Combined Climate and Chemical Stressors: How Spatial Variability Shapes the Response of Ficopomatus enigmaticus (Fauvel, 1923) to Dimethyl Sulfoxide (DMSO) and Heatwaves, and What It Means for Ecotoxicology
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup, Animal Collection, and Exposure
2.2. Biomarker Analysis
2.2.1. Total Protein Content (PROT)
2.2.2. Superoxide Dismutase (SOD)
2.2.3. Glutathione S-Transferase (GST)
2.2.4. Lipid Peroxidation (LPO)
2.3. IBRv2i Index
2.4. Data Analysis and Statistics
3. Results
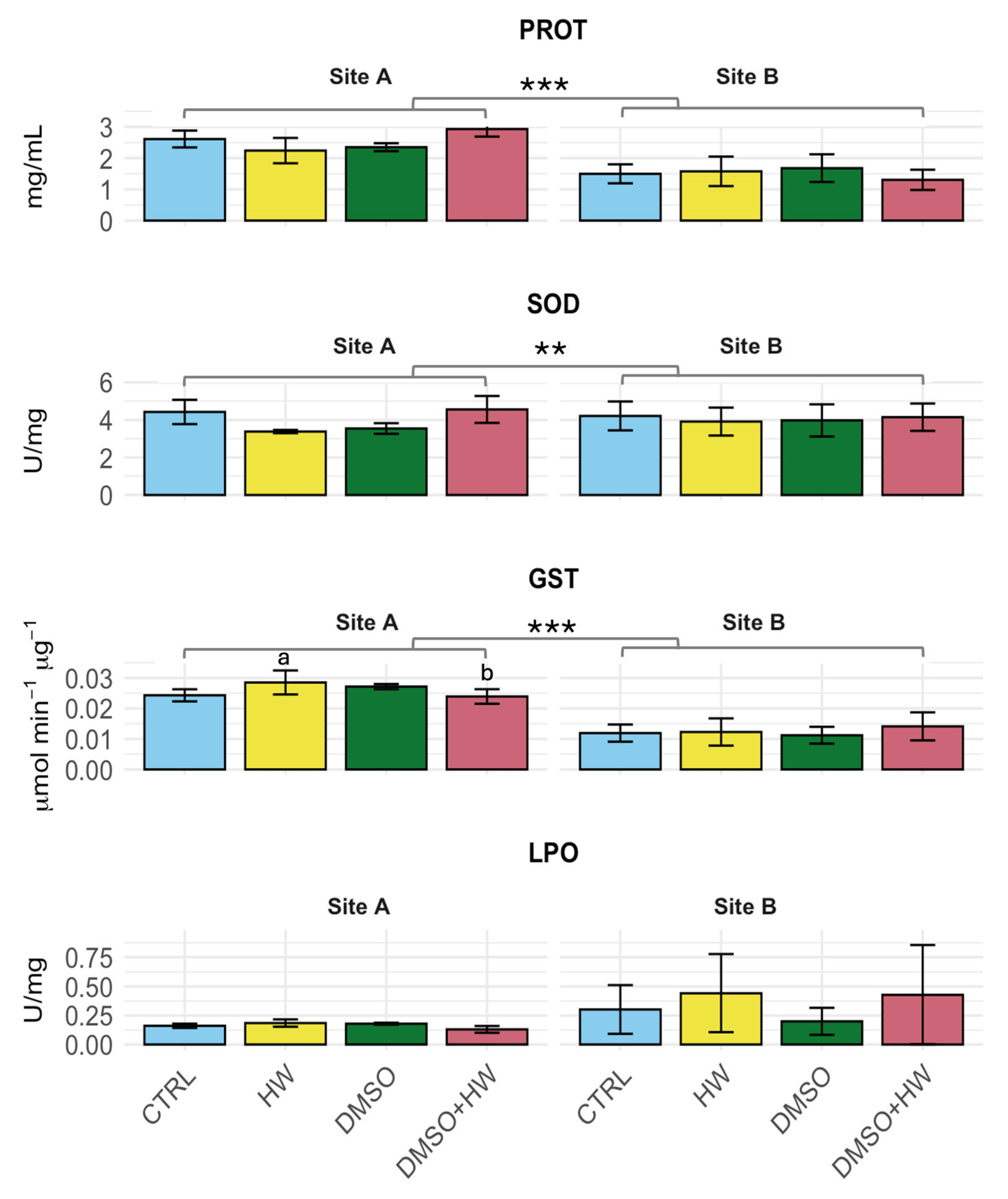
3.1. Biomarker Responses
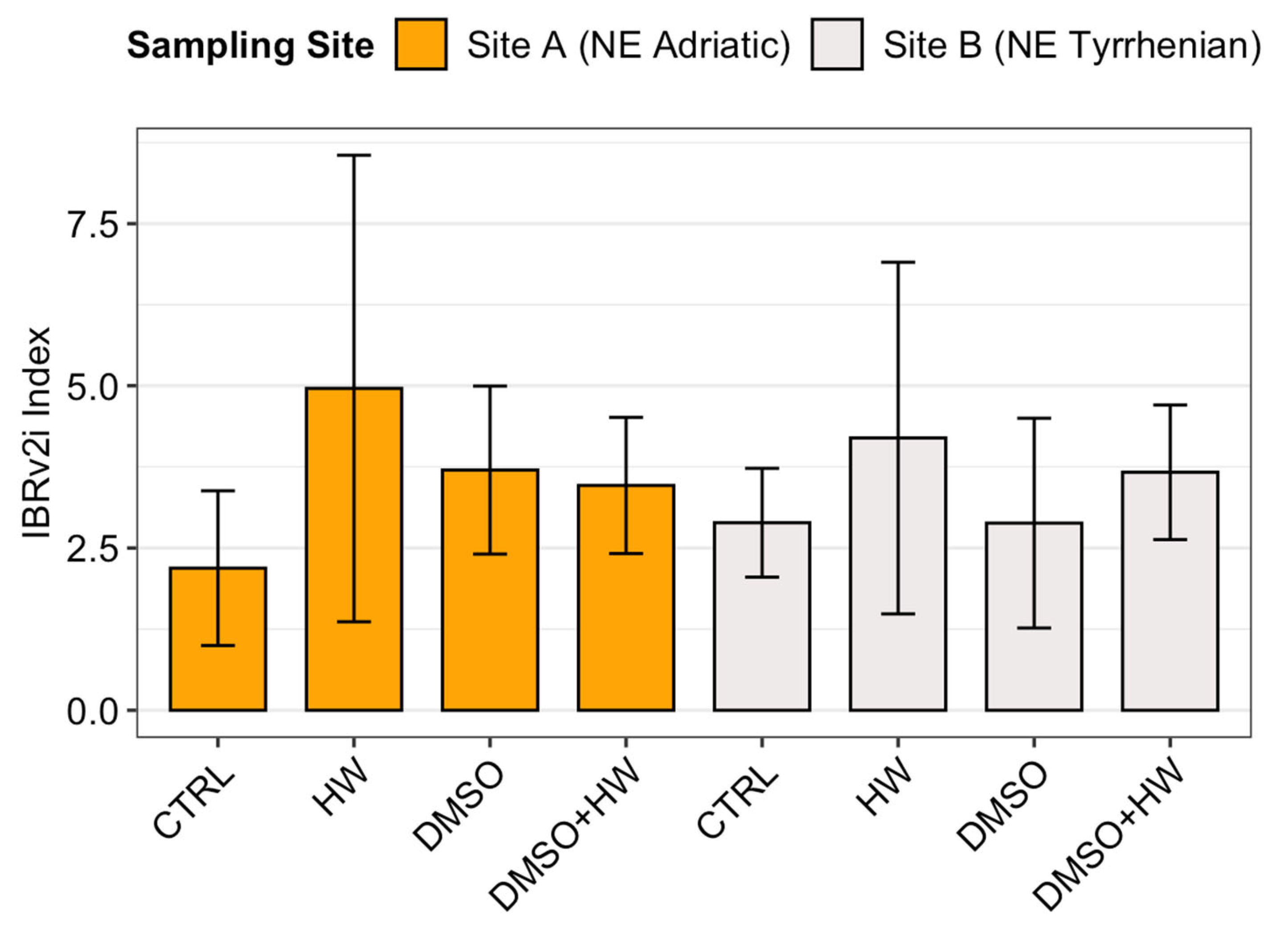
3.2. IBRv2i
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianchi, C.N.; Morri, C. Ficopomatus ‘Reefs’ in the Po River Delta (Northern Adriatic): Their Constructional Dynamics, Biology, and Influences on the Brackish-Water Biota. Mar. Ecol. 1996, 17, 51–66. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Marine Biodiversity of the Mediterranean Sea: Situation, Problems and Prospects for Future Research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Fauvel, P. Un Nouveau Serpulien d’eau Saumatre Mercierella Ng Enigmatica n. Sp. Bull. Soc. Zool. Fr. 1923, 46, 424–430. [Google Scholar]
- Cognetti, G. Forme Della Mercierella Enigmatica Fauvel Nella Nuova Stazione Del Lago Di Patria. Ital. J. Zool. 1954, 21, 41–44. [Google Scholar]
- Langeneck, J.; Lezzi, M.; Pasqua, M.D.; Musco, L.; Gambi, M.C.; Castelli, A.; Giangrande, A. Non-Indigenous Polychaetes along the Coasts of Italy: A Critical Review. Mediterr. Mar. Sci. 2020, 21, 238–275. [Google Scholar] [CrossRef]
- Eno, C.; Clark, R.; Sanderson, W. Non-Native Marine Species in British Waters: A Review and Directory. Jt. Nat. Conserv. Comm. 1997, 6, 215–228. [Google Scholar]
- Oliva, M.; De Marchi, L.; Vieira Sanches, M.; Pires, A.; Cuccaro, A.; Baratti, M.; Chiellini, F.; Morelli, A.; Freitas, R.; Pretti, C. Atlantic and Mediterranean Populations of the Widespread Serpulid Ficopomatus enigmaticus: Developmental Responses to Carbon Nanotubes. Mar. Pollut. Bull. 2020, 156, 111265. [Google Scholar] [CrossRef]
- Ten Hove, H.A.; Kupriyanova, E.K. Taxonomy of Serpulidae (Annelida, Polychaeta): The State of Affairs. Zootaxa 2009, 2036, 1–126. [Google Scholar] [CrossRef]
- Styan, C.; McCluskey, C.; Sun, Y.; Kupriyanova, E. Cryptic Sympatric Species across the Australian Range of the Global Estuarine Invader Ficopomatus enigmaticus (Fauvel, 1923) (Serpulidae, Annelida). Aquat. Invasions 2017, 12, 53–65. [Google Scholar] [CrossRef]
- Dittmann, S.; Rolston, A.; Benger, S.; Kupriyanova, E. Habitat Requirements, Distribution and Colonisation of the Tubeworm Ficopomatus Enigmaticus in the Lower Lakes and Coorong; Report for the South Australian Murray-Darling Basin Natural Resources Management Board: Adelaide, Australia, 2009; p. 104. [Google Scholar]
- Fornós, J.J.; Forteza, V.; Martínez-Taberner, A. Modern Polychaete Reefs in Western Mediterranean Lagoons: Ficopomatus enigmaticus (Fauvel) in the Albufera of Menorca, Balearic Islands. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 128, 175–186. [Google Scholar] [CrossRef]
- Meadows, P.S.; Meadows, A.; Murray, J.M.H. Biological Modifiers of Marine Benthic Seascapes: Their Role as Ecosystem Engineers. Geomorphology 2012, 157–158, 31–48. [Google Scholar] [CrossRef]
- Hille, S.; Kunz, F.; Markfort, G.; Ritzenhofen, L.; Zettler, M. First Record of Mass Occurrence of the Tubeworm Ficopomatus enigmaticus (Fauvel, 1923) (Serpulidae: Polychaeta) in Coastal Waters of the Baltic Sea. BioInvasions Rec. 2021, 10, 859–868. [Google Scholar] [CrossRef]
- Brundu, G.; Magni, P. Context-Dependent Effect of Serpulid Reefs on the Variability of Soft-Bottom Macrobenthic Assemblages in Three Mediterranean Lagoons (Sardinia, Italy). Estuar. Coast. Shelf Sci. 2021, 262, 107589. [Google Scholar] [CrossRef]
- Davies, B.R.; Stuart, V.; de Villiers, M. The Filtration Activity of a Serpulid Polychaete Population (Ficopomatus enigmaticus (Fauvel)) and Its Effects on Water Quality in a Coastal Marina. Estuar. Coast. Shelf Sci. 1989, 29, 613–620. [Google Scholar] [CrossRef]
- Piccardo, M.; Vellani, V.; Anselmi, S.; Bentivoglio, T.; Provenza, F.; Renzi, M.; Bevilacqua, S. The First Evidence of the Water Bioremediation Potential of Ficopomatus enigmaticus (Fauvel 1923): From Threat to Resource? Water 2024, 16, 368. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of Foundation Species: Consequences for the Structure and Dynamics of Forested Ecosystems. Front. Ecol. Environ. Ecol. Soc. Am. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Bruschetti, M.; Bazterrica, M.; Fanjul, E.; Luppi, T.; Iribarne, O. Effect of Biodeposition of an Invasive Polychaete on Organic Matter Content and Productivity of the Sediment in a Coastal Lagoon. J. Sea Res. 2011, 66, 20–28. [Google Scholar] [CrossRef]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of Invasive Alien Marine Species on Ecosystem Services and Biodiversity: A Pan-European Review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Oliva, M.; Mennillo, E.; Barbaglia, M.; Monni, G.; Tardelli, F.; Casu, V.; Pretti, C. The Serpulid Ficopomatus enigmaticus (Fauvel, 1923) as Candidate Organisms for Ecotoxicological Assays in Brackish and Marine Waters. Ecotoxicol. Environ. Saf. 2018, 148, 1096–1103. [Google Scholar] [CrossRef]
- Vellani, V.; Oliva, M.; Pretti, C.; Renzi, M. Stress-Related Molecular Biomarkers to Monitor the Effects of Global Changes on Calcifying Reef-Forming Organisms: A Review in the Mediterranean. J. Mar. Sci. Eng. 2025, 13, 4. [Google Scholar] [CrossRef]
- Cuccaro, A.; De Marchi, L.; Oliva, M.; Sanches, M.V.; Freitas, R.; Casu, V.; Monni, G.; Miragliotta, V.; Pretti, C. Sperm Quality Assessment in Ficopomatus enigmaticus (Fauvel, 1923): Effects of Selected Organic and Inorganic Chemicals across Salinity Levels. Ecotoxicol. Environ. Saf. 2021, 207, 111219. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Manzini, C.; Bontà Pittaluga, G.; Kozinkova, L.; De Marchi, L.; Freitas, R.; Fabi, G.; Pretti, C. Ficopomatus enigmaticus Larval Development Assay: An Application for Toxicity Assessment of Marine Sediments. Mar. Pollut. Bull. 2019, 139, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Vieira Sanches, M.; Oliva, M.; Pires, A.; De Marchi, L.; Cuccaro, A.; Freitas, R.; Baratti, M.; Pretti, C. Relationship between Wild-Caught Organisms for Bioassays and Sampling Areas: Widespread Serpulid Early-Development Comparison between Two Distinct Populations after Trace Element Exposure. Ecotoxicol. Environ. Saf. 2020, 205, 111094. [Google Scholar] [CrossRef]
- Bezuidenhout, M. The Implications of Climate Change for the Invasive Tube Worm Ficopomatus enigmaticus. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2021. [Google Scholar]
- Casu, V.; Tardelli, F.; De Marchi, L.; Monni, G.; Cuccaro, A.; Oliva, M.; Freitas, R.; Pretti, C. Soluble Esterases as Biomarkers of Neurotoxic Compounds in the Widespread Serpulid Ficopomatus enigmaticus (Fauvel, 1923). J. Environ. Sci. Health Part B 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Cuccaro, A.; Oliva, M.; De Marchi, L.; Vieira Sanches, M.; Bontà Pittaluga, G.; Meucci, V.; Battaglia, F.; Puppi, D.; Freitas, R.; Pretti, C. Biochemical Response of Ficopomatus enigmaticus Adults after Exposure to Organic and Inorganic UV Filters. Mar. Pollut. Bull. 2022, 178, 113601. [Google Scholar] [CrossRef]
- De Marchi, L.; Oliva, M.; Freitas, R.; Neto, V.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.M.V.M.; Pretti, C. Toxicity Evaluation of Carboxylated Carbon Nanotubes to the Reef-Forming Tubeworm Ficopomatus enigmaticus (Fauvel, 1923). Mar. Environ. Res. 2019, 143, 1–9. [Google Scholar] [CrossRef]
- Vellani, V.; Cuccaro, A.; Oliva, M.; Pretti, C.; Renzi, M. Assessing Combined Effects of Long-Term Exposure to Copper and Marine Heatwaves on the Reef-Forming Serpulid Ficopomatus enigmaticus through a Biomarker Approach. Mar. Pollut. Bull. 2024, 201, 116269. [Google Scholar] [CrossRef]
- Zebral, Y.D.; da Silva Fonseca, J.; Marques, J.A.; Bianchini, A. Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals. Int. J. Mol. Sci. 2019, 20, 3092. [Google Scholar] [CrossRef]
- Dinh, K.V.; Konestabo, H.S.; Borgå, K.; Hylland, K.; Macaulay, S.J.; Jackson, M.C.; Verheyen, J.; Stoks, R. Interactive Effects of Warming and Pollutants on Marine and Freshwater Invertebrates. Curr. Pollut. Rep. 2022, 8, 341–359. [Google Scholar] [CrossRef]
- Sokolova, I.; Lannig, G. Interactive Effects of Metal Pollution and Temperature on Metabolism in Aquatic Ectotherms: Implications of Global Climate Change. Clim. Res. 2008, 37, 181–201. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef]
- Halpern, B.S.; Frazier, M.; Potapenko, J.; Casey, K.S.; Koenig, K.; Longo, C.; Lowndes, J.S.; Rockwood, R.C.; Selig, E.R.; Selkoe, K.A.; et al. Spatial and Temporal Changes in Cumulative Human Impacts on the World’s Ocean. Nat. Commun. 2015, 6, 7615. [Google Scholar] [CrossRef] [PubMed]
- Brayton, C.F. Dimethyl Sulfoxide (DMSO): A Review. Cornell Vet. 1986, 76, 61–90. [Google Scholar] [PubMed]
- Sum, A.K.; Pablo, J.J. de Molecular Simulation Study on the Influence of Dimethylsulfoxide on the Structure of Phospholipid Bilayers. Biophys. J. 2003, 85, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Szmant, H.H. Physical Properties of Dimethyl Sulfoxide and Its Function in Biological Systems. Ann. N. Y. Acad. Sci. 1975, 243, 20–23. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H. Biological Assay Challenges from Compound Solubility: Strategies for Bioassay Optimization. Drug Discov. Today 2006, 11, 446–451. [Google Scholar] [CrossRef]
- Modrzyński, J.J.; Christensen, J.H.; Brandt, K.K. Evaluation of Dimethyl Sulfoxide (DMSO) as a Co-Solvent for Toxicity Testing of Hydrophobic Organic Compounds. Ecotoxicology 2019, 28, 1136–1141. [Google Scholar] [CrossRef]
- Stibany, F.; Ewald, F.; Miller, I.; Hollert, H.; Schäffer, A. Improving the Reliability of Aquatic Toxicity Testing of Hydrophobic Chemicals via Equilibrium Passive Dosing–A Multiple Trophic Level Case Study on Bromochlorophene. Sci. Total Environ. 2017, 584–585, 96–104. [Google Scholar] [CrossRef]
- OECD. Guidance Document on Aquatic Toxicity Testing of Difficult Substances and Mixtures; Series on Testing and Assessment No. 23; OECD–Organisation for Economic Co-Operation and Development: Paris, France, 2019; Available online: https://www.oecd.org/en/publications/guidance-document-on-aquatic-toxicity-testing-of-difficult-substances-and-mixtures_0ed2f88e-en.html (accessed on 10 March 2023).
- Huang, Y.; Cartlidge, R.; Walpitagama, M.; Kaslin, J.; Campana, O.; Wlodkowic, D. Unsuitable Use of DMSO for Assessing Behavioral Endpoints in Aquatic Model Species. Sci. Total Environ. 2018, 615, 107–114. [Google Scholar] [CrossRef]
- Hedge, J.M.; Hunter, D.L.; Sanders, E.; Jarema, K.A.; Olin, J.K.; Britton, K.N.; Lowery, M.; Knapp, B.R.; Padilla, S.; Hill, B.N. Influence of Methylene Blue or Dimethyl Sulfoxide on Larval Zebrafish Development and Behavior. Zebrafish 2023, 20, 132–145. [Google Scholar] [CrossRef]
- Bigi, S.; Schlappa, K.; Anselmi, S.; Provenza, F.; Renzi, M. Uptake Through Feeding and/or Culture Medium of 0.5% Dimethyl Sulfoxide (DMSO): Biological Response of Daphnia magna and Ceriodaphnia dubia in Ecotoxicity Tests. Water 2025, 17, 191. [Google Scholar] [CrossRef]
- Stevens, A.-S.; Pirotte, N.; Plusquin, M.; Willems, M.; Neyens, T.; Artois, T.; Smeets, K. Toxicity Profiles and Solvent–Toxicant Interference in the Planarian Schmidtea mediterranea after Dimethylsulfoxide (DMSO) Exposure. J. Appl. Toxicol. 2015, 35, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Thorp, J.H.; Gloss, S.P. Field and Laboratory Tests on Acute Toxicity of Cadmium to Freshwater Crayfish. Bull. Environ. Contam. Toxicol. 1986, 37, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Romero-Blanco, A.; Alonso, Á. Laboratory versus Wild Populations: The Importance of Population Origin in Aquatic Ecotoxicology. Environ. Sci. Pollut. Res. 2022, 29, 22798–22808. [Google Scholar] [CrossRef]
- Brouwer, A.; Murk, A.J.; Koeman, J.H. Biochemical and Physiological Approaches in Ecotoxicology. Funct. Ecol. 1990, 4, 275–281. [Google Scholar] [CrossRef]
- Renzi, M. La Laguna di Orbetello. Storia, Ambiente, Gestione e Progetti Futuri; C&P Adver Effigi: Arcidosso, Italy, 2022; ISBN 978-88-5524-448-0. [Google Scholar]
- Bombelli, V.; Lenzi, M. Italy—The Orbetello Lagoon and the Tuscan Coast. In Marine Benthic Vegetation: Recent Changes and the Effects of Eutrophication; Schramm, W., Nienhuis, P.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 331–337. ISBN 978-3-642-61398-2. [Google Scholar]
- Bianchi, C.N.; Morri, C. The Battle Is Not to the Strong: Serpulid Reefs in the Lagoon of Orbetello (Tuscany, Italy). Estuar. Coast. Shelf Sci. 2001, 53, 215–220. [Google Scholar] [CrossRef]
- UNI CEI EN ISO/IEC 17025:2018; General Requirements for the Competence of Testing and Calibration Laboratories 2018. ISO: Geneva, Switzerland, 2018. Available online: https://store.uni.com/uni-cei-en-iso-iec-17025-2018 (accessed on 20 February 2018).
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Chiellini, F.; Soares, A.M.V.M.; Freitas, R. Physiological and Biochemical Responses of Two Keystone Polychaete Species: Diopatra neapolitana and Hediste diversicolor to Multi-Walled Carbon Nanotubes. Environ. Res. 2017, 154, 126–138. [Google Scholar] [CrossRef]
- Provenza, F.; Rampih, D.; Pignattelli, S.; Pastorino, P.; Barceló, D.; Prearo, M.; Specchiulli, A.; Renzi, M. Mussel Watch Program for Microplastics in the Mediterranean Sea: Identification of Biomarkers of Exposure Using Mytilus galloprovincialis. Ecol. Indic. 2022, 142, 109212. [Google Scholar] [CrossRef]
- Vidal-Liñán, L.; Bellas, J. Practical Procedures for Selected Biomarkers in Mussels, Mytilus galloprovincialis—Implications for Marine Pollution Monitoring. Sci. Total Environ. 2013, 461–462, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Gao, R.; Yuan, Z.; Zhao, Z.; Gao, X. Mechanism of Pyrogallol Autoxidation and Determination of Superoxide Dismutase Enzyme Activity. Bioelectrochem. Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of Malonaldehyde Precursor in Tissues by Thiobarbituric Acid Test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Mattos, J.J.; Siebert, M.N.; Bainy, A.C.D. Integrated Biomarker Responses: A Further Improvement of IBR and IBRv2 Indexes to Preserve Data Variability in Statistical Analyses. Environ. Sci. Pollut. Res. 2024, 31, 871–881. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Posit team RStudio: Integrated Development Environment for R 2025.05.0+496. Available online: https://posit.co/download/rstudio-desktop/ (accessed on 19 June 2025).
- Cohen, J. Eta-Squared and Partial Eta-Squared in Fixed Factor Anova Designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Pierce, C.A.; Block, R.A.; Aguinis, H. Cautionary Note on Reporting Eta-Squared Values from Multifactor ANOVA Designs. Educ. Psychol. Meas. 2004, 64, 916–924. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Reprint; Psychology Press: New York, NY, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for t-Tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Louis, Y.D.; Bhagooli, R.; Kenkel, C.D.; Baker, A.C.; Dyall, S.D. Gene Expression Biomarkers of Heat Stress in Scleractinian Corals: Promises and Limitations. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 191, 63–77. [Google Scholar] [CrossRef]
- Monserrat, J.M.; Martínez, P.E.; Geracitano, L.A.; Lund Amado, L.; Martinez Gaspar Martins, C.; Lopes Leães Pinho, G.; Soares Chaves, I.; Ferreira-Cravo, M.; Ventura-Lima, J.; Bianchini, A. Pollution Biomarkers in Estuarine Animals: Critical Review and New Perspectives. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2007, 146, 221–234. [Google Scholar] [CrossRef]
- Knochel, A. The Effects of Thermal Stress on Fluorescent Protein Expression in an Indo-Pacific Scleractinian Coral Species, Acropora tenuis. Indep. Study Proj. ISP Collect. 2017, 2637, 36. [Google Scholar]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy Homeostasis as an Integrative Tool for Assessing Limits of Environmental Stress Tolerance in Aquatic Invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M. Energy-Limited Tolerance to Stress as a Conceptual Framework to Integrate the Effects of Multiple Stressors. Integr. Comp. Biol. 2013, 53, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Singh, K.D.; Singh, A.K. A Study on Superoxide Dismutase Activity in a Freshwater Fish Labeo rohita: A Way of Assessing Aquatic Health. Eur. J. Biomed. Pharm. Sci. 2022, 9, 417–423. [Google Scholar] [CrossRef]
- Manduzio, H.; Monsinjon, T.; Galap, C.; Leboulenger, F.; Rocher, B. Seasonal Variations in Antioxidant Defences in Blue Mussels Mytilus edulis Collected from a Polluted Area: Major Contributions in Gills of an Inducible Isoform of Cu/Zn-Superoxide Dismutase and of Glutathione S-Transferase. Aquat. Toxicol. 2004, 70, 83–93. [Google Scholar] [CrossRef]
- Curd, A.; Boyé, A.; Cordier, C.; Pernet, F.; Firth, L.B.; Bush, L.E.; Davies, A.J.; Lima, F.P.; Meneghesso, C.; Quéré, C.; et al. Environmental Optima for an Ecosystem Engineer: A Multidisciplinary Trait-Based Approach. Sci. Rep. 2021, 11, 22986. [Google Scholar] [CrossRef]
- De Marchi, L.; Pretti, C.; Chiellini, F.; Morelli, A.; Neto, V.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. The Influence of Simulated Global Ocean Acidification on the Toxic Effects of Carbon Nanoparticles on Polychaetes. Sci. Total Environ. 2019, 666, 1178–1187. [Google Scholar] [CrossRef]
- Freitas, R.; de Marchi, L.; Moreira, A.; Pestana, J.L.T.; Wrona, F.J.; Figueira, E.; Soares, A.M.V.M. PPhysiological and Biochemical Impacts Induced by Mercury Pollution and Seawater Acidification in Hediste diversicolor. Sci. Total Environ. 2017, 595, 691–701. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, Q. Oxidative Stress Biomarkers of the Polychaete Nereis diversicolor Exposed to Cadmium and Petroleum Hydrocarbons. Ecotoxicol. Environ. Saf. 2008, 70, 106–114. [Google Scholar] [CrossRef]
- Dias, M.; Ferreira, A.; Gouveia, R.; Madeira, C.; Jogee, N.; Cabral, H.; Diniz, M.; Vinagre, C. Long-Term Exposure to Increasing Temperatures on Scleractinian Coral Fragments Reveals Oxidative Stress. Mar. Environ. Res. 2019, 150, 104758. [Google Scholar] [CrossRef]
- Valente, P.; Cardoso, P.; Giménez, V.; Silva, M.S.S.; Sá, C.; Figueira, E.; Pires, A. Biochemical and Behavioural Alterations Induced by Arsenic and Temperature in Hediste diversicolor of Different Growth Stages. Int. J. Environ. Res. Public. Health 2022, 19, 15426. [Google Scholar] [CrossRef] [PubMed]
- Robillard, S.; Beauchamp, G.; Laulier, M. The Role of Abiotic Factors and Pesticide Levels on Enzymatic Activity in the Freshwater Mussel Anodonta cygnea at Three Different Exposure Sites. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2003, 135, 49–59. [Google Scholar] [CrossRef]
- Domingues, I.; Agra, A.R.; Monaghan, K.; Soares, A.M.V.M.; Nogueira, A.J.A. Cholinesterase and Glutathione-S-Transferase Activities in Freshwater Invertebrates as Biomarkers to Assess Pesticide Contamination. Environ. Toxicol. Chem. 2010, 29, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Hagiwara, A.; Park, H.G.; Lee, J.-S. The Glutathione S-Transferase Genes in Marine Rotifers and Copepods: Identification of GSTs and Applications for Ecotoxicological Studies. Mar. Pollut. Bull. 2020, 156, 111080. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Kim, D.-H.; Lee, M.-C.; Han, J.; Kim, H.-J.; Hagiwara, A.; Hwang, U.-K.; Park, H.G.; Lee, J.-S. Genome-Wide Identification of the Entire 90 Glutathione S-Transferase (GST) Subfamily Genes in Four Rotifer Brachionus Species and Transcriptional Modulation in Response to Endocrine Disrupting Chemicals. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 28, 183–195. [Google Scholar] [CrossRef]
- King, O.C.; van de Merwe, J.P.; Campbell, M.D.; Smith, R.A.; Warne, M.S.J.; Brown, C.J. Interactions Among Multiple Stressors Vary with Exposure Duration and Biological Response. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220348. [Google Scholar] [CrossRef]
- Dias, M.; Madeira, C.; Jogee, N.; Ferreira, A.; Gouveia, R.; Cabral, H.; Diniz, M.; Vinagre, C. Oxidative Stress on Scleractinian Coral Fragments Following Exposure to High Temperature and Low Salinity. Ecol. Indic. 2019, 107, 105586. [Google Scholar] [CrossRef]
- Li, X. Solvent Effects and Improvements in the Deoxyribose Degradation Assay for Hydroxyl Radical-Scavenging. Food Chem. 2013, 141, 2083–2088. [Google Scholar] [CrossRef]
- Sanmartín-Suárez, C.; Soto-Otero, R.; Sánchez-Sellero, I.; Méndez-Álvarez, E. Antioxidant Properties of Dimethyl Sulfoxide and its Viability as a Solvent in the Evaluation of Neuroprotective Antioxidants. J. Pharmacol. Toxicol. Methods 2011, 63, 209–215. [Google Scholar] [CrossRef]
- Ait Alla, A.; Mouneyrac, C.; Durou, C.; Moukrim, A.; Pellerin, J. Tolerance and Biomarkers as Useful Tools for Assessing Environmental Quality in the Oued Souss Estuary (Bay of Agadir, Morocco). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2006, 143, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Durou, C.; Poirier, L.; Amiard, J.-C.; Budzinski, H.; Gnassia-Barelli, M.; Lemenach, K.; Peluhet, L.; Mouneyrac, C.; Roméo, M.; Amiard-Triquet, C. Biomonitoring in a Clean and a Multi-Contaminated Estuary Based on Biomarkers and Chemical Analyses in the Endobenthic Worm Nereis diversicolor. Environ. Pollut. 2007, 148, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Fossi Tankoua, O.; Buffet, P.E.; Amiard, J.C.; Amiard-Triquet, C.; Méléder, V.; Gillet, P.; Mouneyrac, C.; Berthet, B. Intersite Variations of a Battery of Biomarkers at Different Levels of Biological Organisation in the Estuarine Endobenthic Worm Nereis diversicolor (Polychaeta, Nereididae). Aquat. Toxicol. 2012, 114–115, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Jaramillo, M.; Martins da Rocha, A.; Gomes, V.; Bianchini, A.; Monserrat, J.M.; Sáez, K.; Barra, R. Multibiomarker Approach at Different Organization Levels in the Estuarine Perinereis gualpensis (Polychaeta; Nereididae) Under Chronic and Acute pollution Conditions. Sci. Total Environ. 2011, 410–411, 126–135. [Google Scholar] [CrossRef]
- Bastos, F.F.; Hauser-Davis, R.A.; Tobar, S.A.L.; Campos, R.C.; Ziolli, R.L.; Bastos, V.L.F.C.; Bastos, J.C. Enzymatic GST Levels and Overall Health of Mullets from Contaminated Brazilian Lagoons. Aquat. Toxicol. 2013, 126, 414–423. [Google Scholar] [CrossRef]
- European Commission. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive) 2008. Available online: https://www.bsbd.bg/UserFiles/Dir2008-56-EO-EN.pdf (accessed on 5 June 2023).
- Pokhrel, P.; Suzuki, J.; Fujita, M. Integrated Biomarker Responses of a Brackish Water Clam to Global Warming Conditions. J. Water Environ. Technol. 2022, 20, 238–247. [Google Scholar] [CrossRef]
- Mesquita, A.F.; Gonçalves, F.J.M.; Gonçalves, A.M.M. Effects of Inorganic and Organic Pollutants on the Biomarkers’ Response of Cerastoderma Edule under Temperature Scenarios. Antioxidants 2023, 12, 1756. [Google Scholar] [CrossRef]
- Alves, A.V.; Gusso-Choueri, P.K.; Altafim, G.L.; Ferraz, M.A.; Trevizani, T.H.; Felix, C.S.A.; Figueira, R.C.L.; Abessa, D.M.d.S.; Choueri, R.B. Influence of CO2-Induced Acidification and Temperature Increased on the Toxicity of Metals in Sediment in the Mussel Mytella charruana. Front. Ocean Sustain. 2025, 3, 1575728. [Google Scholar] [CrossRef]
- Baag, S.; Mahapatra, S.; Mandal, S. An Integrated and Multibiomarker Approach to Delineate Oxidative Stress Status of Bellamya bengalensis under the Interactions of Elevated Temperature and Chlorpyrifos Contamination. Chemosphere 2021, 264, 128512. [Google Scholar] [CrossRef]
- Dias, M.; Pousão-Ferreira, P.; Diniz, M.S.; Marques, A.; Rosa, R.; Anacleto, P.; Maulvault, A.L. Integrated Multi-Biomarker Responses of Juvenile Zebra Seabream (Diplodus cervinus) to Warming and Acidification Conditions. Oceans 2024, 5, 571–590. [Google Scholar] [CrossRef]

| Source | d.f. | SS | MS | η2 | F | p | |
|---|---|---|---|---|---|---|---|
| PROT | Treatment | 3 | 0.000022 | 0.000008 | 0.010 | 0.595 | 0.622 |
| Site | 1 | 0.001661 | 0.0016613 | 0.740 | 132.035 | 0.000 | |
| Treatment × Site | 3 | 0.000064 | 0.0000212 | 0.030 | 1.687 | 0.185 | |
| Residuals | 40 | 0.000503 | 0.0000126 | ||||
| SOD | Treatment | 3 | 0.1958 | 0.0653 | 0.060 | 1.2 | 0.319 |
| Site | 1 | 0.4618 | 0.4618 | 0.140 | 8.488 | 0.005 | |
| Treatment × Site | 3 | 0.1686 | 0.0562 | 0.050 | 1.033 | 0.386 | |
| Residuals | 52 | 2.8293 | 0.0544 | ||||
| GST | Treatment | 3 | 127,276 | 42,425 | 0.005 | 0.340 | 0.796 |
| Site | 1 | 14,930,468 | 14,930,468 | 0.580 | 119.716 | 0.000 | |
| Treatment × Site | 3 | 2,247,608 | 749,203 | 0.090 | 6.007 | 0.001 | |
| Residuals | 52 | 6,485,213 | 124,716 | ||||
| Tukey post hoc | |||||||
| Site A: | CTRL = DMSO = DMSO + HW; CTRL = DMSO = HW; HW > DMSO + HW | ||||||
| Site B: | CTRL = DMSO = HW = DMSO + HW | ||||||
| LPO | Treatment | 3 | 2.305 | 0.7682 | 0.090 | 1.441 | 0.245 |
| Site | 1 | 0.065 | 0.0653 | 0.003 | 0.123 | 0.728 | |
| Treatment × Site | 3 | 1.479 | 0.4929 | 0.060 | 0.924 | 0.438 | |
| Residuals | 40 | 21.33 | 0.5332 | ||||
| IBRv2i | Treatment | 3 | 12.79 | 4.263 | 0.180 | 1.182 | 0.348 |
| Site | 1 | 0.17 | 0.173 | 0.002 | 0.048 | 0.829 | |
| Treatment × Site | 3 | 2.5 | 0.834 | 0.030 | 0.231 | 0.873 | |
| Residuals | 16 | 57.72 | 3.608 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellani, V.; Piccardo, M.; Provenza, F.; Anselmi, S.; Pitacco, V.; Lipej, L.; Bevilacqua, S.; Renzi, M. Combined Climate and Chemical Stressors: How Spatial Variability Shapes the Response of Ficopomatus enigmaticus (Fauvel, 1923) to Dimethyl Sulfoxide (DMSO) and Heatwaves, and What It Means for Ecotoxicology. J. Xenobiot. 2025, 15, 181. https://doi.org/10.3390/jox15060181
Vellani V, Piccardo M, Provenza F, Anselmi S, Pitacco V, Lipej L, Bevilacqua S, Renzi M. Combined Climate and Chemical Stressors: How Spatial Variability Shapes the Response of Ficopomatus enigmaticus (Fauvel, 1923) to Dimethyl Sulfoxide (DMSO) and Heatwaves, and What It Means for Ecotoxicology. Journal of Xenobiotics. 2025; 15(6):181. https://doi.org/10.3390/jox15060181
Chicago/Turabian StyleVellani, Verdiana, Manuela Piccardo, Francesca Provenza, Serena Anselmi, Valentina Pitacco, Lovrenc Lipej, Stanislao Bevilacqua, and Monia Renzi. 2025. "Combined Climate and Chemical Stressors: How Spatial Variability Shapes the Response of Ficopomatus enigmaticus (Fauvel, 1923) to Dimethyl Sulfoxide (DMSO) and Heatwaves, and What It Means for Ecotoxicology" Journal of Xenobiotics 15, no. 6: 181. https://doi.org/10.3390/jox15060181
APA StyleVellani, V., Piccardo, M., Provenza, F., Anselmi, S., Pitacco, V., Lipej, L., Bevilacqua, S., & Renzi, M. (2025). Combined Climate and Chemical Stressors: How Spatial Variability Shapes the Response of Ficopomatus enigmaticus (Fauvel, 1923) to Dimethyl Sulfoxide (DMSO) and Heatwaves, and What It Means for Ecotoxicology. Journal of Xenobiotics, 15(6), 181. https://doi.org/10.3390/jox15060181










