar] [CrossRef]
- LaPensee Elizabeth, W.; Tuttle Traci, R.; Fox Sejal, R.; Ben-Jonathan, N. Bisphenol A at Low Nanomolar Doses Confers Chemoresistance in Estrogen Receptor-α–Positive and –Negative Breast Cancer Cells. Environ. Health Perspect. 2009, 117, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.R. Trumped Treatment?: BPA Blocks Effects of Breast Cancer Chemotherapy Drugs. Environ. Health Perspect. 2009, 117, A75. [Google Scholar] [CrossRef][Green Version]
- Gonzalez, T.L.; Rae, J.M.; Colacino, J.A. Implication of environmental estrogens on breast cancer treatment and progression. Toxicology 2019, 421, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hongchao, P.; Richard, G.; Jeremy, B.; Christina, D.; Carolyn, T.; Paul, M.; Richard, P.; Kathleen, I.P.; Jonas, B.; Mitch, D.; et al. 20-Year Risks of Breast-Cancer Recur-rence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Chevalier, N.; Bouskine, A.; Fenichel, P. Bisphenol A promotes testicular seminoma cell proliferation through GPER/GPR30. Int. J. Cancer 2012, 130, 241–242. [Google Scholar] [CrossRef]
- Öz, E.; Tüylü Küçükkılınç, T. Combined effect of fulvestrant and low dose BPA: Comparative implications on EMT, apoptosis, and TGF-β1 signaling in HepG2 cells. Drug Chem. Toxicol. 2022, 45, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Song, T.; Che, D.; Li, C.; Jiang, J.; Pang, J.; Yang, Y.; GomaLi, P. The effect of bisphenol a exposure onto endothelial and decidualized stromal cells on regulation of the invasion ability of trophoblastic spheroids in in vitro co-culture model. Biochem. Biophys. Res. Commun. 2019, 516, 506–514. [Google Scholar] [CrossRef]
- Guo, H.; Liu, T.; Uemura, Y.; Jiao, S.; Wang, D.; Lin, Z.; Narita, Y.; Suzuki, M.; Hirosawa, N.; Ichihara, Y.; et al. Bisphenol A in combination with TNF-α selectively induces Th2 cell-promoting dendritic cells in vitro with an estrogen-like activity. Cell. Mol. Immunol. 2010, 7, 227–234. [Google Scholar] [CrossRef]
- Puranik, N.V.; Srivastava, P.; Bhatt, G.; John Mary, D.J.S.; Limaye, A.M.; Sivaraman, J. Determination and analysis of agonist and antagonist potential of naturally occurring flavonoids for estrogen receptor (ERα) by various parameters and molecular modelling approach. Sci. Rep. 2019, 9, 7450. [Google Scholar] [CrossRef]
- Yi, K.; Chen, W.; Zhou, X.; Xie, C.; Zhong, C.; Zhu, J. Bisphenol S exposure promotes stemness of triple-negative breast cancer cells via regulating Gli1-mediated Sonic hedgehog pathway. Environ. Res. 2025, 264, 120293. [Google Scholar] [CrossRef]
- Wen, X.; Zhu, M.; Li, Z.; Li, T.; Xu, X. Dual effects of bisphenol A on wound healing, involvement of estrogen receptor β. Ecotoxicol. Environ. Saf. 2022, 231, 113207. [Google Scholar] [CrossRef]
- Kassotaki, E.; Pijuan, M.; Rodriguez-Roda, I.; Buttiglieri, G. Comparative assessment of endocrine disrupting compounds removal in heterotrophic and enriched nitrifying biomass. Chemosphere 2019, 217, 659–668. [Google Scholar] [CrossRef]
- Long, F.; Ren, Y.; Bi, F.; Wu, Z.; Zhang, H.; Li, J.; Gao, R.; Liu, Z.; Li, H. Contamination Characterization, Toxicological Properties, and Health Risk Assessment of Bisphenols in Multiple Media: Current Research Status and Future Perspectives. Toxics 2025, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Abdennebi-Najar, L.; Barouki, R.; Cranor, C.F.; Etzel, R.A.; Gee, D.; Heindel, J.J.; Hougaard, K.S.; Hunt, P.; Nawrot, T.S.; et al. Timescales of developmental toxicity impacting on research and needs for intervention. Basic Clin. Pharmacol. Toxicol. 2019, 125, 70–80. [Google Scholar] [CrossRef]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Adamovsky, O.; Groh, K.J.; Białk-Bielińska, A.; Escher, B.I.; Beaudouin, R.; Mora Lagares, L.; Tollefsen, K.E.; Fenske, M.; Mulkiewicz, E.; Creusot, N.; et al. Exploring BPA alternatives—Environmental levels and toxicity review. Environ. Int. 2024, 189, 108728. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ani, M.; Al-Ani, Y.; Ibrahim, S.S.; Ibrahim, R.S.; Kubatka, P.; Büsselberg, D. Bisphenol A (BPA) Modifies Cancer Signaling Pathways: A Neglected Global Health Threat. J. Xenobiot. 2025, 15, 207. https://doi.org/10.3390/jox15060207
Al-Ani M, Al-Ani Y, Ibrahim SS, Ibrahim RS, Kubatka P, Büsselberg D. Bisphenol A (BPA) Modifies Cancer Signaling Pathways: A Neglected Global Health Threat. Journal of Xenobiotics. 2025; 15(6):207. https://doi.org/10.3390/jox15060207
Chicago/Turabian StyleAl-Ani, Minatullah, Yassir Al-Ani, Shahad Sabaawi Ibrahim, Raghad Sabaawi Ibrahim, Peter Kubatka, and Dietrich Büsselberg. 2025. "Bisphenol A (BPA) Modifies Cancer Signaling Pathways: A Neglected Global Health Threat" Journal of Xenobiotics 15, no. 6: 207. https://doi.org/10.3390/jox15060207
APA StyleAl-Ani, M., Al-Ani, Y., Ibrahim, S. S., Ibrahim, R. S., Kubatka, P., & Büsselberg, D. (2025). Bisphenol A (BPA) Modifies Cancer Signaling Pathways: A Neglected Global Health Threat. Journal of Xenobiotics, 15(6), 207. https://doi.org/10.3390/jox15060207







