Treatment of Xenobiotic Cyclic Nitramine Explosives in Wastewater
Abstract
1. Introduction
2. Physicochemical and Toxicological Properties
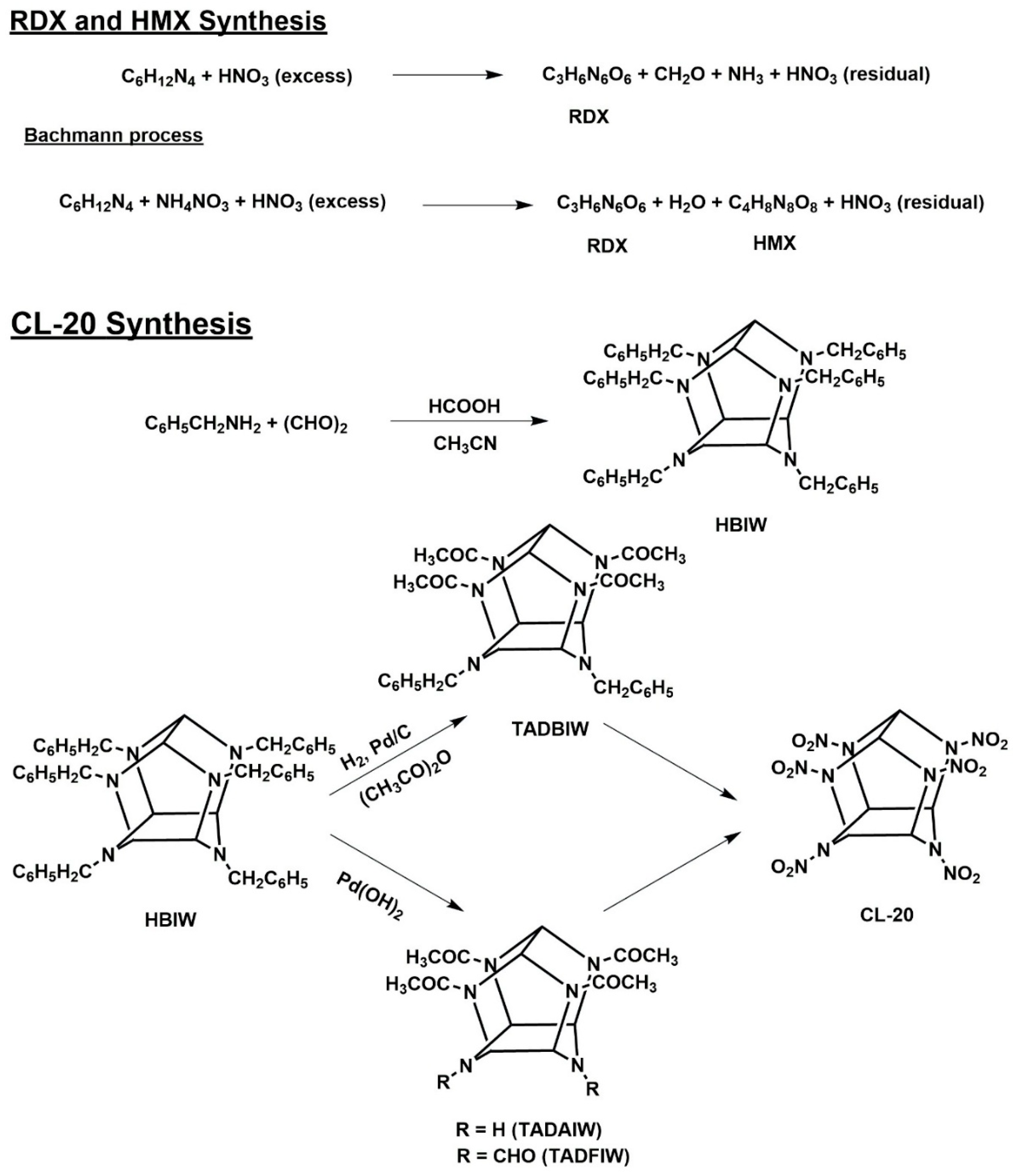
3. Industrial Production and Associated Wastewater
4. Conventional Treatment Approaches
4.1. Adsorption
4.2. Chemical Reduction
4.3. Advanced Oxidation Processes (AOPs)
4.4. Alkaline Hydrolysis
4.5. Limitations of Physical and Chemical Methods
5. Biodegradation Potential and Mechanisms
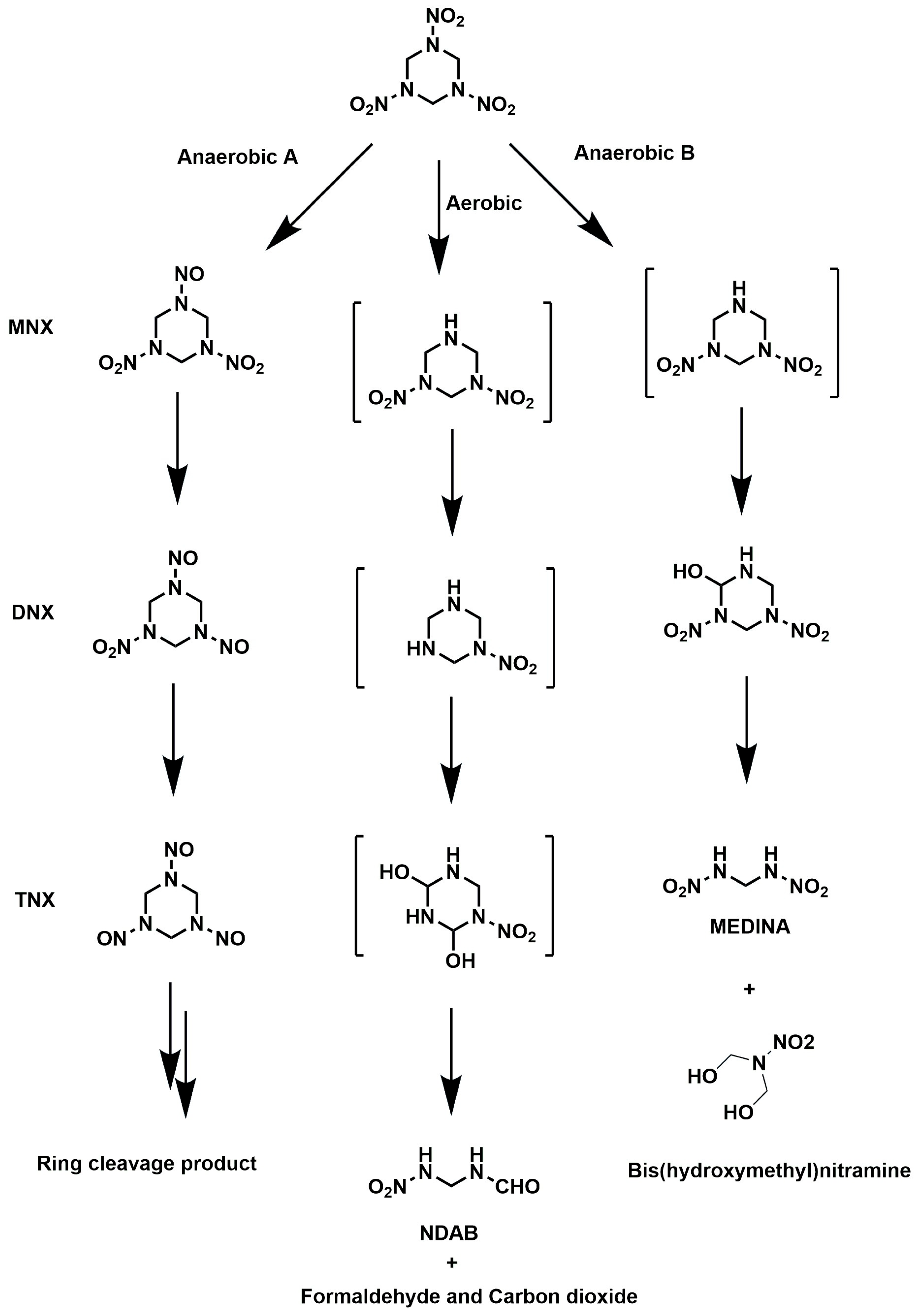
5.1. Biodegradation of RDX
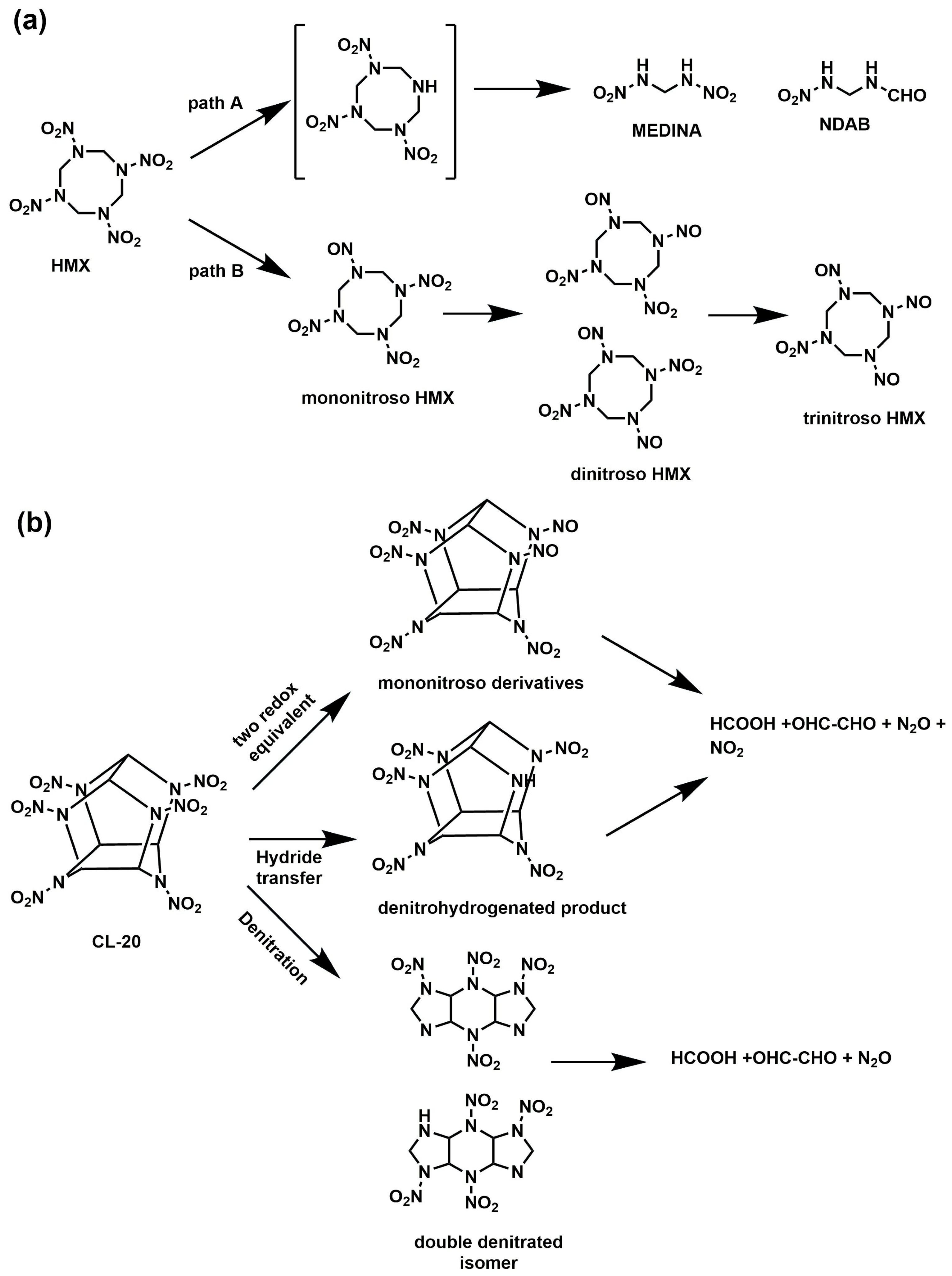
5.2. Biodegradation of HMX
5.3. Biodegradation of CL-20
5.4. Biodegradation of RDX, HMX, and CL-20 by Fungi
6. Biological Explosives Contaminated Wastewater Treatment Technologies
6.1. Activated Sludge/Aerobic Granular Sludge Process
6.2. Membrane Bioreactor
6.3. Up-Flow Anaerobic Sludge Blanket and Modified Reactors
6.4. Biofilm-Based Reactors
6.5. Phytoremediation and Constructed Wetlands
7. Integrated Treatment Using Physical and Chemical Methods
7.1. Adsorption with Biological Treatment
7.2. Ultrafiltration with Biological Treatment
7.3. Advanced Oxidation Integrated with Biological Treatment
7.4. Zero Valent Iron (ZVI) Integrated with Biological Treatment
7.5. Alkaline Hydrolysis Integrated with Biological Treatment
| Treatment Methods | Explosives | Condition | References |
|---|---|---|---|
| Activated sludge | CL-20 hydrolysates | aerobic | [112] |
| Membrane Bioreactor | RDX, HMX | sequential anaerobic–aerobic | [117] |
| RDX hydrolysates | anoxic | [118] | |
| Sequencing Batch Reactors | HMX | aerobic | [58] |
| RDX, HMX | oxygen-depleted conditions | [115] | |
| Up-flow Anaerobic Sludge Blanket and modified reactors | RDX | anaerobic | [121] |
| RDX, HMX | anaerobic | [122] | |
| RDX | anaerobic | [123] | |
| Adsorption with biological treatment: Granulated activated carbon (GAC)-based fluidized bed reactors | RDX | anoxic | [64] |
| Ultrafiltration with biological treatment | RDX hydrolysate | anoxic | [118] |
| Advanced oxidation integrated with biological treatment | RDX | anoxic–oxic | [130] |
| Zero valent iron (ZVI) integrated with biological treatment | RDX | anaerobic | [131] |
| RDX | aerobic or anaerobic | [132] | |
| Alkaline hydrolysis integrated with biological treatment | CL-20 hydrolysates | aerobic | [112] |
| Sequential biological treatment | RDX | sequential anaerobic–aerobic | [116] |
8. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RDX | Hexahydro-1,3,5-trinitro-1,3,5-triazine |
| HMX | Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine |
| CL-20 | 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane |
| MNX | Hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine |
| DNX | Hexahydro-1,3-dinitroso-nitro-1,3,5-triazine |
| TNX | Hexahydro-1,3,5-trinitroso-1,3,5-triazine |
| ROS | Reactive oxygen species |
| MEDINA | Methylene dinitramine |
| BHNA | Bis(hydroxymethyl)nitramine |
| NDAB | 4-Nitro-2,4-diazabutanal |
| TNT | 2,4,6-Trinitrotoluene |
| XO | Xanthine oxidase |
| MBR | Membrane bioreactor |
| COD | Chemical oxygen demand |
| UASB | Up-flow anaerobic sludge bed reactor |
| AFBR | Anaerobic fluidized bed reactors |
| FFBR | Fixed film bioreactor |
| GAC | Granulated activated carbon |
| FBR | Fluidized bed reactors |
| ZVI | Zero valent iron |
| HBIW | Hexabenzylhexaazaisowurtzitane |
| TADBIW | 4,10-Dibenzyl-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazaisowurtzitane |
| TADAIW | 2,6,8,12-Tetraacetyl-2,4,6,8,10,12-hexaazaisowurtzitane |
| TADFIW | 4,10-Diformyl-2,6,8,12-tetraacetyl-2,4,6,8,10,12-hexaazaisowurtzitane |
| ACF | Activated carbon fiber |
| ZVINs | Zero-valent iron nanoparticles |
| PRB | Permeable reactive barrier |
| AOP | Advanced oxidation processes |
| TOC | Total organic carbon |
| TN | Total nitrogen |
References
- Gupta, S.; Goel, S.S.; Siebner, H.; Ronen, Z.; Ramanathan, G. Transformation of 2,4,6-Trinitrotoluene by Stenotrophomonas Strain SG1 under Aerobic and Anaerobic Conditions. Chemosphere 2023, 311, 137085. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Sangwan, P.; Sharma, P. Microbial Degradation of Explosive Manufacturing Facility Wastewater in a Bioreactor. Int. J. Environ. Res. 2023, 17, 49. [Google Scholar] [CrossRef]
- Ndibe, T.O.; Benjamin, B.; Eugene, W.C.; Usman, J.J. A Review on Biodegradation and Biotransformation of Explosive Chemicals. Eur. J. Eng. Res. Sci. 2018, 3, 58–65. [Google Scholar] [CrossRef]
- Aamir Khan, M.; Sharma, A.; Yadav, S.; Celin, S.M.; Sharma, S. A Sketch of Microbiological Remediation of Explosives-Contaminated Soil Focused on State of Art and the Impact of Technological Advancement on Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) Degradation. Chemosphere 2022, 294, 133641. [Google Scholar] [CrossRef]
- Ronen, Z.; Bernstein, A. Biodegradation of the Explosives TNT, RDX and HMX. In Microbial Degradation of Xenobiotics; Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2012; pp. 135–176. [Google Scholar] [CrossRef]
- Steevens, J.A.; Maurice Duke, B.; Lotufo, G.R.; Bridges, T.S. Toxicity of The Explosives 2,4,6-Trinitrotoluene, Hexahydro-1,3,5-Trinitro-1,3,5-Triazine, and Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine in Sediments to Chironomus tentans and Hyalella azteca: Low-Dose Hormesis and High-Dose Mortality. Environ. Toxicol. Chem. 2002, 21, 1475–1482. [Google Scholar] [CrossRef]
- Bhanot, P.; Celin, S.M.; Sreekrishnan, T.R.; Kalsi, A.; Sahai, S.K.; Sharma, P. Application of Integrated Treatment Strategies for Explosive Industry Wastewater—A Critical Review. J. Water Process Eng. 2020, 35, 101232. [Google Scholar] [CrossRef]
- Crocker, F.H.; Indest, K.J.; Fredrickson, H.L. Biodegradation of the Cyclic Nitramine Explosives RDX, HMX, and CL-20. Appl. Microbiol. Biotechnol. 2006, 73, 274–290. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, J.; Singh, K. Microbial Remediation of Explosive Waste. Crit. Rev. Microbiol. 2012, 38, 152–167. [Google Scholar] [CrossRef]
- Rocheleau, S.; Lachance, B.; Kuperman, R.G.; Hawari, J.; Thiboutot, S.; Ampleman, G.; Sunahara, G.I. Toxicity and Uptake of Cyclic Nitramine Explosives in Ryegrass Lolium perenne. Environ. Pollut. 2008, 156, 199–206. [Google Scholar] [CrossRef]
- Chakraborty, N.; Begum, P.; Patel, B.K. Counterbalancing Common Explosive Pollutants (TNT, RDX, and HMX) in the Environment by Microbial Degradation. In Development in Wastewater Treatment Research and Processes: Microbial Degradation of Xenobiotics Through Bacterial and Fungal Approach; Elsevier: Amsterdam, The Netherlands, 2022; pp. 263–310. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, P.; Sangwan, P. Bioremediation of RDX and HMX Contaminated Soil Employing a Biochar-Based Bioformulation. Carbon Res. 2023, 2, 33. [Google Scholar] [CrossRef]
- Van Aken, B.; Yoon, J.M.; Schnoor, J.L. Biodegradation of Nitro-Substituted Explosives 2,4,6-Trinitrotoluene, Hexahydro-1,3,5-Trinitro-1,3,5-Triazine, and Octahydro-1,3,5,7-Tetranitro-1,3,5-Tetrazocine by a Phytosymbiotic Methylobacterium sp. Associated with Poplar Tissues (Populus deltoides × nigra DN34). Appl. Environ. Microbiol. 2004, 70, 508–517. [Google Scholar] [CrossRef]
- Bhushan, B.; Halasz, A.; Thiboutot, S.; Ampleman, G.; Hawari, J. Chemotaxis-Mediated Biodegradation of Cyclic Nitramine Explosives RDX, HMX, and CL-20 by Clostridium sp. EDB2. Biochem. Biophys. Res. Commun. 2004, 316, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.L.; Felt, D.R.; Davis, J.L.; Escalon, L. Analysis of CL-20 in Environmental Matrices: Water and Soil. J. Chromatogr. Sci. 2002, 40, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, R.G.; Checkai, R.T.; Simini, M.; Phillips, C.T.; Anthony, J.S.; Kolakowski, J.E.; Davis, E.A. Toxicity of Emerging Energetic Soil Contaminant CL-20 to Potworm Enchytraeus crypticus in Freshly Amended or Weathered and Aged Treatments. Chemosphere 2006, 62, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, A. Biodegradation of Military Explosives RDX and HMX. In Microbial Degradation of Xenobiotics; Environmental Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2012; pp. 235–261. [Google Scholar] [CrossRef]
- Bhanot, P.; Celin, S.M.; Sharma, P.; Sahai, S.K.; Kalsi, A. Comparative Evaluation of Low- and Medium-Pressure UV Lamps for Photo-Degradation of RDX Wastewater. Water Air Soil Pollut. 2023, 234, 587. [Google Scholar] [CrossRef]
- Hawari, J.; Halasz, A.; Sheremata, T.; Beaudet, S.; Groom, C.; Paquet, L.; Rhofir, C.; Ampleman, G.; Thiboutot, S. Characterization of Metabolites during Biodegradation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) with Municipal Anaerobic Sludge. Appl. Environ. Microbiol. 2000, 66, 2652–2657. [Google Scholar] [CrossRef]
- Srivastava, V.; Boczkaj, G.; Lassi, U. An Overview of Treatment Approaches for Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX) Explosive in Soil, Groundwater, and Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 15948. [Google Scholar] [CrossRef]
- Yang, X.; Lai, J.L.; Li, J.; Zhang, Y.; Luo, X.G.; Han, M.W.; Zhu, Y.B.; Zhao, S.P. Biodegradation and Physiological Response Mechanism of Bacillus aryabhattai to Cyclotetramethylenete-Tranitramine (HMX) Contamination. J. Environ. Manage. 2021, 288, 112247. [Google Scholar] [CrossRef]
- Meda, A.; Sangwan, P.; Bala, K. In-Vessel Composting of HMX and RDX Contaminated Sludge Using Microbes Isolated from Contaminated Site. Environ. Pollut. 2021, 285, 117394. [Google Scholar] [CrossRef]
- Trott, S.; Nishino, S.F.; Hawari, J.; Spain, J.C. Biodegradation of the Nitramine Explosive CL-20. Appl. Environ. Microbiol. 2003, 69, 1871–1874. [Google Scholar] [CrossRef]
- Hawari, J.; Deschamps, S.; Beaulieu, C.; Paquet, L.; Halasz, A. Photodegradation of CL-20: Insights into the Mechanisms of Initial Reactions and Environmental Fate. Water Res. 2004, 38, 4055–4064. [Google Scholar] [CrossRef]
- Meda, A.; Sangwan, P. Optimization and Degradation Studies on Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) with Selected Indigenous Microbes under Aerobic Conditions. Water 2021, 13, 1257. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Paquet, L.; Deschamps, S.; Balakrishnan, V.K.; Beaulieu, C.; Hawari, J. Physico-Chemical Measurements of CL-20 for Environmental Applications: Comparison with RDX and HMX. J. Chromatogr. A 2004, 1025, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Panikov, N.S.; Sizova, M.V.; Ros, D.; Christodoulatos, C.; Balas, W.; Nicolich, S. Biodegradation Kinetics of the Nitramine Explosive CL-20 in Soil and Microbial Cultures. Biodegradation 2007, 18, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Halasz, A.; Hawari, J. Effect of Iron(III), Humic Acids and Anthraquinone-2,6-Disulfonate on Biodegradation of Cyclic Nitramines by Clostridium sp. EDB2. J. Appl. Microbiol. 2006, 100, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.A.; Halasz, A.; Paquet, L.; D’Cruz, P.; Hawari, J. Cyclodextrin-Assisted Capillary Electrophoresis for Determination of the Cyclic Nitramine Explosives RDX, HMX and CL-20: Comparison with High-Performance Liquid Chromatography. J. Chromatogr. A 2003, 999, 17–22. [Google Scholar] [CrossRef]
- Gupta, S.; Siebner, H.; Ramanathan, G.; Ronen, Z. Inhibition Effect of 2,4,6-Trinitrotoluene (TNT) on RDX Degradation by Rhodococcus Strains Isolated from Contaminated Soil and Water. Environ. Pollut. 2022, 311, 120018. [Google Scholar] [CrossRef]
- Bernstein, A.; Adar, E.; Ronen, Z.; Lowag, H.; Stichler, W.; Meckenstock, R.U. Quantifying RDX Biodegradation in Groundwater Using d15N Isotope Analysis. J. Contam. Hydrol. 2010, 111, 25–35. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, Y.; Wang, C.; Nie, Y.; Xu, A.; Wu, L. Multi-Generation Reproductive Toxicity of RDX and the Involved Signal Pathways in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2023, 260, 115074. [Google Scholar] [CrossRef]
- Simini, M.; Checkai, R.T.; Kuperman, R.G.; Phillips, C.T.; Kolakowski, J.E.; Kurnas, C.W.; Sunahara, G.I. Reproduction and Survival of Eisenia fetida in a Sandy Loam Soil Amended with the Nitro-Heterocyclic Explosives RDX and HMX. Pedobiologia 2003, 47, 657–662. [Google Scholar] [CrossRef]
- Gogal, R.M.; Johnson, M.S.; Larsen, C.T.; Prater, M.R.; Duncan, R.B.; Ward, D.L.; Lee, R.B.; Salice, C.J.; Jortner, B.; Holladay, S.D. Dietary Oral Exposure to 1,3,5-Trinitro-1,3,5-Triazine in the Northern Bobwhite (Colinus virginianus). Environ. Toxicol. Chem. 2003, 22, 381–387. [Google Scholar] [CrossRef]
- Mukhi, S.; Patiño, R. Effects of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) in Zebrafish: General and Reproductive Toxicity. Chemosphere 2008, 72, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Gust, K.A.; Wilbanks, M.S.; Guan, X.; Pirooznia, M.; Habib, T.; Yoo, L.; Wintz, H.; Vulpe, C.D.; Perkins, E.J. Investigations of Transcript Expression in Fathead Minnow (Pimephales promelas) Brain Tissue Reveal Toxicological Impacts of RDX Exposure. Aquat. Toxicol. 2011, 101, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.N.; Liu, J.; Espino, M.A.; Cobb, G.P. Age Dependent Acute Oral Toxicity of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) and Two Anaerobic N-Nitroso Metabolites in Deer Mice (Peromyscus maniculatus). Chemosphere 2007, 67, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.K.; Deng, S.; Basta, N.T.; Clarkson, W.W.; Wilber, G.G. Long-Term Explosive Contamination in Soil: Effects on Soil Microbial Community and Bioremediation. Soil Sediment Contam. 2007, 16, 61–77. [Google Scholar] [CrossRef]
- McMurry, S.T.; Jones, L.E.; Smith, P.N.; Cobb, G.P.; Anderson, T.A.; Lovern, M.B.; Cox, S.; Pan, X. Accumulation and Effects of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX) Exposure in the Green Anole (Anolis carolinensis). Ecotoxicology 2012, 21, 304–314. [Google Scholar] [CrossRef]
- Johnson, M.S.; McFarland, C.A.; Bazar, M.A.; Quinn, M.J.; Lafiandra, E.M.; Talent, L.G. Toxicity of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX) in Three Vertebrate Species. Arch. Environ. Contam. Toxicol. 2010, 58, 836–843. [Google Scholar] [CrossRef]
- Johnson, M.S.; Gogal, R.M., Jr.; Larsen, C.T. Food Avoidance Behavior to Dietary Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX) Exposure in the Northern Bobwhite (Colinus virginianus). J. Toxicol. Environ. Health Part A 2005, 68, 1349–1357. [Google Scholar] [CrossRef]
- Robidoux, P.Y.; Hawari, J.; Thiboutot, S.; Ampleman, G.; Sunahara, G.I. Chronic Toxicity of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX) in Soil Determined Using the Earthworm (Eisenia andrei) Reproduction Test. Environ. Pollut. 2001, 111, 283–292. [Google Scholar] [CrossRef]
- Robidoux, P.Y.; Hawari, J.; Bardai, G.; Paquet, L.; Ampleman, G.; Thiboutot, S.; Sunahara, G.I. TNT, RDX, and HMX Decrease Earthworm (Eisenia andrei) Life-Cycle Responses in a Spiked Natural Forest Soil. Arch. Environ. Contam. Toxicol. 2002, 43, 379–388. [Google Scholar] [CrossRef]
- Neuwoehner, J.; Schofer, A.; Erlenkaemper, B.; Steinbach, K.; Hund-Rinke, K.; Eisentraeger, A. Toxicological Characterization of 2,4,6-Trinitrotoluene, Its Transformation Products, and Two Nitramine Explosives. Environ. Toxicol. Chem. 2007, 26, 1090–1099. [Google Scholar] [CrossRef]
- Qasim, M.M.; Furey, J.; Fredrickson, H.L.; Szecsody, J.; McGrath, C.; Bajpai, R. Semiempirical Predictions of Chemical Degradation Reaction Mechanisms of CL-20 as Related to Molecular Structure. Struct. Chem. 2004, 15, 493–499. [Google Scholar] [CrossRef]
- Kuperman, R.G.; Checkai, R.T.; Phillips, C.T.; Simini, M.; Anthony, J.S. An Emerging Energetic Soil Contaminant, CL-20, Can Affect the Soil Invertebrate Community in a Sandy Loam Soil. Appl. Soil Ecol. 2014, 83, 210–218. [Google Scholar] [CrossRef]
- Robidoux, P.Y.; Sunahara, G.I.; Savard, K.; Berthelot, Y.; Dodard, S.; Martel, M.; Gong, P.; Hawari, J. Acute and Chronic Toxicity of the New Explosive CL-20 to the Earthworm (Eisenia andrei) Exposed to Amended Natural Soils. Environ. Toxicol. Chem. 2004, 23, 1026–1034. [Google Scholar] [CrossRef]
- Gong, P.; Escalon, B.L.; Hayes, C.A.; Perkins, E.J. Uptake of Hexanitrohexaazaisowurtzitane (CL-20) by the Earthworm Eisenia fetida through Dermal Contact. Sci. Total Environ. 2008, 390, 295–299. [Google Scholar] [CrossRef]
- Gong, P.; Guan, X.; Pirooznia, M.; Liang, C.; Perkins, E.J. Gene Expression Analysis of CL-20-Induced Reversible Neurotoxicity Reveals GABA A Receptors as Potential Targets in the Earthworm Eisenia fetida. Environ. Sci. Technol. 2012, 46, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Dodard, S.G.; Sunahara, G.I.; Kuperman, R.G.; Sarrazin, M.; Gong, P.; Ampleman, G.; Thiboutot, S.; Hawari, J. Survival and Reproduction of Enchytraeid Worms, Oligochaeta, in Different Soil Types Amended with Energetic Cyclic Nitramines. Environ. Toxicol. Chem. 2005, 24, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Bardai, G.; Sunahara, G.I.; Spear, P.A.; Martel, M.; Gong, P.; Hawari, J. Effects of Dietary Administration of CL-20 on Japanese Quail Coturnix Coturnix japonica. Arch. Environ. Contam. Toxicol. 2005, 49, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, L.M.; Okolica, M.R.; Gut, C.P.; Gargas, M.L. Cancer Mode of Action, Weight of Evidence, and Proposed Cancer Reference Value for Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX). Regul. Toxicol. Pharmacol. 2012, 64, 205–224. [Google Scholar] [CrossRef]
- Hassan, M.; Adegboyega, T.T.; Bala, S.Z.; Saleh, J.; Komolafe, M.B.; Muhammad, M.Y.; Gaiya, D.D.; Bature, A.S.; Livinus, M.U. The Carcinogenic Risks of Military Explosives: Mechanisms, Health Impacts, and Environmental Consequences of Chemical Munitions. Toxicol. Environ. Health Sci. 2025, 17, 349–363. [Google Scholar] [CrossRef]
- Howa, J.D.; Lott, M.J.; Chesson, L.A.; Ehleringer, J.R. Carbon and Nitrogen Isotope Ratios of Factory-Produced RDX and HMX. Forensic Sci. Int. 2014, 240, 80–87. [Google Scholar] [CrossRef]
- Lewis, T.A.; Newcombe, D.A.; Crawford, R.L. Bioremediation of Soils Contaminated with Explosives. J. Environ. Manag. 2004, 70, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Nair, U.R.; Sarwade, D.B.; Gore, G.M.; Asthana, S.N.; Singh, H. Studies on CL-20: The Most Powerful High Energy Material. J. Therm. Anal. Calorim. 2003, 73, 913–922. [Google Scholar] [CrossRef]
- Nair, U.R.; Sivabalan, R.; Gore, G.M.; Geetha, M.; Asthana, S.N.; Singh, H. Hexanitrohexaazaisowurtzitane (CL-20) and CL-20-Based Formulations (Review). Combust. Explos. Shock Waves 2005, 41, 121–132. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Zhu, X. Treatment of HMX-Production Wastewater in an Aerobic Granular Reactor. Water Environ. Res. 2013, 85, 301–307. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Liu, N.; Wang, X.; Li, C.; Ma, J.; Wu, K.; Zhang, M.; Liu, J.; Song, J.; et al. Study on the Adsorption Mechanism of CL-20 in Waste Acid by Silicic Acid: Adsorption Model and DFT Calculation. Environ. Res. 2025, 277, 121595. [Google Scholar] [CrossRef]
- Ming-chun, X.; Hui, L.; Zhen, Y.; Cheng, H.; Xin-bai, J.; Jin-you, S. Development and Application of Hexanitrohexaazaisowurtzitane Wastewater Treatment Combination Process. Chin. J. Energ. Mater. 2022, 30, 980. [Google Scholar] [CrossRef]
- Bhanot, P.; Celin, S.M.; Kalsi, A.; Singh, S.K.; Sahai, S.K.; Sharma, P. Treatment of High Explosive HMX (Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine) Production Effluent by Advanced Oxidation Processes. Int. J. Environ. Sci. Technol. 2022, 19, 1775–1784. [Google Scholar] [CrossRef]
- Kanekar, S.P.; Kanekar, P.P.; Sarnaik, S.S.; Gujrathi, N.P.; Shede, P.N.; Kedargol, M.R.; Reardon, K.F. Bioremediation of Nitroexplosive Wastewater by an Yeast Isolate Pichia sydowiorum MCM Y-3 in Fixed Film Bioreactor. J. Ind. Microbiol. Biotechnol. 2009, 36, 253–260. [Google Scholar] [CrossRef]
- Tanvanit, P.; Anotai, J.; Su, C.C.; Lu, M.C. Treatment of Explosive-Contaminated Wastewater Through the Fenton Process. Desalin. Water Treat. 2013, 51, 2820–2825. [Google Scholar] [CrossRef]
- Fuller, M.E.; Hatzinger, P.B.; Condee, C.W.; Togna, A.P. Combined Treatment of Perchlorate and RDX in Ground Water Using a Fluidized Bed Reactor. Groundw. Monit. Remediat. 2007, 27, 59–64. [Google Scholar] [CrossRef]
- Morley, M.C.; Henke, J.; Speitel, G. Adsorption of RDX and HMX in Rapid Small-Scale Column Tests: Implications for Full-Scale Adsorbers. J. Environ. Eng. 2005, 131, 29–37. [Google Scholar] [CrossRef]
- Liu, Z.; He, Y.; Li, F.; Liu, Y. Photocatalytic Treatment of RDX Wastewater with Nano-Sized Titanium Dioxide (5 pp). Environ. Sci. Pollut. Res. 2006, 13, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gao, D.; Huang, H.; Huang, B.; Cai, H. Mesoporous Silicas Prepared by Ammonium Perchlorate Oxidation and Their Application in the Selective Adsorption of High Explosives. Microporous Mesoporous Mater. 2013, 168, 46–50. [Google Scholar] [CrossRef]
- Boddu, V.M.; Naismith, N.K.; Patel, H.R. Environmentally Responsive Poly(N-Isopropylacrylamide)-Co-Poly(Acrylic Acid) Hydrogels for Separation of Toxic Metals and Organic Explosive Compounds from Water. J. Polym. Environ. 2019, 27, 571–580. [Google Scholar] [CrossRef]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Lin, K.S.; Dehvari, K.; Hsien, M.J.; Hsu, P.J.; Kuo, H. Degradation of TNT, RDX, and HMX Explosive Wastewaters Using Zero-Valent Iron Nanoparticles. Propellants Explos. Pyrotech. 2013, 38, 786–790. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Jiang, X.; Liu, X.; Sun, X.; Li, J.; Han, W.; Wang, L.; Shen, J. Assembling Nanoscale Zero-Valent Iron on Magnetic Fe3O4/Reduced Graphene Oxide Composite for Efficient Reduction of Hexanitrohexaazaisowurtzitane (Cl-20). Desalin. Water Treat. 2020, 182, 225–236. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.S.; Hsien, M.J.; Chang, C.J.; Kunene, S.C. Synthesis, Characterization, and Application of Zero-Valent Iron Nanoparticles for TNT, RDX, and HMX Explosives Decontamination in Wastewater. J. Taiwan Inst. Chem. Eng. 2020, 114, 186–198. [Google Scholar] [CrossRef]
- Zoh, K.-D.; Stenstrom, M.K. Fenton Oxidation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) and Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX). Water Res. 2002, 36, 1331–1341. [Google Scholar] [CrossRef]
- Celin, S.M.; Pandit, M.; Kapoor, J.C. Photochemical Oxidation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) in Aqueous Phase. WIT Trans. Built Environ. 2009, 108, 93–101. [Google Scholar] [CrossRef]
- Zappi, M.E.; Hernandez, R.; Gang, D.; Bajpai, R.; Kuo, C.H.; Hill, D.O. Treatment of Groundwater Contaminated with High Levels of Explosives Using Advanced Oxidation Processes. Int. J. Environ. Sci. Technol. 2016, 13, 2767–2778. [Google Scholar] [CrossRef]
- Choi, J.K.; Son, H.S.; Kim, T.S.; Stenstrom, M.K.; Zoh, K.D. Degradation Kinetics and Mechanism of RDX and HMX in TiO2 Photocatalysis. Environ. Technol. 2006, 27, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Anotai, J.; Tanvanit, P.; Garcia-Segura, S.; Lu, M.-C. Electro-Assisted Fenton Treatment of Ammunition Wastewater Containing Nitramine Explosives. Process Saf. Environ. Prot. 2017, 109, 429–436. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, K.; Chai, G.; Xi, P.; Yang, H.; Xie, L.; Qin, L.; Lin, Y.; Li, X.; Yan, W.; et al. Performance Optimization and Toxicity Effects of the Electrochemical Oxidation of Octogen. Catalysts 2022, 12, 815. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, H.; Chen, Y. Recent Advances in Biological Removal of Nitroaromatics from Wastewater. Environ. Pollut. 2022, 307, 119570. [Google Scholar] [CrossRef]
- Balakrishnan, V.K.; Halasz, A.; Hawari, J. Alkaline Hydrolysis of the Cyclic Nitramine Explosives RDX, HMX, and CL-20: New Insights into Degradation Pathways Obtained by the Observation of Novel Intermediates. Environ. Sci. Technol. 2003, 37, 1838–1843. [Google Scholar] [CrossRef]
- Heilmann, H.M.; Wiesmann, U.; Stenstrom, M.K. Kinetics of the Alkaline Hydrolysis of High Explosives RDX and HMX in Aqueous Solution and Adsorbed to Activated Carbon. Environ. Sci. Technol. 1996, 30, 1485–1492. [Google Scholar] [CrossRef]
- Panz, K.; Miksch, K. Phytoremediation of Explosives (TNT, RDX, HMX) by Wild-Type and Transgenic Plants. J. Environ. Manag. 2012, 113, 85–92. [Google Scholar] [CrossRef]
- Claus, H. Microbial Degradation of 2,4,6-Trinitrotoluene In Vitro and in Natural Environments. In Biological Remediation of Explosive Residues; Environmental Science and Engineering; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Chatterjee, S.; Deb, U.; Datta, S.; Walther, C.; Gupta, D.K. Common Explosives (TNT, RDX, HMX) and Their Fate in the Environment: Emphasizing Bioremediation. Chemosphere 2017, 184, 438–451. [Google Scholar] [CrossRef]
- Fuller, M.E.; Heraty, L.; Condee, C.W.; Vainberg, S.; Sturchio, N.C.; Böhlke, J.K.; Hatzinger, P.B. Relating Carbon and Nitrogen Isotope Effects to Reaction Mechanisms during Aerobic or Anaerobic Degradation of RDX (Hexahydro-1,3,5-Trinitro-1,3,5-Triazine) by Pure Bacterial Cultures. Appl. Environ. Microbiol. 2016, 82, 3297–3309. [Google Scholar] [CrossRef]
- Bernstein, A.; Ronen, Z.; Gelman, F. Insight on RDX Degradation Mechanism by Rhodococcus Strains Using 13C and 15N Kinetic Isotope Effects. Environ. Sci. Technol. 2013, 47, 479–484. [Google Scholar] [CrossRef]
- Sagi-Ben Moshe, S.; Ronen, Z.; Dahan, O.; Weisbrod, N.; Groisman, L.; Adar, E.; Nativ, R. Sequential Biodegradation of TNT, RDX and HMX in a Mixture. Environ. Pollut. 2009, 157, 2231–2238. [Google Scholar] [CrossRef]
- Ronen, Z.; Yanovich, Y.; Goldin, R.; Adar, E. Metabolism of the Explosive Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) in a Contaminated Vadose Zone. Chemosphere 2008, 73, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, A.; Celin, S.M.; Sharma, J.G. Aerobic Biodegradation of High Explosive Hexahydro-1,3,5-Trinitro-1,3,5-Triazine by Janibacter cremeus Isolated from Contaminated Soil. Biotechnol. Lett. 2020, 42, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Perreault, N.N.; Crocker, F.H.; Indest, K.J.; Hawari, J. Involvement of Cytochrome c CymA in the Anaerobic Metabolism of RDX by Shewanella oneidensis MR-1. Can. J. Microbiol. 2012, 58, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, Y.; Kwon, M.J.; Lee, W. Riboflavin-Mediated RDX Transformation in the Presence of Shewanella putrefaciens CN32 and Lepidocrocite. J. Hazard. Mater. 2014, 274, 24–31. [Google Scholar] [CrossRef]
- Zhao, J.S.; Paquet, L.; Halasz, A.; Hawari, J. Metabolism of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine through Initial Reduction to Hexahydro-1-Nitroso-3,5-Dinitro-1,3,5-Triazine Followed by Denitration in Clostridium bifermentans HAW-1. Appl. Microbiol. Biotechnol. 2003, 63, 187–193. [Google Scholar] [CrossRef]
- Crocker, F.H.; Blakeney, G.A.; Jung, C.M. Complete Degradation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) by a Co-Culture of Gordonia sp. KTR9 and Methylobacterium sp. JS178. Remediation 2016, 26, 51–58. [Google Scholar] [CrossRef]
- Fuller, M.E.; Perreault, N.; Hawari, J. Microaerophilic Degradation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) by Three Rhodococcus Strains. Lett. Appl. Microbiol. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Bernstein, A.; Adar, E.; Nejidat, A.; Ronen, Z. Isolation and Characterization of RDX-Degrading Rhodococcus Species from a Contaminated Aquifer. Biodegradation 2011, 22, 997–1005. [Google Scholar] [CrossRef]
- Brenner, A.; Ronen, Z.; Harel, Y.; Abeliovich, A. Use of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine as a Nitrogen Source in Biological Treatment of Munitions Wastes. Water Environ. Res. 2000, 72, 469–475. [Google Scholar] [CrossRef]
- Nejidat, A.; Kafka, L.; Tekoah, Y.; Ronen, Z. Effect of Organic and Inorganic Nitrogenous Compounds on RDX Degradation and Cytochrome P-450 Expression in Rhodococcus Strain YH1. Biodegradation 2008, 19, 313–320. [Google Scholar] [CrossRef]
- Yadav, A.; Gupta, S.; Istvan, P.; Ronen, Z. Effects of Perchlorate and Other Groundwater Inorganic Co-Contaminants on Aerobic RDX Degradation. Microorganisms 2022, 10, 663. [Google Scholar] [CrossRef]
- Zhao, J.S.; Paquet, L.; Halasz, A.; Manno, D.; Hawari, J. Metabolism of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine by Clostridium bifermentans Strain HAW-1 and Several Other H2-Producing Fermentative Anaerobic Bacteria. FEMS Microbiol. Lett. 2004, 237, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Nagar, S.; Shaw, A.K.; Anand, S.; Celin, S.M.; Rai, P.K. Aerobic Biodegradation of HMX by Planomicrobium flavidum. 3 Biotech 2018, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Paquet, L.; Halasz, A.; Spain, J.C.; Hawari, J. Mechanism of Xanthine Oxidase Catalyzed Biotransformation of HMX under Anaerobic Conditions. Biochem. Biophys. Res. Commun. 2003, 306, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Soni, P.; Kumar, P.; Purohit, S.; Singh, A. Biodegradation of High Explosive Production Effluent Containing RDX and HMX by Denitrifying Bacteria. World J. Microbiol. Biotechnol. 2009, 25, 269–275. [Google Scholar] [CrossRef]
- Liu, Z.; Dang, K.; Li, C.; Gao, J.; Wang, H.; Gao, Y.; Zhao, B.; Fan, P.; Qian, A. Isolation and Identification of a Novel Bacterium, Pseudomonas sp. ZyL-01, Involved in the Biodegradation of CL-20. AMB Express 2020, 10, 196. [Google Scholar] [CrossRef]
- Bhushan, B.; Halasz, A.; Hawari, J. Nitroreductase Catalyzed Biotransformation of CL-20. Biochem. Biophys. Res. Commun. 2004, 322, 271–276. [Google Scholar] [CrossRef]
- Bhushan, B.; Halasz, A.; Hawari, J. Stereo-Specificity for pro-(R) Hydrogen of NAD(P)H during Enzyme-Catalyzed Hydride Transfer to CL-20. Biochem. Biophys. Res. Commun. 2005, 337, 1080–1083. [Google Scholar] [CrossRef]
- Bhatt, M.; Zhao, J.S.; Halasz, A.; Hawari, J. Biodegradation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine by Novel Fungi Isolated from Unexploded Ordnance Contaminated Marine Sediment. J. Ind. Microbiol. Biotechnol. 2006, 33, 850–858. [Google Scholar] [CrossRef]
- Fournier, D.; Halasz, A.; Thiboutot, S.; Ampleman, G.; Manno, D.; Hawari, J. Biodegradation of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX) by Phanerochaete chrysosporium: New Insight into the Degradation Pathway. Environ. Sci. Technol. 2004, 38, 4130–4133. [Google Scholar] [CrossRef] [PubMed]
- Fournier, D.; Monteil-Rivera, F.; Halasz, A.; Bhatt, M.; Hawari, J. Degradation of CL-20 by White-Rot Fungi. Chemosphere 2006, 63, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, D.; Mishra, V.; Kushwaha, J.; Sengar, M.; Sinha, S.; Singh, S.; Giri, B.S. Strategies for Biological Treatment of Waste Water: A Critical Review. J. Clean. Prod. 2024, 454, 142266. [Google Scholar] [CrossRef]
- Gupta, S.; Ronen, Z. Biological Treatment of Nitroaromatics in Wastewater. Water 2024, 16, 901. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I. Wastewater Treatment by Biological Methods. Environ. Water 2013, 7, 179–204. [Google Scholar] [CrossRef]
- Karakaya, P.; Christodoulatos, C.; Koutsospyros, A.; Balas, W.; Nicolich, S.; Sidhoum, M. Biodegradation of the High Explosive Hexanitrohexaazaiso-Wurtzitane (CL-20). Int. J. Environ. Res. Public Health 2009, 6, 1371–1392. [Google Scholar] [CrossRef]
- Stein, N.; Podder, A.; Goel, R. Biodegradation of Insensitive Munition (IM) Formulations: IMX-101 and IMX-104 Using Aerobic Granule Technology. J. Hazard. Mater. 2023, 449, 130942. [Google Scholar] [CrossRef]
- Dutta, A.; Sarkar, S. Sequencing Batch Reactor for Wastewater Treatment: Recent Advances. Curr. Pollut. Reports 2015, 1, 177–190. [Google Scholar] [CrossRef]
- Morley, M.C.; Shammas, S.N.; Speitel, G.E. Biodegradation of RDX and HMX Mixtures: Batch Screening Experiments and Sequencing Batch Reactors. Environ. Eng. Sci. 2002, 19, 237–250. [Google Scholar] [CrossRef]
- Ibeanusi, V.; Jeilani, Y.; Houston, S.; Doss, D.; Coley, B. Sequential Anaerobic-Aerobic Degradation of Munitions Waste. Biotechnol. Lett. 2009, 31, 65–69. [Google Scholar] [CrossRef]
- Fuller, M.E.; Hedman, P.C.; Chu, K.H.; Webster, T.S.; Hatzinger, P.B. Evaluation of a Sequential Anaerobic-Aerobic Membrane Bioreactor System for Treatment of Traditional and Insensitive Munitions Constituents. Chemosphere 2023, 340, 139887. [Google Scholar] [CrossRef]
- Zoh, K.D.; Stenstrom, M.K. Application of a Membrane Bioreactor for Treating Explosives Process Wastewater. Water Res. 2002, 36, 1018–1024. [Google Scholar] [CrossRef]
- Fuller, M.E.; Hedman, P.C.; Chu, K.H.; Webster, T.S.; Schaefer, C.E.; Tran, D.N.; Hatzinger, P.B. Effective Treatment of Energetic Containing Wastewater in a Sequential Anaerobic-Aerobic Membrane Bioreactor (MBR) System-Part 1: Treatment of IMX-104 Wastewater. Chemosphere 2025, 386, 144606. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, U.E.; Abeykoon, A.M.W.D.C.B.; Sewmini, P.M.N.; Weerasekara, W.M.R.B.; Darshani, N.P.; Jayasanka, J.; Weerasekara, N.A.; Hewawasam, C.; Sanjeewa, K.K.A.; Jayawardena, T.U. Up-Flow Anaerobic Sludge Bed Reactors for Sustainable Wastewater Management: Challenges, Innovations, and Future Directions. Water 2025, 17, 476. [Google Scholar] [CrossRef]
- An, C.J.; He, Y.L.; Huang, G.H.; Yang, S.C. Degradation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) by Anaerobic Mesophilic Granular Sludge from a UASB Reactor. J. Chem. Technol. Biotechnol. 2010, 85, 831–838. [Google Scholar] [CrossRef]
- An, C.; Shi, Y.; He, Y.; Huang, G.; Liu, Y.; Yang, S. Biotransformation of RDX and HMX by Anaerobic Granular Sludge with Enriched Sulfate and Nitrate. Water Environ. Res. 2017, 89, 472–479. [Google Scholar] [CrossRef]
- Atikovic, E.; Suidan, M.T.; Maloney, S.W. Anaerobic Treatment of Army Ammunition Production Wastewater Containing Perchlorate and RDX. Chemosphere 2008, 72, 1643–1648. [Google Scholar] [CrossRef]
- Young, T.S.; Morley, M.C.; Snow, D.D. Anaerobic Biodegradation of RDX and TCE: Single- and Dual-Contaminant Batch Tests. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2006, 10, 94–101. [Google Scholar] [CrossRef]
- Zheng, C.-W.; Long, M.; Luo, Y.-H.; Long, X.; Bi, Y.; Zhou, D.; Zhou, C.; Rittmann, B.E. Reductive Destruction of Multiple Nitrated Energetics over Palladium Nanoparticles in the H2-Based Membrane Catalyst-Film Reactor (MCfR). J. Hazard. Mater. 2022, 423, 127055. [Google Scholar] [CrossRef]
- Jha, P.; Sen, R.; Jobby, R.; Sachar, S.; Bhatkalkar, S.; Desai, N. Biotransformation of Xenobiotics by Hairy Roots. Phytochemistry 2020, 176, 112421. [Google Scholar] [CrossRef]
- Yoon, J.M.; Oh, B.-T.; Just, C.L.; Schnoor, J.L. Uptake and Leaching of Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine by Hybrid Poplar Trees. Environ. Sci. Technol. 2002, 36, 4649–4655. [Google Scholar] [CrossRef]
- Low, D.; Tan, K.; Anderson, T.; Cobb, G.P.; Liu, J.; Jackson, W.A. Treatment of RDX Using Down-Flow Constructed Wetland Mesocosms. Ecol. Eng. 2008, 32, 72–80. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Bai, Q.; Yan, N.; Xu, H.; Rittmann, B.E. Intimately Coupling of Photolysis Accelerates Nitrobenzene Biodegradation, but Sequential Coupling Slows Biodegradation. J. Hazard. Mater. 2015, 287, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hong, L.; Han, W.; Wang, L.; Sun, X.; Li, J. Treatment of High Explosive Production Wastewater Containing RDX by Combined Electrocatalytic Reaction and Anoxic-Oxic Biodegradation. Chem. Eng. J. 2011, 168, 1256–1262. [Google Scholar] [CrossRef]
- Oh, B.-T.; Just, C.L.; Alvarez, P.J.J. Hexahydro-1,3,5-Trinitro-1,3,5-Triazine Mineralization by Zerovalent Iron and Mixed Anaerobic Cultures. Environ. Sci. Technol. 2001, 35, 4341–4346. [Google Scholar] [CrossRef]
- Wildman, M.J.; Alvarez, P.J. RDX Degradation Using an Integrated Fe(0)-Microbial Treatment Approach. Water Sci. Technol. 2001, 43, 25–33. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Chiu, P.C.; Kim, B.J.; Cha, D.K. Zero-Valent Iron Pretreatment for Enhancing the Biodegradability of RDX. Water Res. 2005, 39, 5027–5032. [Google Scholar] [CrossRef]
- Zoh, K.-D.; Daniels, J.I.; Knezovich, J.P.; Stenstrom, M.K. Treatment of Hydrolysates of the High Explosives Hexahydro-1,3,5,-Trinitro-1,3,5-Trianize and Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine Using Biological Denitrification. Water Environ. Res. 1999, 71, 148–155. [Google Scholar] [CrossRef]
- An, C.; Shi, Y.; He, Y.; Huang, G.; Liang, J.; Liu, Z. Effect of Different Carbon Substrates on the Removal of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) and Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX) by Anaerobic Mesophilic Granular Sludge. Water Air Soil Pollut. 2014, 225, 2174. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Liu, Y.; He, Y. Anaerobic Biodegradation of RDX and HMX with Different Co-Substrates. Chin. J. Chem. Eng. 2015, 23, 704–709. [Google Scholar] [CrossRef]
- Lee, B.U.; Choi, M.S.; Kim, D.M.; Oh, K.H. Genome Shuffling of Stenotrophomonas maltophilia OK-5 for Improving the Degradation of Explosive RDX (Hexahydro-1,3,5-Trinitro-1,3,5-Triazine). Curr. Microbiol. 2017, 74, 268–276. [Google Scholar] [CrossRef]
- Cary, T.J.; Rylott, E.L.; Zhang, L.; Routsong, R.M.; Palazzo, A.J.; Strand, S.E.; Bruce, N.C. Field Trial Demonstrating Phytoremediation of the Military Explosive RDX by XplA/XplB-Expressing Switchgrass. Nat. Biotechnol. 2021, 39, 1216–1219. [Google Scholar] [CrossRef]
- Dang, H.; Cupples, A.M. Diversity and Abundance of the Functional Genes and Bacteria Associated with RDX Degradation at a Contaminated Site Pre- and Post-Biostimulation. Appl. Microbiol. Biotechnol. 2021, 105, 6463–6475. [Google Scholar] [CrossRef]
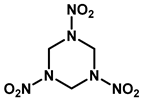 | 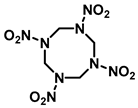 | 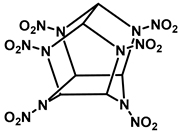 | |
|---|---|---|---|
| Common Name | RDX | HMX | CL-20 |
| Chemical Formula | C3H6N6O6 | C4H8N8O8 | C6H6N12O12 |
| Molecular Weight (g/mol) | 222.26 | 296.16 | 438.19 |
| Melting Point (℃) | 205 | 276–286 | 260 (with decomposition) |
| Water Solubility (mg/L) at 25 °C | 40.0 | 6.6 | 3.6 |
| Octanol/Water Partition Coefficient (log Kw) | 0.90 | 0.17 | 1.92 |
| Henry’s Law Constant at 25 °C (atm m3/mol) | 1.96 × 10−11 | 2.60 × 10−15 | Not determined |
| Vapor Pressure at 25 °C (mm Hg) | 4.0 × 10−9 | 3.3 × 10−14 | Not determined |
| Permissible limit (mg/L) in drinking water | 0.002 | 0.04 | Not determined |
| Compounds | Species | Effects | References |
|---|---|---|---|
| RDX | Nematodes (Caenorhabditis elegans) | generation of reactive oxygen species, death of germ cells, and a reduction in the number of eggs produced | [32] |
| Earthworm (Eisenia fetida) | negative impact on reproduction | [33] | |
| Northern bobwhites (Colinus virginianus) | decline in total feed consumption, total egg production, and hen-housed production characteristics | [34] | |
| Zebrafish | lethally poisoned | [35] | |
| Fathead minnows | overt toxicity | [36] | |
| Deer mice (Peromyscus maniculatus) | age-dependent toxicity | [37] | |
| HMX | Green anoles (Anolis carolinensis) | lower hatching success rate | [39] |
| Three vertebrate species (rabbit (Oryctolagus cuniculus)), amphibians (redbacked salamander (Plethodon cinereus)), and reptiles (Western fence lizard (Sceloporus occidentalis)) | While Salamanders showed no effects at ≤1970 mg/kg HMX, lizards died at high oral dosages, due to gastrointestinal impaction. Rabbits showed neurological symptoms, more sensitive than amphibians and reptiles | [40] | |
| Northern bobwhites (Colinus virginianus) | concentration-dependent reduction in food intake, body mass, and egg production | [41] | |
| Earthworm (Eisenia andrei) | some reproductive consequences, such as the quantity of juveniles and their biomass | [42,43] | |
| CL-20 | Nematodes and microarthropods | Nematodes remained the same, but microarthropods drastically decreased | [46] |
| Earthworm (Eisenia andrei) | reproductive toxicity | [47] | |
| Earthworm (Eisenia fetida) | neurotoxicity through dermal contact | [48,49] | |
| Potworms (Enchytraeus albidus and Enchytraeus crypticus) | dramatically decreasing adult survival and juvenile production | [50] | |
| Japanese quail embryos (Coturnix coturnix japonica) | produce adverse developmental effects | [51] |
| Wastewater | Parameters | Value | References |
|---|---|---|---|
| RDX wastewater | pH | 2.0 | [18] |
| Nitrate (mg/L) | 13,869.9 | ||
| COD (mg/L) | 6153.6 | ||
| RDX (mg/L) | 135.77 | ||
| RDX and HMX wastewater | pH | 1–3 | [7,20,58,61,62] |
| Acetate (mg/L) | 2700–227,000 | ||
| Sulfate (mg/L) | 350 | ||
| Ammonium nitrate (mg/L) | 200–250 | ||
| Nitrate (mg/L) | 400–342,350 | ||
| COD (mg/L) | 350–430,000 | ||
| BOD (mg/L) | 1238 | ||
| RDX (mg/L) | 400–500 | ||
| HMX (mg/L) | 200–500 | ||
| CL-20 wastewater | pH | 5.4 | [60] |
| COD (mg/L) | 39,333 | ||
| CL-20 (mg/L) | 15.7 | ||
| Ethyl acetate (mg/L) | 11,250 | ||
| Chloroform (mg/L) | 1120 | ||
| Total nitro compound (mg/L) | 65 |
| Treatment Methods | Compounds | References | |
|---|---|---|---|
| Adsorption | granular activated carbon | RDX and HMX from polluted groundwater | [65] |
| activated carbon fiber (ACF) cloth filled with nanoscale TiO2 particles | RDX wastewater | [66] | |
| mesoporous silicas | HMX | [67] | |
| Chemical Reduction | nano-zero-valent iron (nZVIs) | RDX and HMX | [70] |
| nZVI@MG (nZVI assembled on magnetic Fe3O4/reduced graphene oxides composite) | CL-20 | [71] | |
| Advanced Oxidation Processes (AOPs) | photo-peroxidation and photo-Fenton oxidation | RDX | [74] |
| Fenton process | explosive-polluted wastewater | [63] | |
| UV irradiation used along with hydrogen peroxide and ozone | groundwater from a US Army facility contaminated by explosives | [75] | |
| direct photolysis, photo-peroxidation, and photo-Fenton oxidation | RDX wastewater | [18] | |
| photolyzed in a Rayonet photoreactor at 300 nm | CL-20 | [24] | |
| Using nano-TiO2 as the photocatalytic catalyst | RDX wastewater | [66] | |
| UV lamp and TiO2 | RDX and HMX | [76] | |
| electro-assisted Fenton method | RDX and HMX from real wastewater | [77] | |
| Alkaline Hydrolysis | Alkaline hydrolysis in an aqueous solution (pH 10–12.3) | RDX, HMX, and CL-20 | [80] |
| Compounds | Organism | Pathways and Degradation Products | References |
|---|---|---|---|
| RDX | Gordonia sp. | NDAB | [88] |
| Janibacter cremeus | BHNA and MDNA | [89] | |
| Shewanella oneidensis MR-1 | nitro reduction | [90] | |
| Shewanella putrefaciens CN32 | ammonium, formaldehyde, and nitroso intermediates | [91] | |
| Clostridium bifermentans strain HAW-1 | MNX | [92] | |
| Clostridium sp. strain EDB2 | Denitration pathway | [14] | |
| Gordonia sp. KTR9 | NDAB | [93] | |
| Rhodococcus strains, DN22, 11Y, and A | MEDINA and NDAB | [94] | |
| Rhodococcus strains, YH1, T7, and T9N | NDAB | [86,95,96] | |
| HMX | Clostridium bifermentans strain HAW-1 | MEDINA and the mononitroso derivatives | [99] |
| Planomicrobium flavidum strain S5-TSA-19 | MEDINA and N-methyl-N,N′-dinitromethanediamine | [100] | |
| Bacillus strains (HPB2, HPB3) and Pseudomonas (HPB1) | Products not identified | [102] | |
| CL-20 | Pseudomonas sp. ZyL-01 | Mechanism not identified | [103] |
| Escherichia coli nitroreductase | denitration pathway | [104] | |
| Two enzymes, a diaphorase from Clostridium kluyveri and a dehydrogenase from Clostridium sp. EDB2 | N-denitrohydrogenated products | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Ronen, Z. Treatment of Xenobiotic Cyclic Nitramine Explosives in Wastewater. J. Xenobiot. 2025, 15, 188. https://doi.org/10.3390/jox15060188
Gupta S, Ronen Z. Treatment of Xenobiotic Cyclic Nitramine Explosives in Wastewater. Journal of Xenobiotics. 2025; 15(6):188. https://doi.org/10.3390/jox15060188
Chicago/Turabian StyleGupta, Swati, and Zeev Ronen. 2025. "Treatment of Xenobiotic Cyclic Nitramine Explosives in Wastewater" Journal of Xenobiotics 15, no. 6: 188. https://doi.org/10.3390/jox15060188
APA StyleGupta, S., & Ronen, Z. (2025). Treatment of Xenobiotic Cyclic Nitramine Explosives in Wastewater. Journal of Xenobiotics, 15(6), 188. https://doi.org/10.3390/jox15060188