Microplastic Polymer Mass Fractions in Marine Bivalves: From Isolation to Hazard Risk
Abstract
1. Introduction
2. Microplastic Polymers and Marine Bivalves
3. Measurements and Data Analysis
3.1. Analytical Approaches/Techniques for the Separation and Identification of Microplastic Polymers
| Targeted Microplastic Polymers | Matrix | Microwave-Assisted Procedure (MAD/MAE), System, and Total Microplastic Polymer Recovery (%) | Filtration Filters/Filtration Performance | Identification/ Quantification Method | Reference |
|---|---|---|---|---|---|
| PET, PS, EPS, PP, HDPE, LDPE, PC, PVC, PU | Seafood tissue (acoupa weakfish, tuna fish, trahira, shrimp) | MAD with HNO3; System: Ultrawave™, Milestone, Sorisole, Italy; Recovery range: 103–106% | Vacuum system filtration (2 μm linter fiber filter paper (Whatman No. 589/3) Florham Park, MI) | Gravimetry | [49] |
| PE, PP, PS, PMMA, PVC, PC | Mussels, shrimp, salmon fillets | MAE of GF filters in DCM after sample digestion in KOH; System: Mars6 system (CEM, Matthews, NC, USA); Recovery range: 51–151% | Matrix-dependent filters, sequential filtration with 1 μm GF filters, 20 μm and 10 μm polycarbonate filters | Pyr-GC-MS | [21] |
| HDPE, PP, PET, PA, PS | Dried Mytilus galloprovincialis | MAD multireagent digestion: KOH, H2O2, and methanol; System: Ethos system (Milestone, Sorisole, Italy); Recovery range: 99.4–100.5% | 1.2 μm GF filters | Optical microscopy and Raman spectroscopy | [49] |
| PVC, HDPE, LDPE, PP, PS, PET | Unique food matrix (fiber, apple, courgette, cucumber, kidney beans, lettuce, potatoes, tomatoes, bread, cheese, yoghurt) | MAD with HNO3; System: Mars Xpress Microwave (CEM Corporation, Matthews, NC, USA); Recovery: 102.2% | 3 μm Duran glass filter (VWR) | ATR FTIR | [50] |
| PE, PP, PS ABS, SBR, PMMA, PC, PVC, PET, N6, and N66 | Mussels (commercial lyophilized flour of Perna canaliculus) | MAD with HCl/or HNO3; System: Ethos X Advanced Microwave Extraction System (Milestone Srl, Italy); Recovery: 87–138% | 0.7 μm quartz fiber filter | Pyr-GC-MS | [44] |
3.2. Risk Assessment Studies for Microplastic Intake in Humans Through Bivalve Consumption
4. Conclusions and Perspectives
- Specifically optimized, robust, and relatively rapid methods of quantifying small MPs in selected environmental samples relevant to the marine food web;
- The accurate identification of plastic polymers in microplastic samples and assessment of the potential risks posed by MPs to humans and ecosystems;
- Comprehensive monitoring and risk assessment strategies to mitigate the impacts of MPs on both food safety and marine ecosystems, as similar polymer contamination levels have been reported in marine bivalves.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Pyr-GC/MS | pyrolysis gas chromatography–mass spectrometry |
| TED-GC/MS | thermal extraction–desorption combined with gas chromatography–mass spectrometry |
| TGA-DSC | thermogravimetric analysis coupled with differential scanning calorimetry |
| PP | polypropylene |
| PE | polyethylene |
| PVC | polyvinyl chloride |
| PS | polystyrene |
| HDPE | high-density polyethylene |
| LDPE | low-density polyethylene |
| PET | polyethylene terephthalate |
| EPS | expanded polystyrene |
| XPS | extruded polystyrene |
| PC | polycarbonate |
| ABS | acrylonitrile butadiene styrene |
| PA | polyamides |
| PEST | polyester |
| PVDC | polyvinylidene chloride |
| PMMA | polymethyl methacrylate |
| PSU | polyarylsulfone |
| PAN | polyacrylonitrile |
| PVA | polyvinyl alcohol |
| PTFE | polytetrafluoroethylene |
References
- Hahn, S.; Hennecke, D. WP2C—Framework on Persistence Assessment for Polymers and Microplastics; European Chemicals Industry Council: Brussels, Belgium, 2022; pp. 1–59. [Google Scholar]
- Law, K.L.; Moret-Ferguson, S.; Maximenko, N.A.; Proskurowski, G.; Peacock, E.E.; Hafner, J.; Reddy, C.M. Plastic Accumulation in the North Atlantic Subtropical Gyre. Science 2010, 329, 1185–1188. [Google Scholar] [CrossRef]
- Rai, M.; Pant, G.; Pant, K.; Aloo, B.N.; Kumar, G.; Singh, H.B.; Tripathi, V. Microplastic Pollution in Terrestrial Ecosystems and Its Interaction with Other Soil Pollutants: A Potential Threat to Soil Ecosystem Sustainability. Resources 2023, 12, 67. [Google Scholar] [CrossRef]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris. In Proceedings of the NOAA Technical Memorandum NOS-OR&R-30, Tacoma, WA, USA, 9–11 September 2008. [Google Scholar]
- Lusher, A.L.; Hollman, P.C.H.; Mendoza-Hill, J.J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO, Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2017; Volume 615, pp. 1–147. [Google Scholar]
- Koelmans, A.A.; Diepens, N.J.; Velzeboer, I.; Besseling, E.; Quik, J.T.K.; van de Meent, D. Guidance for the prognostic risk assessment of nanomaterials in aquatic ecosystems. Sci. Total Environ. 2015, 535, 141–149. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Jang, Y.C.; Hong, S.Y.; Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Kang, D.; et al. Distribution and Size Relationships of Plastic Marine Debris on Beaches in South Korea. Arch. Environ. Contam. Toxicol. 2015, 69, 288–298. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Plastics, E. Plastics the Facts 2019: An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2019; pp. 1–39. [Google Scholar]
- Arredondo-Navarro, A.; Dronjak, L.; Solano, J.R.; Navarro-Amador, R.; Martinez-Tavera, E.; Flores-Cervantes, D.X. A Review for Prioritizing Microplastic Regulation and Research: An Integral Approach. Air Soil. Water Res. 2024, 17, 1–22. [Google Scholar] [CrossRef]
- Goldberg, E.D. The Mussel Watch Concept. Environ. Monit. Assess. 1986, 7, 91–103. [Google Scholar] [CrossRef]
- O’Connor, T.P. Mussel Watch results from 1986 to 1996. Mar. Pollut. Bull. 1998, 37, 14–19. [Google Scholar] [CrossRef]
- Nakata, H.; Shinohara, R.I.; Nakazawa, Y.; Isobe, T.; Sudaryanto, A.; Subramanian, A.; Tanabe, S.; Zakaria, M.P.; Zheng, G.J.; Lam, P.K.S.; et al. Asia-Pacific mussel watch for emerging pollutants: Distribution of synthetic musks and benzotriazole UV stabilizers in Asian and US coastal waters. Mar. Pollut. Bull. 2012, 64, 2211–2218. [Google Scholar] [CrossRef]
- Bogdanovic, T.; Ujevic, I.; Sedak, M.; Listes, E.; Simat, V.; Petricevic, S.; Poljak, V. As, Cd, Hg and Pb in four edible shellfish species from breeding and harvesting areas along the eastern Adriatic Coast, Croatia. Food Chem. 2014, 146, 197–203. [Google Scholar] [CrossRef] [PubMed]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Pipkin, W.; Belganeh, R.; Robberson, W.; Allen, H.L.; Cook, A.M.; Watanabe, A. Identification of Microplastics in Environmental Monitoring Using Pyrolysis-GC-MS Analysis. LC GC Eur. 2021, 34, 306–314. [Google Scholar]
- ECHA. Guidance for Monomers and Polymers, Version 3.0; Guidance Documents: Helsinki, Finland, 2023; p. 26. [Google Scholar]
- Besseling, E.; Redondo-Hasselerharm, P.; Foekema, E.M.; Koelmans, A.A. Quantifying ecological risks of aquatic micro- and nanoplastic. Crit. Rev. Environ. Sci. Technol. 2019, 49, 32–80. [Google Scholar] [CrossRef]
- Ribeiro, F.; Okoffo, E.D.; O’Brien, J.W.; Fraissinet-Tachet, S.; O’Brien, S.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Mueller, J.F.; Galloway, T.; et al. Quantitative Analysis of Selected Plastics in High-Commercial-Value Australian Seafood by Pyrolysis Gas Chromatography Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 9408–9417. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Rochman, C.M. Microwave-Assisted Extraction for Quantification of Microplastics Using Pyrolysis-Gas Chromatography/Mass Spectrometry. Environ. Toxicol. Chem. 2021, 40, 2733–2741. [Google Scholar] [CrossRef]
- Albignac, M.; Ghiglione, J.F.; Labrune, C.; Ter Halle, A. Determination of the microplastic content in Mediterranean benthic macrofauna by pyrolysis-gas chromatography-tandem mass spectrometry. Mar. Pollut. Bull. 2022, 181, 113882. [Google Scholar] [CrossRef]
- Halbach, M.; Vogel, M.; Tammen, J.K.; Rüdel, H.; Koschorreck, J.; Scholz-Böttcher, B.M. 30 years trends of microplastic pollution: Mass-quantitative analysis of archived mussel samples from the North and Baltic Seas. Sci. Total Environ. 2022, 826, 154179. [Google Scholar] [CrossRef]
- Zhong, Y.; Bao, Q.; Yuan, L.; Liu, J.; Cai, Y.; Chen, X. Analysis of Microplastics in Aquatic Shellfish by Pyrolysis-Gas Chromatography/Mass Spectrometry after Alkali Digestion and Solvent Extraction. Polymers 2022, 14, 3888. [Google Scholar] [CrossRef]
- Liu, S.; Sam, K. Quantitative Analysis of Microplastics in Shellfish Using Pyrolysis-GC-MS. CDS Analytical. Available online: https://www.cdsanalytical.com/single-post/277-quantitative-analysis-of-microplastics-in-shellfish-using-pyrolysis-gc-ms (accessed on 4 August 2025).
- Cabernard, L.; Roscher, L.; Lorenz, C.; Gerdts, G.; Primpke, S. Comparison of Raman and Fourier Transform Infrared Spectroscopy for the Quantification of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2018, 52, 13279–13288. [Google Scholar] [CrossRef]
- Primpke, S.; Fischer, M.; Lorenz, C.; Gerdts, G.; Scholz-Bottcher, B.M. Comparison of pyrolysis gas chromatography/mass spectrometry and hyperspectral FTIR imaging spectroscopy for the analysis of microplastics. Anal. Bioanal. Chem. 2020, 412, 8283–8298. [Google Scholar] [CrossRef]
- Li, J.N.; Yang, D.Q.; Li, L.; Jabeen, K.; Shi, H.H. Microplastics in commercial bivalves from China. Environ. Pollut. 2015, 207, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, T.; Di Renzo, L.; SOKOLIĆ, D.; Mascilongo, G.; Brkljaca, M.; Cito, F.; Calistri, P.; Notarstefano, V.; Gioacchini, G.; Giorgini, E.; et al. Microplastics and Bisphenol A in Mussels along Italian and Croatian Coast of the Adriatic Sea. In Proceedings of the Book of Abstracts of the EFSA Colloquium 2021 Micro and Nanoplastics in Food, Online, 6–7 May 2021. [Google Scholar]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, I.A.; Rivadeneira, M.M.; Thiel, M. Temporal and spatial distribution of floating objects in coastal waters of central-southern Chile and Patagonian fjords. Cont. Shelf Res. 2011, 31, 172–186. [Google Scholar] [CrossRef]
- Phaksopa, J.; Sukhsangchan, R.; Keawsang, R.; Tanapivattanakul, K.; Asvakittimakul, B.; Thamrongnawasawat, T.; Worachananant, S. Assessment of Microplastics in Green Mussel (Perna viridis) and Surrounding Environments around Sri Racha Bay, Thailand. Sustainability 2023, 15, 9. [Google Scholar] [CrossRef]
- Pequeno, J.; Antunes, J.; Dhimmer, V.; Bessa, F.; Sobral, P. Microplastics in Marine and Estuarine Species From the Coast of Portugal. Front. Environ. Sci. 2021, 9, 579127. [Google Scholar] [CrossRef]
- Marques, F.; Vale, C.; Rudnitskaya, A.; Moreirinha, C.; Costa, S.T.; Botelho, M.J. Major characteristics of microplastics in mussels from the Portuguese coast. Environ. Res. 2021, 197, 110993. [Google Scholar] [CrossRef]
- Suaria, G.; Perold, V.; Lee, J.R.; Lebouard, F.; Aliani, S.; Ryan, P.G. Floating macro- and microplastics around the Southern Ocean: Results from the Antarctic Circumnavigation Expedition. Environ. Int. 2020, 136, 105494. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef]
- White, E.M.; Clark, S.; Manire, C.A.; Crawford, B.; Wang, S.; Locklin, J.; Ritchie, B.W. Ingested Micronizing Plastic Particle Compositions and Size Distributions within Stranded Post-Hatchling Sea Turtles. Environ. Sci. Technol. 2018, 52, 10307–10316. [Google Scholar] [CrossRef]
- Prata, J.C. Plastic Pollution: Challenges and Innovative Solutions. Environments 2024, 11, 271. [Google Scholar] [CrossRef]
- Expósito, N.; Rovira, J.; Sierra, J.; Folch, J.; Schuhmacher, M. Microplastics levels, size, morphology and composition in marine water, sediments and sand beaches. Case study of Tarragona coast (western Mediterranean). Sci. Total Environ. 2021, 786, 147453. [Google Scholar] [PubMed]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozo, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cho, J.L.; Sohn, J.; Kim, C. Health Effects of Microplastic Exposures: Current Issues and Perspectives in South Korea. Yonsei Med. J. 2023, 64, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Seeley, M.E.; Lynch, J.M. Previous successes and untapped potential of pyrolysis-GC/MS for the analysis of plastic pollution. Anal. Bioanal. Chem. 2023, 415, 2873–2890. [Google Scholar] [CrossRef]
- Biale, G.; La Nasa, J.; Fiorentini, L.; Ceccarini, A.; Carnaroglio, D.; Mattonai, M.; Modugno, F. Characterization and quantification of microplastics and organic pollutants in mussels by microwave-assisted sample preparation and analytical pyrolysis. Environ. Sci.-Adv. 2024, 3, 76–84. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frere, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Riviere, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Rani, M.; Ducoli, S.; Depero, L.E.; Prica, M.; Tubic, A.; Ademovic, Z.; Morrison, L.; Federici, S. A Complete Guide to Extraction Methods of Microplastics from Complex Environmental Matrices. Molecules 2023, 28, 5710. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef]
- Bitencourt, G.R.; Mello, P.A.; Flores, E.M.M.; Pirola, C.; Carnaroglio, D.; Bizzi, C.A. Determination of microplastic content in seafood: An integrated approach combined with the determination of elemental contaminants. Sci. Total Environ. 2020, 749, 142301. [Google Scholar] [CrossRef] [PubMed]
- Lievens, S.; Slegers, T.; Mees, M.A.; Thielemans, W.; Poma, G.; Covaci, A.; van der Borght, M. A simple, rapid and accurate method for the sample preparation and quantification of meso- and microplastics in food and food waste streams. Environ. Pollut. 2022, 307, 119511. [Google Scholar] [CrossRef] [PubMed]
- La Nasa, J.; Biale, G.; Fabbri, D.; Modugno, F. A review on challenges and developments of analytical pyrolysis and other thermoanalytical techniques for the quali-quantitative determination of microplastics. J. Anal. Appl. Pyrolysis 2020, 149, 104841. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous Trace Identification and Quantification of Common Types of Microplastics in Environmental Samples by Pyrolysis-Gas Chromatography-Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Matsueda, M.; Mattonai, M.; Iwai, I.; Watanabe, A.; Teramae, N.; Robberson, W.; Ohtani, H.; Kim, Y.M.; Watanabe, C. Preparation and test of a reference mixture of eleven polymers with deactivated inorganic diluent for microplastics analysis by pyrolysis-GC-MS. J. Anal. Appl. Pyrolysis 2021, 154, 104993. [Google Scholar] [CrossRef]
- Fischer, M.; Gossmann, I.; Scholz-Böttcher, B.M. Fleur de Sel-An interregional monitor for microplastics mass load and composition in European coastal waters? J. Anal. Appl. Pyrolysis 2019, 144, 104711. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Brits, M.; van Poelgeest, B.; Nijenhuis, W.; van Velzen, M.J.M.; Been, F.M.; Gruter, G.J.M.; Brandsma, S.H.; Lamoree, M.H. Quantitation of polystyrene by pyrolysis-GC-MS: The impact of polymer standards on micro and nanoplastic analysis. Polym. Test. 2024, 137, 108511. [Google Scholar] [CrossRef]
- Okoffo, E.D.; Ribeiro, F.; O’Brien, J.W.; O’Brien, S.; Tscharke, B.J.; Gallen, M.; Samanipour, S.; Mueller, J.F.; Thomas, K.V. Identification and quantification of selected plastics in biosolids by pressurized liquid extraction combined with double-shot pyrolysis gas chromatography-mass spectrometry. Sci. Total Environ. 2020, 715, 136924. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Bogdanović, T.; Petričević, S.; Di Giacinto, F.; Listeš, I.; Sokolić, D.; Listeš, E.; Gjerde, J.; Pleadin, J. The bisphenol microplastics issue in marine bivalves. Vet. Stanica 2025, 56, 13–17. [Google Scholar] [CrossRef]
- Ferreira, O.; Barboza, L.G.A.; Rudnitskaya, A.; Moreirinha, C.; Vieira, L.R.; Botelho, M.J.; Vale, C.; Fernandes, J.O.; Cunha, S.; Guilhermino, L. Microplastics in marine mussels, biological effects and human risk of intake: A case study in a multi-stressor environment. Mar. Pollut. Bull. 2023, 197, 115704. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Otero, X.L.; Guilhermino, L. Microplastic contamination in marine mussels from the Atlantic coast of North Portugal and human risk of microplastic intake through mussel consumption. Environ. Pollut. 2024, 352, 124133. [Google Scholar] [CrossRef]
- ECHA CHEM Database; ECHA: Helsinki, Finland, 2024.
- Xu, P.; Peng, G.Y.; Su, L.; Gao, Y.Q.; Gao, L.; Li, D.J. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef]
- Chinfak, N.; Charoenpong, C.; Sampanporn, A.; Wongpa, C.; Sompongchaiyakul, P. Microplastics in commercial bivalves from coastal areas of Thailand and health risk associated with microplastics in ingested bivalves. Mar. Pollut. Bull. 2024, 208, 116937. [Google Scholar] [CrossRef]
- Malloggi, C.; Nalbone, L.; Bartalena, S.; Guidi, M.; Corradini, C.; Foti, A.; Gucciardi, P.G.; Giarratana, F.; Susini, F.; Armani, A. The Occurrence of Microplastics in Donax trunculus (Mollusca: Bivalvia) Collected along the Tuscany Coast (Mediterranean Sea). Animals 2024, 14, 618. [Google Scholar] [CrossRef]
- Chen, B. Characteristics and hazard risk of microplastics in Sinonovacula constricta: From farming to market. Front Mar Sci 2023, 10, 1151523. [Google Scholar] [CrossRef]
- Ding, J.F.; Sun, Y.M.; He, C.F.; Li, J.X.; Li, F.M. Towards Risk Assessments of Microplastics in Bivalve Mollusks Globally. J. Mar. Sci. Eng. 2022, 10, 288. [Google Scholar] [CrossRef]
- Fang, C.; Zheng, R.; Chen, H.; Hong, F.; Lin, L.; Lin, H.; Guo, H.; Bailey, C.; Segner, H.; Mu, J.; et al. Comparison of microplastic contamination in fish and bivalves from two major cities in Fujian province, China and the implications for human health. Aquaculture 2019, 51, 734322. [Google Scholar] [CrossRef]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]

| Bivalve Species and Sample Origin (Single Marine Shellfish Polymer Content in µg/g Wet Weight) | |||||||
|---|---|---|---|---|---|---|---|
| Crassostrea gigas Australian Local Fish Market | Mussel (Mytillus spp.) Environmental Samples | Acanthocardia spp. Northwestern Mediterranean | Fustiaria rubescens (Scaphopoda) Northwestern Mediterranean | M. edulis Baltic Sea (BS), North Sea (NS) | Commercially Available Mussels Chinese Seafood Market | Oysters, Stimpson’s Surf Clams, Asian Clams, Scallops | |
| Investigated polymers | PS, PMMA, PVC, PE, PET, PP | PC, PE, PMMA, PP, PS, PVC | PC, PE, PET, PMMA, PP, PS | PC, PE, PET, PMMA, PP, PS | PA6, PC, PE, PET, PMMA, PP, PS, PVC, PUR | PA6, PA66 | PE, PET, PMMA, PP, PS, PVC |
| PA66 | - ** | - | - | - | - | 0.2 | - |
| PA6 | - | - | - | - | - | 0.06 | - |
| PC | - | <5.8 | - | - | 0.002 *–0.495 * (BS) 0.001 *–0.43 * (NS) | - | - |
| PE | not detected | 18.0–32.9 | 92 * | 169 * | 0.45 *–5.83 * (BS) 0.03 *–2.69 * (NS) | - | 10–15 |
| PET | - | - | - | - | 0.13 *–5.09 * (BS) 0.05 *–10.39 * (NS) | - | not detected |
| PVC | <10.93–23.5 | <2.3–41.6 | - | - | 0.33 *–9.84 * (BS) 0.59 *–7.61 * (NS) | - | not detected |
| PMMA | not detected | not detected | - | - | 0.06 *–2.99 * (BS) 0.09 *–1.12 * (NS) | - | not detected |
| PS | not detected | not detected | - | - | 0.03 *–2.44 * (BS) 0.09 *–1.12 * (NS) | - | not detected |
| PP | <2.45 | <1.2 | 13 * | 0.04 *–1.54 * (BS) 0.02 *–0.87 * (NS) | - | not detected | |
| Reference | [20] | [21] | [22] | [22] | [23] | [24] | [25] |
| Bivalve Species | Characteristic Compound (Decomposition Product)/Quantitative Ions (m/z) | Calibration Curve | Internal Standard | Linear Range (µg) | Recovery Range (%) | LOQ (µg) | Reference |
|---|---|---|---|---|---|---|---|
| Oyster (Crassostrea gigas) | PE: 1-Decene (C10), 3-tetradecne (NIST) or 1-dodecene (C12), 1-tetradecane (C14), m/z = 83 PET PMMA: Methylmethacrylate, m/z = 100 PP: 2,4,6-Dimethyl-1-heptene, m/z = 126 PS: Styrene dimer: 3-butene-1,3-diyldibenzene, m/z = 130 PVC: Benzene, m/z= 78 | External, ASE extraction of solid standards | - | 0.02–10 | Expressed as RSD: 3–12 | 0.96–24.29 | [33] |
| Mussel (Mytilus spp.) | PC: 4-Isopropenylphenol, m/z = 134 PE: Alkene C17 peak, m/z = 83 PMMA: Methyl-methacrylate, m/z = 100 PP: 2,4-Dimethyl-1-heptene, m/z = 70 PS: 3-Butene-1,3-diyldibenzene (styrene trimer), m/z = 91 PVC: Indene, m/z = 115 | Internal, MAE dissolution of solid standards | Styrene-d5 (m/z = 109) | 0.5–10 (PE, PVC) 0.25–5 (PP, PS, PMMA, PC) | Mean recovery: 51.1–150.9% | 1.2 (PP, PS)–5.8 (PC) | [34] |
| Acanthocardia spp. Fustiaria rubescens | PC: Methyl-bisphenol A, 241- > 133 (quantification), 256-> 241 (confirmation) PE: 1,12-Tridecadiene, 95- > 67 (quantification), 109- > 67 (confirmation) PET: Dimethylterephthalate, 163- > 135 (quantification), 163- > 103 (confirmation) PMMA: Methylmethacrylate, 100- > 41 (quantification), 100- > 69 (confirmation) PP: Dimethyleheptane, 70- > 55 (quantification), 126- > 83 (confirmation) PS: Styrene trimer, 207- > 129 (quantification), 207- > 71 (confirmation) | External, solid cryo-milled polymers diluted in a calcined powdered glass microfiber filter | 0.025–1.360 | Recovery: 82 –129% | 0.13 AL | [35] | |
| M. edulis | PA6: ε-Caprolactam, m/z = 113 N-methyl caprolactam *, m/z = 127 PC: 2,2-Bis(4’-methoxyphenyl)propane *, m/z = 241 PE: α,ω-Alkanes (e.g., C20), m/z = 82 PET: Dimethylterephthalate*, m/z = 163 PMMA: Methylmethacrylate, m/z = 100 PP: 2,4-Dimethylhept-1-ene, m/z = 70 PS: 2,4,6-Triphenyl-1-hexene, m/z = 91 PVC: Naphthalene, m/z = 128 PUR: 4,4’-Methylenbis(N,N-dimethylaniline) *, m/z = 254 | Internal, solid standards | 4 internal standards: 9-tetradecyl-1,2,3,4,5,6,7,8-octahydro anthracene (TOHA), deuterated anthracene, cholanic acid, and deuterated polystyrene | 0.5 to 50 μg, 4 internal standards (9-tetradecyl-1,2,3,4,5,6,7,8-octahydro, 0.5 µg; cholanic acid, 0.5 µg; anthracene (d10), 1 µg; polystyrene (d8), 1 µg) | - | 0.3–1.4 | [20] |
| Commercially available mussels | PA66: Cyclopentanone, m/z = 84 PA6: Caprolactam, m/z = 113 | External, dissolved standards | - | 2–64 | Average recovery: 81.5–94.5 | 0.6 (PA6)–2.0 (PA66) | [36] |
| Oysters, Stimpson’s surf clams, Asian clams, scallops | PE: 1-Undecene, m/z = 55 PET: Biphenyl, m/z = 154 PMMA: Methylmethacrylate, m/z = 100 PP: 2,4-Dimethylhept-1-ene, m/z = 70 PVC: Naphthalene, m/z = 128 PS: Styrene, m/z = 104 | External, solid standards | - | 4.4–16. 27 | Recovery: 82–85 | - | [37] |
| Mussels (commercial lyophilized flour of Perna canaliculus) | ABS: 2-Phenethyl-4- phenylpent-4-enenitrile (SAS), m/z = 170 PA6: Caprolactam, m/z = 133 PA66: Cyclopentanone, m/z = 84 PC: Bisphenol A (BPA), m/z = 213 PE: α,ω-Alkanes C15–C25 (average of the areas), m/z = 82 PET: Benzoic acid (BA), m/z= 122 PMMA: Methylmethacrylate (MMA), m/z = 100 PP: 2,4-Dimethyl-1-heptene, m/z = 126 PS: 3-Butene-1,3-diyldibenzene (styrene dimer), m/z = 91 SBR: Butadiene trimer, m/z = 79 | Internal, solid standard diluted in SiO2 | Anthracene-d10 | 0.3–39 | Recovery: 87–138 | 0.003–0.04 | [43] |
| Shellfish Species/Location | Human Risk of Microplastic Intake, HRI (MPs/Day)/(MPs/Year) | Dominant Polymers | Polymer Hazard Index Caused by MPs (PHI) | Hazard Level/Risk Category | Reference |
|---|---|---|---|---|---|
| Wild mussels (Mytilus Galloprovincialis), Portugal | 2-6/680–2342 | PE, PA, PS, PP, PEVA | 1505–2850 | 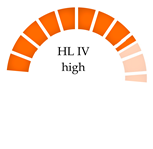 | [62] |
| Farmed bivalves: Green mussels (Perna viridis), oysters (Saccostrea cucullata), cockles (Tegillarca granosa), and clams (Meretrix meretrix), coastal areas of Thailand | 0.52 (MPs/day) | PET, cotton as cellulosic polymer, rayon, PP, PE, PP, acrylic, PA, PS | 149–964 | 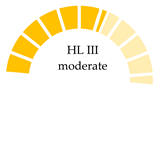 | [65] |
| Donax trunculus, Italy—Tuscany Coast (Mediterranean Sea) | 19.2 (MPs/year) | PE, PET | NR | 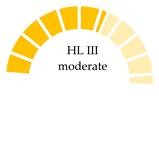 | [66] |
| Farmed and market samples of Sinonovacula constricta, Zhangzhou City, China | NA | PP, PE, PA, PET, PC | 623 (farmed)–819 (market samples) | 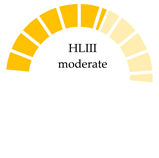 | [67] |
| Mussels (Mytilus galloprovincialis), Portugal | 7/2438–2650 | Cellulose, polyacrylonitrile, PP, PS, PA, PET, LDPE | NA | NA | [61] |
| Bivalves from 14 countries across the world | 0–8369 (MPs/year) | PVC, PET, PE, PS, PMMA, PA, PP | 220– 12,050 | 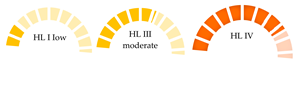 | [68] |
| Farmed and wild samples: Clams (Meretrix meretrix), mussels (Mytilus edulis), green mussels (Perna viridis), Fujian Province, China | NA | PET, PAN | 3813–3928 (farmed) 3925–4478 (wild) | 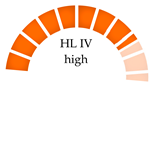 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanović, T.; Listeš, I.; Gjerde, J.; Petričević, S.; Jažo, Z.; Listeš, E.; Pleadin, J.; Sokolić, D.; Jadrešin, I.; di Giacinto, F. Microplastic Polymer Mass Fractions in Marine Bivalves: From Isolation to Hazard Risk. J. Xenobiot. 2025, 15, 186. https://doi.org/10.3390/jox15060186
Bogdanović T, Listeš I, Gjerde J, Petričević S, Jažo Z, Listeš E, Pleadin J, Sokolić D, Jadrešin I, di Giacinto F. Microplastic Polymer Mass Fractions in Marine Bivalves: From Isolation to Hazard Risk. Journal of Xenobiotics. 2025; 15(6):186. https://doi.org/10.3390/jox15060186
Chicago/Turabian StyleBogdanović, Tanja, Irena Listeš, Jennifer Gjerde, Sandra Petričević, Zvonimir Jažo, Eddy Listeš, Jelka Pleadin, Darja Sokolić, Ivona Jadrešin, and Federica di Giacinto. 2025. "Microplastic Polymer Mass Fractions in Marine Bivalves: From Isolation to Hazard Risk" Journal of Xenobiotics 15, no. 6: 186. https://doi.org/10.3390/jox15060186
APA StyleBogdanović, T., Listeš, I., Gjerde, J., Petričević, S., Jažo, Z., Listeš, E., Pleadin, J., Sokolić, D., Jadrešin, I., & di Giacinto, F. (2025). Microplastic Polymer Mass Fractions in Marine Bivalves: From Isolation to Hazard Risk. Journal of Xenobiotics, 15(6), 186. https://doi.org/10.3390/jox15060186









