Polycyclic Aromatic Hydrocarbons in Honey: A Systematic Review of Occurrence, Concentrations, and Health Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Risk Assessment
2.5. Limitations of the Risk Assessment
3. Results
3.1. Processing the Systematic Review
3.2. Characteristics of Reviewed Studies
3.3. PAH Species Detected
3.4. PAH Residues in Honey
3.5. Health Risk Assessment
4. Discussion
4.1. Comparison with Previous Findings
4.2. Consistency in Target Compounds
4.3. Risk Assessment and Safety Implications
4.4. Comparison of PAHs in Honey with Other Food and Environmental Sources
4.5. Strengths and Limitations
4.6. Implications and Future Directions
- Sampling: Adopt a standardized and transparent protocol for site selection (urban/rural/industrial), sample size, and storage, ensuring representativeness and minimizing contamination.
- Analytical Methods: Adopt a unified QuEChERS–GC–MS/MS or GC–MS protocol, adhere to agreed-upon quality assurance/quality control standards (limits of detection/quantification, recovery), and mandate reporting of ΣPAHs, BaP, and PAH4.
- Risk Assessment: Adopt internationally recognized TEQ/MEQ procedures with explicit quantification of uncertainties (e.g., confidence intervals, scenario analysis), and, where feasible, derive a PTWI.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ace | Acenaphthene |
| Acy | Acenaphthylene |
| Ant | Anthracene |
| BaA | Benzo[a]anthracene |
| BaP | Benzo[a]pyrene |
| BaPEQ | Benzo[a]pyrene Equivalent |
| BbF | Benzo[b]fluoranthene |
| BkF | Benzo[k]fluoranthene |
| Chr | Chrysene |
| CR | Cancer Risk |
| DahA | Dibenzo[a,h]anthracene |
| DDI | Daily Dietary Intake |
| EDI | Estimated Daily Intake |
| EFSA | European Food Safety Authority |
| EPA 16 | The USEPA list of 16 priority PAHs |
| EU | European Union |
| ECR | Excess Cancer Risk |
| FL | Fluoranthene |
| FLu | Fluorene |
| GC–FID | Gas Chromatography–Flame Ionization Detection |
| GC–MS | Gas Chromatography–Mass Spectrometry |
| GC–MS/MS | Gas Chromatography–Tandem Mass Spectrometry |
| HI | Hazard Index |
| HQ | Hazard Index |
| HPLC | High Performance Liquid Chromatography |
| IcdP | Indeno[1,2,3–cd]pyrene |
| ILCR | Incremental Lifetime Cancer Risk |
| LC–MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MEF | Mutagenic Equivalency Factor |
| MEQBaP | Mutagenic Equivalent concentration relative to BaP |
| ND | Not Detected |
| NPAHs | Nitrated Polycyclic Aromatic Hydrocarbons |
| NR | Not Reported |
| OPAHs | Oxygenated Polycyclic Aromatic Hydrocarbons |
| PAH4 | Sum of BaP, BbF, BaA, and Chr (EU indicator) |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| Phe | Phenanthrene |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta–Analyses |
| Pyr | Pyrene |
| QuEChERS | Easy, Cheap, Effective, Rugged, and Safe (sample preparation) |
| ΣPAHs | Sum of Polycyclic Aromatic Hydrocarbons |
| SPME | Solid-Phase Microextraction |
| TEF | Toxic Equivalency Factor |
| TEQBaP | Toxic Equivalent concentration relative to BaP |
| USEPA | United States Environmental Protection Agency |
| WHO | World Health Organization |
| dw | Dry Weight |
References
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. A Multiresidue Method for the Analysis of 90 Pesticides, 16 PAHs, and 22 PCBs in Honey Using QuEChERS–SPME. Anal. Bioanal. Chem. 2017, 409, 5157–5169. [Google Scholar] [CrossRef]
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. Determination of 16 PAHs and 22 PCBs in Honey Samples Originated from Different Region of Lebanon and Used as Environmental Bimonitors Sentinel. J. Environ. Sci. Health A 2018, 54, 9–15. [Google Scholar] [CrossRef]
- Ciemniak, A.; Witczak, A.; Mocek, K. Assessment of Honey Contamination with Polycyclic Aromatic Hydrocarbons. J. Environ. Sci. Health B 2013, 48, 993–998. [Google Scholar] [CrossRef]
- Jovetić, M.S.; Raičević, A.; Nedić, N.M.; Vojt, D.; Đurđić, S.Z.; Brčeski, I.D.; Milojković-Opsenica, D. Urban Honey—The Aspects of Its Safety. Arh. Hig. Rada Toksikol. 2018, 69, 264–274. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Polycyclic Aromatic Hydrocarbons (PAHs) Fact Sheet; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2008. Available online: https://archive.epa.gov/epawaste/hazard/wastemin/web/pdf/pahs.pdf (accessed on 5 June 2025).
- World Health Organization (WHO). Characterization of Risk: Integrated Risk Assessment; IPCS Harmonization Project Document No. 8; World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.inchem.org/documents/harmproj/harmproj/harmproj8.pdf (accessed on 6 June 2025).
- Derrar, S.; Lo Turco, V.; Albergamo, A.; Sgrò, B.; Ayad, M.A.; Litrenta, F.; Saim, M.S.; Potortì, A.G.; Aggad, H.; Rando, R.; et al. Study of Physicochemical Quality and Organic Contamination in Algerian Honey. Foods 2024, 13, 1413. [Google Scholar] [CrossRef]
- Mandelli, A.; Guiñez, M.; Cerutti, S. Evaluation of Environmentally Relevant Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbons in Honey. Foods 2023, 12, 2205. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.A.; Tesi, G.O.; Obi, G.; Obi-Iyeke, G.E.; Igbuku, U.A.; Martincigh, B.S. Concentrations, Health Risks and Sources of Polycyclic Aromatic Hydrocarbons in Nigerian Honey. Toxicol. Environ. Health Sci. 2016, 8, 28–42. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons; Office of Research and Development: Washington, DC, USA, 1993; EPA/600/R-93/089. Available online: https://19january2021snapshot.epa.gov/sites/static/files/2015-11/1993_epa_600_r-93_c89.pdf (accessed on 6 June 2025).
- U.S. Environmental Protection Agency (USEPA). Exposure Factors Handbook: 2011 Edition (Final); National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 2011. Available online: https://www.epa.gov/expobox/exposure-factors-handbook-2011-edition (accessed on 6 June 2025).
- European Commission. Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs; European Union: Brussels, Belgium, 2011; pp. 4–8. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32011R0835 (accessed on 6 June 2025).
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; Di Domenico, A.; Fernández-Cruz, M.; Fink-Gremmels, J.; Fürst, P.; Galli, C.; et al. Polycyclic Aromatic Hydrocarbons in Food. Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 724, 1–114. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Shoaei, F.; Talebi-Ghane, E.; Amirsadeghi, S.; Mehri, F. The Investigation of Polycyclic Aromatic Hydrocarbons (PAHs) in Milk and Its Products: A Global Systematic Review, Meta-Analysis and Health Risk Assessment. Int. Dairy J. 2023, 142, 105645. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A). Interim Final; Office of Emergency and Remedial Response: Washington, DC, USA, 1989. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/rags_a.pdf (accessed on 7 June 2025).
- Russo, M.V.; Avino, P.; Notardonato, I. PAH Residues in Honey by Ultrasound-Vortex-Assisted Liquid-Liquid Micro-Extraction Followed by GC-FID/IT-MS. Food Anal. Methods 2017, 10, 2132–2142. [Google Scholar] [CrossRef]
- Khatami, A.; Dabbagh Moghaddam, A.; Dini Talatappeh, H.; Mohammadimehr, M. Simultaneous Extraction of Polycyclic Aromatic Hydrocarbons and Tetracycline Antibiotics from Honey Samples Using Dispersive Solid Phase Extraction Combined with Dispersive Liquid-Liquid Microextraction before Their Determination with HPLC-DAD. J. Food Compos. Anal. 2024, 131, 106179. [Google Scholar] [CrossRef]
- Kamankesh, M.; Ghanati, K.; Shahdoostkhany, M.; Mohammadi, A.; Hadian, Z. Investigation of 33 Types of Honey Samples: Application of an Efficient Dispersive Liquid-Liquid Microextraction Technique Coupled with Gas Chromatography-Mass Spectrometry to Determine 16 Polycyclic Aromatic Hydrocarbons. J. Apic. Res. 2022, 63, 997–1003. [Google Scholar] [CrossRef]
- Murcia-Morales, M.; Tzanetou, E.N.; Garcia-Gallego, G.; Kasiotis, K.M.; Vejsnaes, F.; Brodschneider, R.; Hatjina, F.; Machera, K.; Van der Steen, J.J.M. Environmental Assessment of PAHs through Honey Bee Colonies—A Matrix Selection Study. Heliyon 2024, 10, e23564. [Google Scholar] [CrossRef]
- Ozoani, H.; Ezejiofor, A.N.; Amadi, C.N.; Chijioke-Nwauche, I.; Orisakwe, O.E. Safety of Honey Consumed in Enugu State, Nigeria: A Public Health Risk Assessment of Lead and Polycyclic Aromatic Hydrocarbons. Rocz. Panstw. Zakl. Hig. 2020, 71, 57–66. [Google Scholar] [CrossRef]
- Di Fiore, C.; De Cristofaro, A.; Nuzzo, A.; Notardonato, I.; Ganassi, S.; Iafigliola, L.; Sardella, G.; Ciccone, M.; Nugnes, D.; Passarella, S.; et al. Biomonitoring of Polycyclic Aromatic Hydrocarbons, Heavy Metals, and Plasticizers Residues: Role of Bees and Honey as Bioindicators of Environmental Contamination. Environ. Sci. Pollut. Res. Int. 2023, 30, 44234–44250. [Google Scholar] [CrossRef]
- Ek-Huchim, J.P.; Rodríguez-Cab, E.M.; López-Torres, E.; Dzul-Caamal, R.; Canepa-Pérez, I.M.; Osten, J.R.-V. Pesticides and Polycyclic Aromatic Hydrocarbons in Honey and Apis mellifera from the Yucatán Peninsula, Mexico. J. Food Compos. Anal. 2024, 132, 106293. [Google Scholar] [CrossRef]
- Marcolin, L.C.; de Oliveira Arias, J.L.; Kupski, L.; Barbosa, S.C.; Primel, E.G. Polycyclic Aromatic Hydrocarbons (PAHs) in Honey from Stingless Bees (Meliponinae) in Southern Brazil. Food Chem. 2023, 405, 134944. [Google Scholar] [CrossRef]
- Surma, M.; Sadowska-Rociek, A.; Draszanowska, A. Levels of Contamination by Pesticide Residues, Polycyclic Aromatic Hydrocarbons (PAHs), and 5-Hydroxymethylfurfural (HMF) in Honeys Retailed in Europe. Arch. Environ. Contam. Toxicol. 2023, 84, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Kazazic, M.; Djapo-Lavic, M.; Mehic, E.; Jesenkovic-Habul, L. Monitoring of Honey Contamination with Polycyclic Aromatic Hydrocarbons in Herzegovina Region. Chem. Ecol. 2020, 36, 726–732. [Google Scholar] [CrossRef]
- Hungerford, N.L.; Fletcher, M.T.; Tsai, H.H.; Hnatko, D.; Swann, L.J.; Kelly, C.L.; Anuj, S.R.; Tinggi, U.; Webber, D.C.; Were, S.T.; et al. Occurrence of Environmental Contaminants (Pesticides, Herbicides, PAHs) in Australian/Queensland Apis mellifera Honey. Food Addit. Contam. Part B Surveill. 2021, 14, 193–205. [Google Scholar] [CrossRef]
- Lambert, O.; Veyrand, B.; Durand, S.; Marchand, P.; Le Bizec, B.; Piroux, M.; Puyo, S.; Thorin, C.; Delbac, F.; Pouliquen, H. Polycyclic Aromatic Hydrocarbons: Bees, Honey and Pollen as Sentinels for Environmental Chemical Contaminants. Chemosphere 2012, 86, 98–104. [Google Scholar] [CrossRef]
- Mohebbi, A.; Fathi, A.A.; Afshar Mogaddam, M.R.; Farajzadeh, M.A.; Yaripour, S.; Fattahi, N. Application of Magnetic Dispersive Solid Phase Extraction Combined with Solidification of Floating Organic Droplet-Based Dispersive Liquid-Liquid Microextraction and GC-MS in the Extraction and Determination of Polycyclic Aromatic Hydrocarbons in Honey. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2024, 41, 175–187. [Google Scholar] [CrossRef]
- Passarella, S.; Guerriero, E.; Quici, L.; Ianiri, G.; Cerasa, M.; Notardonato, I.; Protano, C.; Vitali, M.; Russo, M.V.; De Cristofaro, A.; et al. PAHs Presence and Source Apportionment in Honey Samples: Fingerprint Identification of Rural and Urban Contamination by Means of Chemometric Approach. Food Chem. 2022, 382, 132361. [Google Scholar] [CrossRef]
- Saitta, M.; Di Bella, G.; Fede, M.R.; Lo Turco, V.; Potortì, A.G.; Rando, R.; Russo, M.T.; Dugo, G. Gas Chromatography-Tandem Mass Spectrometry Multi-Residual Analysis of Contaminants in Italian Honey Samples. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 800–808. [Google Scholar] [CrossRef]
- Sari, M.F.; Esen, F. Polycyclic Aromatic Hydrocarbon (PAH) Residues in the Honeybee, Honey, and Pollen and Estimation of Atmospheric Concentrations in Bursa, Turkey. Polycycl. Aromat. Compd. 2023, 44, 457–472. [Google Scholar] [CrossRef]
- Sari, M.F.; Esen, F. Concentration Levels and an Assessment of Human Health Risk of Polycyclic Aromatic Hydrocarbons (PAHs) and Polychlorinated Biphenyls (PCBs) in Honey and Pollen. Environ. Sci. Pollut. Res. Int. 2022, 29, 66913–66921. [Google Scholar] [CrossRef]
- Sawicki, T.; Surma, M.; Sadowska-Rociek, A. Characteristics of Contaminants in the Polish-Origin Bee Products and Cancer Risk Assessment. Food Chem. Toxicol. 2023, 175, 113693. [Google Scholar] [CrossRef] [PubMed]
- Toptancı, İ.; Bayrak, A.; Kiralan, M.; Ramadan, M.F. Application of QuEChERS with GC/MS/MS for Monitoring Polycyclic Aromatic Hydrocarbon (PAHs) Contaminants in Turkish Flora Honey Produced in Urban and Rural Areas. Chem. Ecol. 2022, 38, 252–264. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.; Li, Z.; Li, J.; Yang, X.; Wang, C.; Wang, Z. Construction of Covalent Triazine-Based Frameworks and Application to Solid Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Honey Samples. Food Chem. 2020, 322, 126770. [Google Scholar] [CrossRef]
- Ayyildiz, E.G.; Sari, M.F.; Gunes, M.E.; Tasdemir, Y.; Esen, F. Determination of Atmospheric PAHs Concentration by Using Honeybee and Passive Air Sampler. In Proceedings of the 4th World Congress on Civil, Structural, and Environmental Engineering (CSEE’19), Rome, Italy, 7–9 April 2019; Available online: https://avestia.com/CSEE2019_Proceedings/files/CSEE19_Proceedings.pdf (accessed on 15 June 2025).
- Antwi-Boasiako, S. Assessment of Polycyclic Aromatic Hydrocarbons in Honey. Master’s Thesis, Department of Food Science and Technology, Faculty of Biosciences, College of Science, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana, 2017. [Google Scholar]
- European Commission. Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 Relating to Arsenic, Cadmium, Mercury, Nickel and Polycyclic Aromatic Hydrocarbons in Ambient Air; European Union: Brussels, Belgium, 2005; pp. 3–16. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32004L0107 (accessed on 15 June 2025).
- Zhang, Y.; Tao, S. Seasonal Variation of Polycyclic Aromatic Hydrocarbons (PAHs) Emissions in China. Environ. Pollut. 2008, 156, 657–663. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric Polycyclic Aromatic Hydrocarbons: Source Attribution, Emission Factors and Regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Shen, G.; Wang, W.; Yang, Y.; Ding, J.; Xue, M.; Min, Y.; Zhu, C.; Shen, H.; Li, W.; Wang, B.; et al. Emissions of PAHs from Indoor Crop Residue Burning in a Typical Rural Stove: Emission Factors, Size Distributions, and Gas-Particle Partitioning. Environ. Sci. Technol. 2011, 45, 1206–1212. [Google Scholar] [CrossRef]
- Du, W.; Yun, X.; Chen, Y.; Zhong, Q.; Wang, W.; Wang, L.; Qi, M.; Shen, G.; Tao, S. PAHs Emissions from Residential Biomass Burning in Real-World Cooking Stoves in Rural China. Environ. Pollut. 2020, 267, 115592. [Google Scholar] [CrossRef]
- Collaud Coen, M.; Andrews, E.; Aliaga, D.; Andrade, M.; Angelov, H.; Bukowiecki, N.; Ealo, M.; Fialho, P.; Flentje, H.; Hallar, A.G.; et al. Identification of Topographic Features Influencing Aerosol Observations at High Altitude Stations. Atmos. Chem. Phys. 2018, 18, 12289–12313. [Google Scholar] [CrossRef]
- Kaplan, O.B.; Litman, R. Polycyclic Aromatic Hydrocarbons in Soils of a Mountain Valley: Correlation with Highway Traffic and Cancer Incidence. Environ. Sci. Technol. 1978, 12, 599. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 92, Available online: https://publications.iarc.fr/106 (accessed on 18 June 2025).
- Lundstedt, S.; White, P.A.; Lemieux, C.L.; Lynes, K.D.; Lambert, I.B.; Öberg, L.; Haglund, P.; Tysklind, M. Sources, Fate, and Toxic Hazards of Oxygenated Polycyclic Aromatic Hydrocarbons (PAHs) at PAH-Contaminated Sites. AMBIO J. Hum. Environ. 2007, 36, 475–485. [Google Scholar] [CrossRef]
- Andersson, J.T.; Achten, C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Meusel, H. Nitrated Polycyclic Aromatic Hydrocarbons (Nitro-PAHs) in the Environment—A Review. Sci. Total Environ. 2017, 581–582, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Nanaobi, H.; Sarojnalini, C.; Naganathan, D. Determination of Polycyclic Aromatic Hydrocarbons in Smoked Fishes and Their Carcinogenic Health Risks. Polycycl. Aromat. Compd. 2023, 44, 4699–4710. [Google Scholar] [CrossRef]
- Kim, M.-J.; Hwang, J.-H.; Shin, H.-S. Evaluation of Polycyclic Aromatic Hydrocarbon Contents and Risk Assessment for Fish and Meat Products in Korea. Food Sci. Biotechnol. 2014, 23, 991–998. [Google Scholar] [CrossRef]
- Wu, S.; Gong, G.; Yan, K.; Sun, Y.; Zhang, L. Polycyclic Aromatic Hydrocarbons in Edible Oils and Fatty Foods: Occurrence, Formation, Analysis, Change and Control. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 93, pp. 59–112. [Google Scholar] [CrossRef]
- Einolghozati, M.; Talebi-Ghane, E.; Amirsadeghi, S.; Fereshteh, M. Evaluation of Polycyclic Aromatic Hydrocarbons (PAHs) in Processed Cereals: A Meta-Analysis Study, Systematic Review, and Health Risk Assessment. Heliyon 2022, 8, e12168. [Google Scholar] [CrossRef]
- Ahmadi, S.; Talebi-Ghane, E.; Mehri, F.; Naderifar, H. Evaluation of Polycyclic Aromatic Hydrocarbons (PAHs) in Various Teas: A Meta-Analysis Study, Systematic Review, and Health Risk Assessment. J. Food Compos. Anal. 2024, 133, 106402. [Google Scholar] [CrossRef]
- Ravindra, K.; Wauters, E.; Van Grieken, R. Variation in Particulate PAHs Levels and Their Relation with the Transboundary Movement of the Air Masses. Sci. Total Environ. 2008, 396, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, S. Global Atmospheric Emission Inventory of Polycyclic Aromatic Hydrocarbons (PAHs) for 2004. Atmos. Environ. 2009, 43, 812–819. [Google Scholar] [CrossRef]
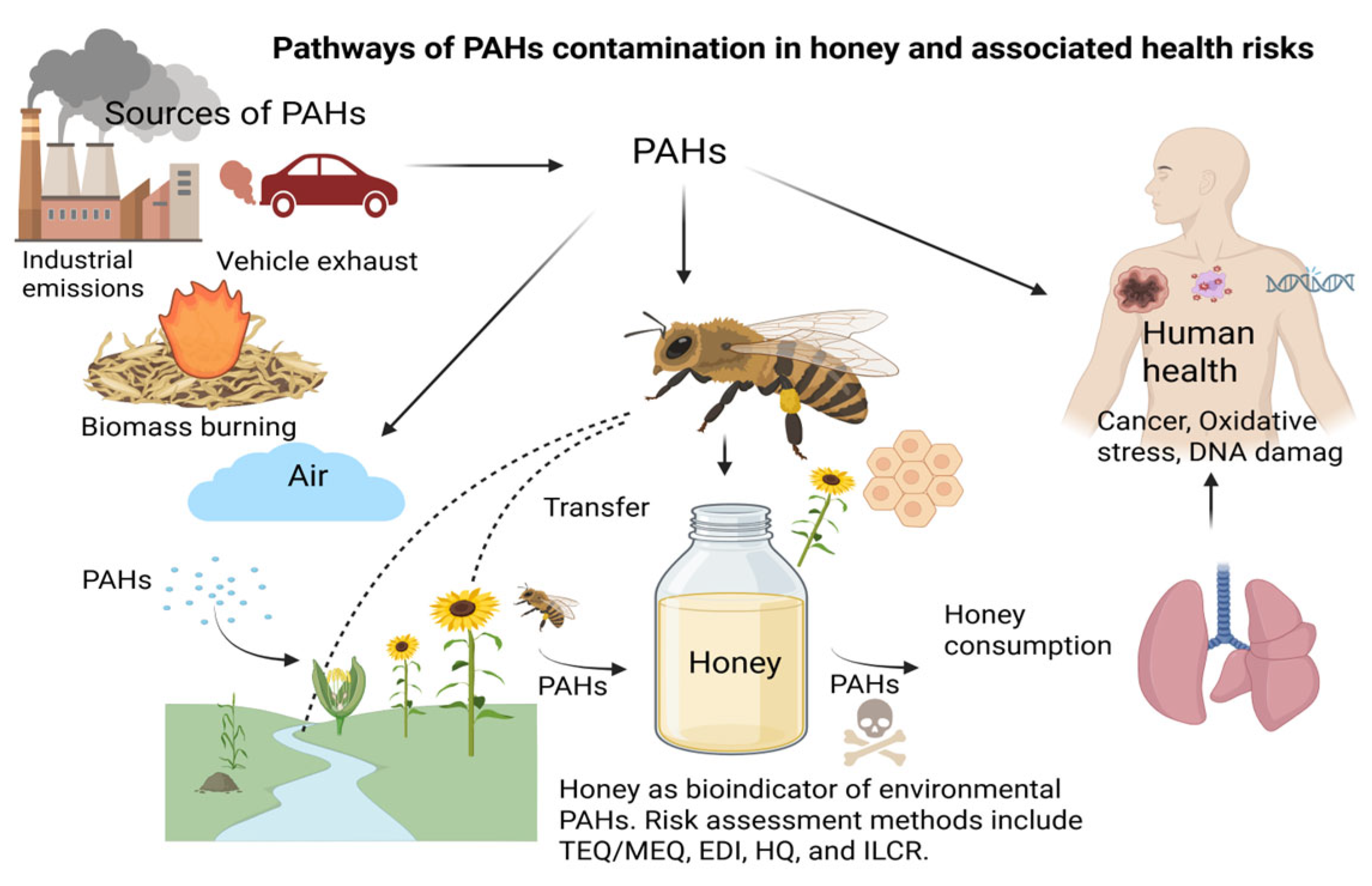

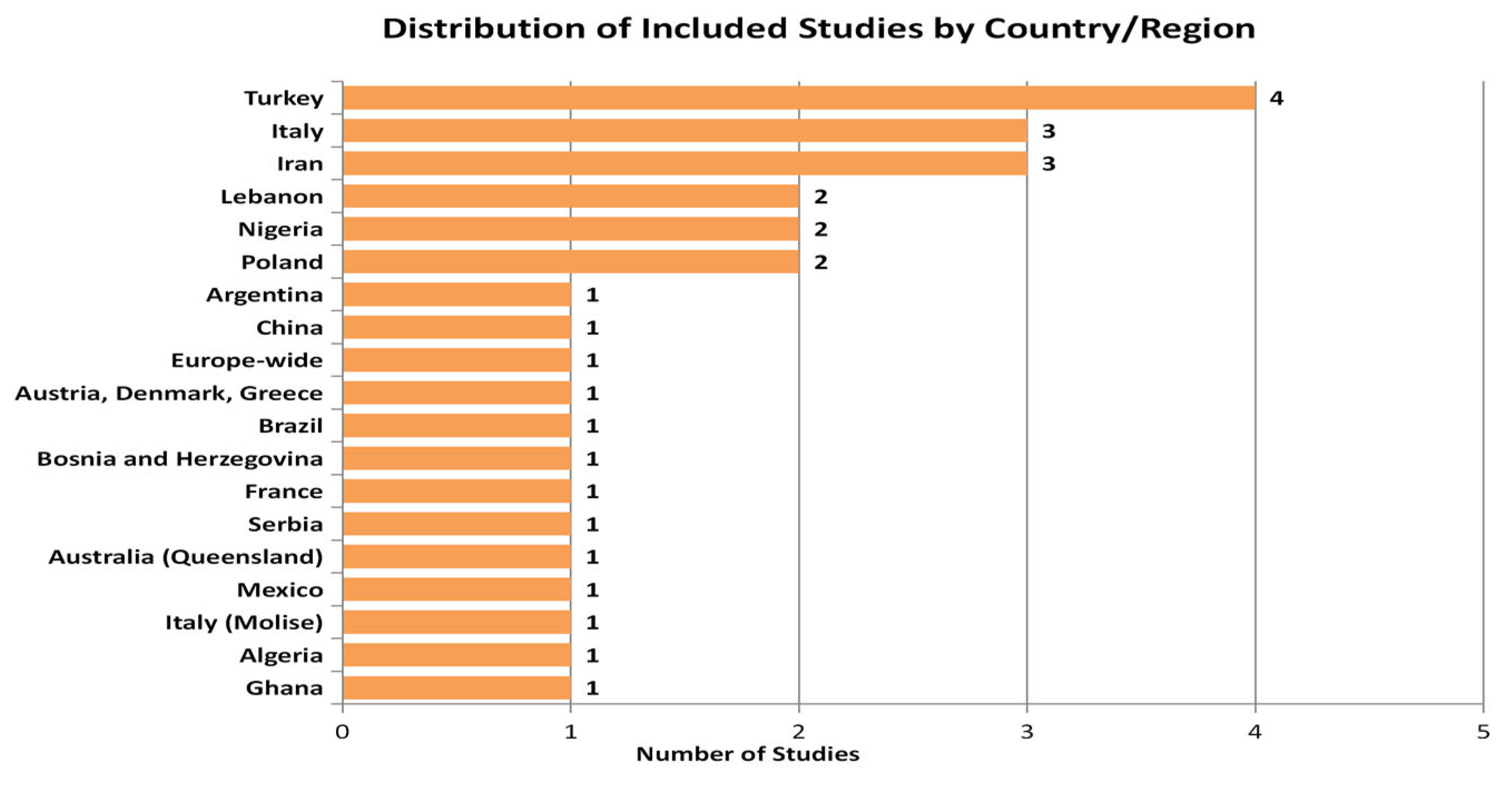
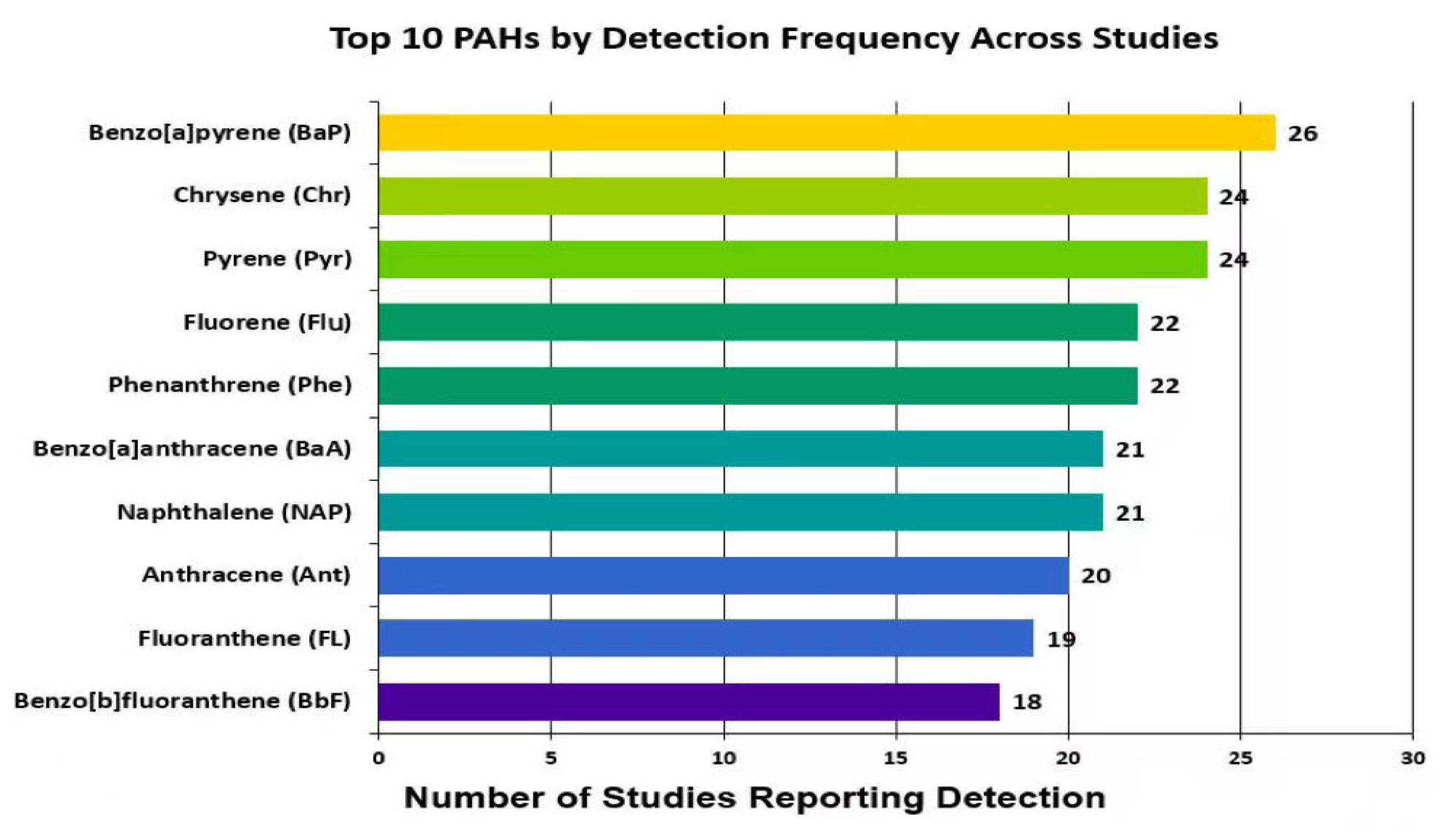
| (a) | |||
| Variable | Definition | Value/Unit | Ref. |
| IR (Ingestion rate) | Daily honey intake | 10 g/day (WHO default) | [11] |
| EF (Exposure frequency) | Number of exposure days/year | 350 days/year | [16] |
| ED (Exposure duration) | Period of exposure for adults | 30 years (screening); 70 years (lifetime, carcinogenic risk) | [16] |
| BW (Body weight) | Average adult weight | 70 kg | [16] |
| AT (Averaging time) | ED × 365 (non-carcinogenic); 70 years × 365 (carcinogenic) | 10,950 days/25,550 days | [16] |
| SF_BaP | Oral carcinogenic slope factor for BaP | 7.30 (mg/kg/day)−1 | [11] |
| (b) | |||
| Compound | TEF | MEF | Ref. |
| Benzo[a]anthracene (BaA) | 0.1 | 0.082 | [11] |
| Benzo[a]pyrene (BaP) | 1.0 | 1.0 | [11] |
| Benzo[b]fluoranthene (BbF) | 0.1 | 0.25 | [11] |
| Benzo[k]fluoranthene (BkF) | 0.01 | 0.11 | [11] |
| Chrysene (Chr) | 0.001 | 0.017 | [11] |
| Dibenzo[a,h]anthracene (DahA) | 1.0 | 0.290 | [11] |
| Indeno[1,2,3–cd]pyrene (IcdP) | 0.1 | 0.310 | [11] |
| Author | Region | PAHs Detected (n) | Matrix Type | Extraction Method | Detection Method | LOD/LOQ (µg/kg) | Ref. |
|---|---|---|---|---|---|---|---|
| Al-Alam, J. | Lebanon | 16 (9 detected) | Honey | QuEChERS + SPME | GC–MS/MS | LOD = 3 × S/N; LOQ = 10 × S/N (matrix-matched calibration) | [2] |
| Al-Alam, J. | Lebanon | 16 | Honey | QuEChERS + SPME | GC–MS/MS (ion-trap) | LOD: 0.00007–0.012; LOQ: 0.00023–0.040 | [1] |
| Ciemniak, A. | Poland | 23 (16 + additional) | Honey | Liquid–liquid extraction with n-hexane | GC–MS (HP 6890/5973, Agilent Technologies, Santa Clara, USA; SIM mode) | LOD: 0.022–0.109; LOQ: 0.066–0.329 (BaP: LOD 0.023, LOQ 0.07) | [3] |
| Derrar, S. | Algeria | 13 (6 detected) | Honey | QuEChERS (MgSO4 + NaCl; d-SPE cleanup) | GC–MS/MS (Shimadzu GCMS-TQ8030, Shimadzu Corporation, Kyoto, Japan; EI mode, MRM) | LOD: 0.12–1.23; LOQ: 0.20–1.55 (compound-dependent) | [7] |
| Di Fiore, C. | Italy (Molise) | 16 | Honey, bees | Ultrasonication with dichloromethane/acetone (1:1 v/v), rotavapor, SPE cleanup | GC–IT/MS | NR | [22] |
| Ek-Huchim, J.P. | Mexico | 16 | Honey, Apis mellifera | Liquid–liquid extraction + clean-up (florisil, alumina, silica gel, Na2SO4) | GC–MS/MS (Thermo TSQ 8000 Evo, Thermo Fisher Scientific, Waltham, USA; SRM mode) | LOD: 0.009–0.099; LOQ: 0.02–0.26 | [23] |
| Hungerford, N.L. | Australia (Queensland) | 33 | Apis mellifera honey (urban, peri-urban, rural, blends) | Solid-phase extraction (SPE) | GC–MS/MS (QHFSS method QIS34973) | LOQ: 0.5 | [27] |
| Iwegbue, C.M.A. | Nigeria | 16 | Honey (Apis mellifera) | Ultrasonication with n-hexane and dichloromethane | GC-MS | NR | [9] |
| Jovetić, M.S. | Serbia | 15 | Honey, pollen | QuEChERS (with dSPE cleanup) | HPLC-FLD (Thermo Spectra System, Thermo Fisher Scientific, Waltham, USA; PAH C18 column) | LOD: <0.1–0.4 (e.g., BaA, Chr); LOQ: <0.2–2.0 | [4] |
| Kamankesh, M. | Iran | 16 | Honey | High-density solvent-dispersive liquid–liquid microextraction (HDS/DLLME) | GC–MS (SIM mode) | LOD: 0.0003–0.0008; LOQ: 0.0009–0.0024 | [19] |
| Kazazic, M. | Bosnia and Herzegovina | 16 (9 detected) | Honey (Apis mellifera) | Ultrasonic bath extraction with n-hexane/acetone (1:1) | HPLC-UV/Vis (255 nm) | NR | [26] |
| Khatami, A. | Iran | 4 (1 detected) | Honey (Apis mellifera) | Dispersive Solid Phase Extraction (DSPE) + Dispersive Liquid–Liquid Microextraction (DLLME) using Co–GA MOF and MDES | HPLC–DAD | LOD: 0.00018–0.00026; LOQ: 0.00060–0.00087 | [18] |
| Lambert, O. | France | 16 | Honey, pollen, bee | Pressurized Liquid Extraction (ASE) + LLE (cyclohexane/ethyl acetate for honey) + SPE cleanup | GC–MS/MS (Triple Quadrupole System, Thermo Fisher Scientific, Waltham, USA) | LOD: 0.008–0.017; LOQ: 0.026–0.055 | [28] |
| Mandelli, A. | Argentina | 31 (16 PAHs + OPAHs + NPAHs) | Honey | SALLE (salting-out assisted liquid–liquid extraction) | UHPLC-(+)APCI-MS/MS | LOD: 0.00004–0.00977 µg/kg LOQ: NR | [8] |
| Marcolin, L.C. | Brazil | 16 | Stingless bee honey (Meliponinae) | QuEChERS | GC–MS/MS (Triple Quadrupole System, Agilent Technologies, Santa Clara, USA; SIM mode) | LOD 0.3–3; LOQ 1–10 | [24] |
| Mohebbi, L. | Iran | 16 | Honey | Magnetic dispersive solid phase extraction (DSPE) + Solidification of floating organic droplet-based dispersive liquid–liquid microextraction (SFOD-DLLME) | GC–MS (Agilent 6890 GC + 5973 MS, HP-5 MS column; Agilent Technologies, Santa Clara, CA, USA) | LOD: 0.08–0.17; LOQ: 0.27–0.57 | [29] |
| Murcia-Morales, M. | Austria, Denmark, Greece | 27 (parent + alkylated PAHs) | Honey bees, pollen, propolis | QuEChERS | GC–MS/MS | iLOQ: 0.5–1; up to 5 for nitro-PAHs; values < iLOQ reported as trace | [20] |
| Ozoani, H.A. | Nigeria | 16 | Honey | Solvent extraction (acetone + dichloromethane, 50:50) | GC–FID (HP-5 column, dual detector; Agilent Technologies, Santa Clara, CA, USA; US EPA Method 8100) | LOD: <0.015; LOQ: 0.05 | [21] |
| Passarella, S. | Italy | 22 (EPA + additional) | Honey | Ultrasound–vortex assisted DLLME | GC–MS/MS (Thermo Trace 1310/TSQ 8000 Evo, SIM & full scan; Thermo Fisher Scientific, Waltham, MA, USA) | LOD: 0.003–0.029; LOQ: 0.009–0.095 | [30] |
| Russo, M.V. | Italy | 9 | Honey | Ultrasound–Vortex Assisted DLLME (UVALLME), | GC–FID; GC–IT/MS (ion trap) | GC-FID: LOD 36–63; LOQ 41–74. GC-IT/MS: LOD 0.030–0.199; LOQ 0.069–0.466 | [17] |
| Saitta, M. | Italy | 16 | Honey | QuEChERS | GC–MS/MS (Thermo Trace GC Ultra + TSQ Quantum XLS triple quadrupole, SRM mode; Thermo Fisher Scientific, Waltham, MA, USA) | LOD:0.10–5.21; LOQ: 0.33–17.19 | [31] |
| Sari, M.F. | Turkey | 16 | Honey, pollen | Liquid–liquid extraction | GC–MS (Agilent 7890A/5975C; Agilent Technologies, Santa Clara, CA, USA) | NR | [32] |
| Sari, M.F. | Turkey | 16 | honey | Liquid–liquid extraction (MeOH, DCM), GPC + silica/alumina clean-up | GC–MS (SIM) | LOD: 0.0001–0.0044 µg/kg | [33] |
| Sawicki, T. | Poland | 16 | Honey, Bee bread, Bee pollen, Beeswax | QuEChERS | GC–MS (Ion Trap, SIM mode) | LOD: 0.08–0.26; LOQ: 0.24–0.78 | [34] |
| Surma, M. | Europe-wide | 16 | Honey | QuEChERS | GC–MS (Varian 4000 Ion Trap, DB-5MS column, SIM mode; Varian Inc., Palo Alto, CA, USA) | LOD: 0.76–18.98 (ΣPAHs) | [25] |
| Toptanci, İ. | Turkey | 16 | Honey | QuEChERS | GC–MS/MS (Agilent 7890A + 7000B Triple Quadrupole, HP-5MS column, MRM mode; Agilent Technologies, Santa Clara, CA, USA) | LOD: 0.03–0.29; LOQ: 0.11–0.27 | [35] |
| Wang, W. | China | 16 | Honey | Headspace-SPME | GC–MS (Agilent 7820A–5977E, SIM mode; Agilent Technologies, Santa Clara, CA, USA) | LOD: 0.00003–0.00019; LOQ: 0.00010–0.00063 | [36] |
| Ayyildiz, E.G. | Turkey | 16 | Honey, bees | DCM/PE extraction + ultrasound | GC–MS (Agilent 7890A/5975C, SIM mode, HP-5MS column; Agilent Technologies, Santa Clara, CA, USA) | NR | [37] |
| Antwi-Boasiako, S. | Ghana | 14 | Honey | QuEChERS (ACN + MgSO4/NaOAc; d-SPE cleanup with PSA/C18/MgSO4) | HPLC–FLD | LOD: 0.10–6.46; LOQ: NR | [38] |
| Author | Region | PAHs Detected (n) | Honey Type | Individual PAH Concentration (µg/kg) | ΣPAHs (µg/kg, Mean/Range) | Max PAH (Compound, µg/kg) | Ref. |
|---|---|---|---|---|---|---|---|
| Al-Alam, J. | Lebanon | 16 (9 detected) | NR | Naph: 33.3; Ace: 25.3; FLu: 33.03; Phe: 10.33; Ant: 15.87; FL: 20.2; Pyr: 14.7; BaA: 8.74; Chr: 5.36 | Mean 166.83; Range 5.36–33.03 | Naph, 33.03 | [2] |
| Al-Alam, J. | Lebanon | 16 | Multifloral | BaP: 0.17; Naph: <LOQ–1.71; most PAHs <LOQ–0.56 | Range < LOQ–6.05 | Naph, 1.71 | [1] |
| Ciemniak, A. | Poland | 23 (16 + additional) | Multifloral | BaP: <0.005–0.024 | Range < 0.005–0.311 (up to 0.311) | NR, 0.076 | [3] |
| Derrar, S. | Algeria | 13 (6 detected) | Mixed: Unifloral & Multifloral | FLu: 5.73; Phe: 2.33; Ant: 1.55; BaA; Chr; Acy | NR | FLu, 5.73 | [7] |
| Di Fiore, C. | Italy (Molise) | 16 | Multifloral | BaP: 0.19–0.38; FL: 0.34–2.20; Chr: 0.27–0.62 | Range 2.48–9.58 | FL, 2.20 | [22] |
| Ek-Huchim, J.P. | Mexico | 16 | NR | FLu: 2.1–6.2; Phe: 1.3–4.2; Ant: 1.4–3.1; Pyr: 1.9–4.6; BaP: 0.3–0.9 | Range 3.2–14.6 | FLu, 6.2 | [23] |
| Hungerford, N.L. | Australia (Queensland) | 33 | Multifloral | BaP: 0.0079; Chr: 0.0049; FL: 0.0109 | Range 0.0102–0.0297 | FL, 0.0109 | [27] |
| Iwegbue, C.M.A. | Nigeria | 16 | Multifloral | NW: BaP: 1.83–3.26; DahA: 2.66–5.29; SW: BaP: 1.92–2.73; DahA: 3.27–5.85; SE: BaP: 2.15–4.32; DahA: 4.17–6.86; ND: BaP: 4.65–7.91; DahA: 6.24–11.4 | Range NW: 17.46–22.97; SW: 22.07–29.64; SE: 26.32–35.51; ND: 37.62–47.15 | DahA, 11.4 | [9] |
| Jovetić, M.S. | Serbia | 15 | Multifloral | BaP: 0.53; | Range 2.8–18 | Naph, ≈5 | [4] |
| Kamankesh, M. | Iran | 16 | Mixed: Unifloral & Multifloral | Min: 1.22; Max: 11.63; Mean: ≈4.6 | Mean ≈ 4.6; Range 1.22–11.63 | NR, 11.63 | [19] |
| Kazazic, M. | Bosnia and Herzegovina | 16 (9 detected) | Multifloral | BaP: ND–6.12 (most samples ND; one sample 6.12) | Range 2.68–12.58 (in 4 positive samples) | BaP, 6.12 | [26] |
| Khatami, A. | Iran | 4 (1 detected) | Mixed: Unifloral & Multifloral | Pyr: 0.00556–0.01298 | Range 0.00556–0.01298 | Pyr, 0.01298 | [18] |
| Lambert, O. | France | 16 | Multifloral | ND–0.155 | Range ND–0.155 | NR, 0.155 | [28] |
| Mandelli, A. | Argentina | 31 (16 PAHs + OPAHs + NPAHs) | Multifloral | Parent PAHs: ND–91.5; OPAHs: ND–103; NPAHs: ND–22.1 | Range ND–103 (across all PAH classes) | OPAHs, 103 | [8] |
| Marcolin, L.C. | Brazil | 16 | NR | 1.4–23.3 | Range 1.4–23.3 | NR, 23.3 | [24] |
| Mohebbi, L. | Iran | 16 | NR | ND–118.25 | Range ND–118.25 | IcdP, 118.25 | [29] |
| Murcia-Morales, M. | Austria, Denmark, Greece | 27 (parent + alkylated PAHs) | NR | ND–7.67 | Range ND–7.67 | Chr, 7.67 | [20] |
| Ozoani, H.A. | Nigeria | 16 | Multifloral | 0.439–3.22 | 2.51–3.08 | PAH4, 3.22 | [21] |
| Passarella, S. | Italy | 22 (EPA + additional) | Multifloral | 0.003–5.91 | NR | FL, 5.91 | [30] |
| Russo, M.V. | Italy | 9 | Mixed: Unifloral & Multifloral | <LOQ–38.7 | Range < LOQ–38.7 | NR, 38.7 | [17] |
| Saitta, M. | Italy | 16 | Mixed: Unifloral & Multifloral | 0.11–16.0 | Mean 0.16; Range 0.11–16.0 | NR, 16 | [31] |
| Sari, M.F. | Turkey | 16 | Multifloral | 0.10–5.19 | Mean 1.71; Range 0.10–5.19 | BaP, 0.45 | [32] |
| Sari, M.F. | Turkey | 16 | Multifloral | NR | Range 1.22–11.63 | NR, 11.63 | [33] |
| Sawicki, T. | Poland | 16 | Multifloral | 0.02–16.0 | Mean 1.03; Range 0.02–16.0 | BaP (in propolis), 3.58 | [34] |
| Surma, M. | Europe-wide | 16 | Multifloral | Phe up to 1.19 | Mean 1.96; Range 0.14–5.03 | Phe, 1.19 | [25] |
| Toptanci, İ. | Turkey | 16 | Multifloral | Urban: 3.81–16.14; Rural: 2.27–7.24 | Urban: Mean 10.12; Range 3.81–16.14; Rural: Mean 4.11; Range 2.27–7.24 | Σ16PAHs (urban), 16.14 | [35] |
| Wang, W. | China | 16 | Unifloral | 1.22–11.63 | Range 1.22–11.63 | NR, 11.63 | [36] |
| Ayyildiz, E.G. | Turkey | 16 | NR | Individual PAHs: 0.0249–0.3809 | Ovaakca: 380.87; Cumalikizik: 217.96 (µg/kg dw) | ΣPAHs (Bee Ovaakca, dw), 380.87 | [37] |
| Antwi-Boasiako, S. | Ghana | 14 | Multifloral | Mean: 1.29. Range: 0.10–5.80 | Mean 1.29; Range 0.10–5.80 | NR, 5.80 | [38] |
| Study (Author, Year) | Country/Region | Matrix | Risk Assessment Method(s) | Risk Characterization | Ref. |
|---|---|---|---|---|---|
| Derrar, S. (2024) | Algeria | Honey | EDI, HQ | Acceptable Risk (HQ < 1, EDI low) | [7] |
| Iwegbue, C.M.A. (2016) | Nigeria | Honey | ILCR, TEQ, BaP threshold | Moderate Risk (BaP > 1 µg/kg) | [9] |
| Jovetić, M.S. (2018) | Serbia | Honey & bee Products | BaP threshold (EU/Serbia) | Below threshold (BaP < 1 µg/kg) | [4] |
| Kamankesh, M. (2022) | Iran | Honey | EDI, HI, ILCR | Some exceed EU BaP limits; overall moderate risk | [19] |
| Marcolin, L.C. (2023) | Brazil | Honey & bee Products | TEQBaP, DDI, ECR | 23% exceeded dietary limits (BaP–TEQ > threshold) | [24] |
| Ozoani, H.A. (2020) | Nigeria | Honey | BaP threshold (EU) | Below threshold | [21] |
| Sari, M.F. (2022) | Turkey | Honey | ILCR (adults, US–EPA model)) | Low Risk | [33] |
| Sawicki, T. (2023) | Poland | Honey | HQ (children) | No significant risk | [34] |
| Ayyildiz, E.G (2019) | Turkey | Honey & bee tissue | TEQ (Bee tissue) | Data not directly for honey | [37] |
| Antwi-Boasiako, S (2017) | Ghana | Honey | ILCR (USEPA model) | Acceptable Risk (ILCR < 10−6–10−5) | [38] |
| Study | Country | BaA | BaP | BbF | BkF | Chr | DahA | IndP | TEQBaP | MEQBaP | BaPEQ –TEQ | BaPEQ –MEQ | CR– TEQ | CR–MEQ | Risk Level | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al–Alam, J. | Lebanon | 0.44 | 0.37 | 0.88 | 0.61 | 0.34 | 0.21 | 0.57 | 0.78 | 0.94 | 1.91 × 101 | 2.31 × 101 | 1.40 × 10−1 | 1.69 × 10−1 | Risk | [2] |
| Al–Alam, J. | Lebanon | 0 | 0.17 | 0 | 0 | 0 | 0 | 0 | 0.17 | 0.17 | 4.19 × 100 | 4.19 × 100 | 3.06 × 10−2 | 3.06 × 10−2 | Risk | [1] |
| Ciemniak, A. | Poland | 0.07 | 0.06 | 0.05 | 0.04 | 0.06 | 0.4 | 0.05 | 0.48 | 0.22 | 1.18 × 101 | 5.31 × 100 | 8.59 × 10−2 | 3.87 × 10−2 | Risk | [3] |
| Derrar, S. | Algeria | 1.41 | 2.16 | 0.68 | 0.17 | 0.3 | 0 | 0 | 2.37 | 2.47 | 6.10 × 10−5 | 6.35 × 10−5 | 4.45 × 10−4 | 4.64 × 10−4 | Acceptable | [7] |
| Di Fiore, C. | Italy (Molise) | 0.19 | 0.14 | 0.04 | 0.02 | 0.05 | 0.01 | 0.01 | 0.17 | 0.17 | 4.30 × 100 | 4.31 × 100 | 3.14 × 10−2 | 3.14 × 10−2 | Risk | [22] |
| Ek-Huchim, J.P. | Mexico | 0.05 | 0.1 | 0 | 0.01 | 0.04 | 0 | 0.02 | 0.11 | 0.11 | 2.64 × 100 | 2.76 × 100 | 1.93 × 10−2 | 2.02 × 10−2 | Risk | [23] |
| Hungerford, N.L. | Australia (Queensland) | 0.006 | 0.017 | 0.003 | 0.002 | 0.004 | 0 | 0.006 | 0.02 | 0.02 | 4.57 × 10−1 | 5.03 × 10−1 | 3.33 × 10−3 | 3.67 × 10−3 | Risk | [27] |
| Iwegbue, C.M.A. | Nigeria | 1.22 | 7.91 | 3.94 | 1.76 | 2.19 | 0.96 | 4.41 | 9.85 | 10.87 | 2.43 × 102 | 2.68 × 102 | 1.77 × 100 | 1.96 × 100 | Risk | [9] |
| Jovetić, M.S. | Serbia | 0.07 | 0.1 | 0.22 | 0.11 | 0.06 | 0.17 | 0.06 | 0.31 | 0.24 | 7.55 × 100 | 5.96 × 100 | 5.51 × 10−2 | 4.35 × 10−2 | Risk | [4] |
| Kamankesh, M. | Iran | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [19] |
| Kazazic, M. | Bosnia and Herzegovina | 0.44 | 6.12 | 0.05 | 0.02 | 0.2 | 0.05 | 2.56 | 6.48 | 6.98 | 1.60 × 102 | 1.72 × 102 | 1.17 × 100 | 1.26 × 100 | Risk | [26] |
| Khatami, A. | Iran | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [18] |
| Lambert, O. | France | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [28] |
| Mandelli, A. | Argentina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [8] |
| Marcolin, L.C. | Brazil | 0.555 | 0.205 | 0.74 | 0.46 | 0.46 | 0.14 | 0.475 | 0.53 | 0.68 | 1.30 × 101 | 1.68 × 101 | 9.49 × 10−2 | 1.23 × 10−1 | Risk | [24] |
| Mohebbi, L. | Iran | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [29] |
| Murcia-Morales, M. | Austria, Denmark, Greece | 0.705 | 0.585 | 0.645 | 0.435 | 0.795 | 0 | 0 | 0.73 | 0.87 | 1.79 × 101 | 2.13 × 101 | 1.31 × 10−1 | 1.56 × 10−1 | Risk | [20] |
| Ozoani, H.A. | Nigeria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [21] |
| Passarella, S. | Italy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [30] |
| Russo, M.V. | Italy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [17] |
| Saitta, M. | Italy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [31] |
| Sari, M.F. | Turkey | 0.2665 | 0.099 | 0.1045 | 0.0845 | 0.1795 | 0.0635 | 0.0785 | 0.21 | 0.20 | 5.14 × 100 | 4.98 × 100 | 3.75 × 10−2 | 3.64 × 10−2 | Risk | [32] |
| Sari, M.F. | Turkey | 0.174 | 0.067 | 0.0825 | 0.061 | 0.061 | 0.045 | 0.123 | 0.15 | 0.16 | 3.71 × 100 | 3.97 × 100 | 2.71 × 10−2 | 2.89 × 10−2 | Risk | [33] |
| Sawicki, T. | Poland | 0.115 | 0.1 | 0.145 | 0.08 | 0.095 | 0 | 0 | 0.13 | 0.16 | 3.13 × 100 | 3.85 × 100 | 2.28 × 10−2 | 2.81 × 10−2 | Risk | [34] |
| Surma, M. | Europe–wide | 0.315 | 0.145 | 0.275 | 0.195 | 0.23 | 0 | 0 | 0.21 | 0.26 | 5.08 × 100 | 6.53 × 100 | 3.71 × 10−2 | 4.77 × 10−2 | Risk | [25] |
| Toptanci, İ. | Turkey | 0.94 | 0.52 | 1.34 | 0.59 | 0.865 | 0 | 0 | 0.75 | 1.01 | 1.86 × 101 | 2.49 × 101 | 1.36 × 10−1 | 1.82 × 10−1 | Risk | [35] |
| Wang, W. | China | 0.575 | 0.515 | 0.79 | 0 | 0.625 | 0 | 0 | 0.65 | 0.77 | 1.61 × 101 | 1.90 × 101 | 1.17 × 10−1 | 1.39 × 10−1 | Risk | [36] |
| Ayyildiz, E.G. | Turkey | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 | Safe | [37] |
| Antwi-Boasiako, S. | Ghana | 0 | 0.18 | 0 | 0 | 0 | 0 | 0 | 0.18 | 0.18 | 4.44 × 100 | 4.44 × 100 | 3.24 × 10−2 | 3.24 × 10−2 | Risk | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Hongsibsong, S. Polycyclic Aromatic Hydrocarbons in Honey: A Systematic Review of Occurrence, Concentrations, and Health Risk Assessment. J. Xenobiot. 2025, 15, 179. https://doi.org/10.3390/jox15060179
Li W, Hongsibsong S. Polycyclic Aromatic Hydrocarbons in Honey: A Systematic Review of Occurrence, Concentrations, and Health Risk Assessment. Journal of Xenobiotics. 2025; 15(6):179. https://doi.org/10.3390/jox15060179
Chicago/Turabian StyleLi, Wenting, and Surat Hongsibsong. 2025. "Polycyclic Aromatic Hydrocarbons in Honey: A Systematic Review of Occurrence, Concentrations, and Health Risk Assessment" Journal of Xenobiotics 15, no. 6: 179. https://doi.org/10.3390/jox15060179
APA StyleLi, W., & Hongsibsong, S. (2025). Polycyclic Aromatic Hydrocarbons in Honey: A Systematic Review of Occurrence, Concentrations, and Health Risk Assessment. Journal of Xenobiotics, 15(6), 179. https://doi.org/10.3390/jox15060179







