Uncovering Exposure Patterns of Metals, PFAS, Phthalates, and PAHs and Their Combined Effect on Liver Injury Markers
Abstract
1. Introduction
1.1. Characterizing Exposure Patterns: Methodological Challenges and Solutions
1.2. Role of Biomonitoring and Urinary Biomarkers
1.3. Challenges in Analyzing Mixtures
1.4. Objectives
- To identify latent patterns of chemical co-exposure across multiple classes of environmental pollutants.
- To examine the associations between these exposure patterns and key hepatic health biomarkers including AST, ALT, GGT, total bilirubin, and MASLD.
2. Materials and Methods
2.1. Data Source and Preprocessing
2.2. Quality Assurance and Control (QA/QC)
2.3. Implementation of Principal Component Pursuit with Limit of Detection (PCP-LOD)
- L represents the low-rank component, capturing systematic co-exposure patterns across the population.
- S represents the sparse component, capturing unique or extreme exposure events at the individual level.
2.4. Cross-Validation Objective, Robust Loss Function, and Parameter Selection
2.5. Bayesian Kernel Machine Regression
2.6. Assessment of Liver Function and Steatosis
3. Results
3.1. Descriptive Statistics of Exposure Variables and Hepatic Biomarkers
3.2. PCP-LOD Model Results
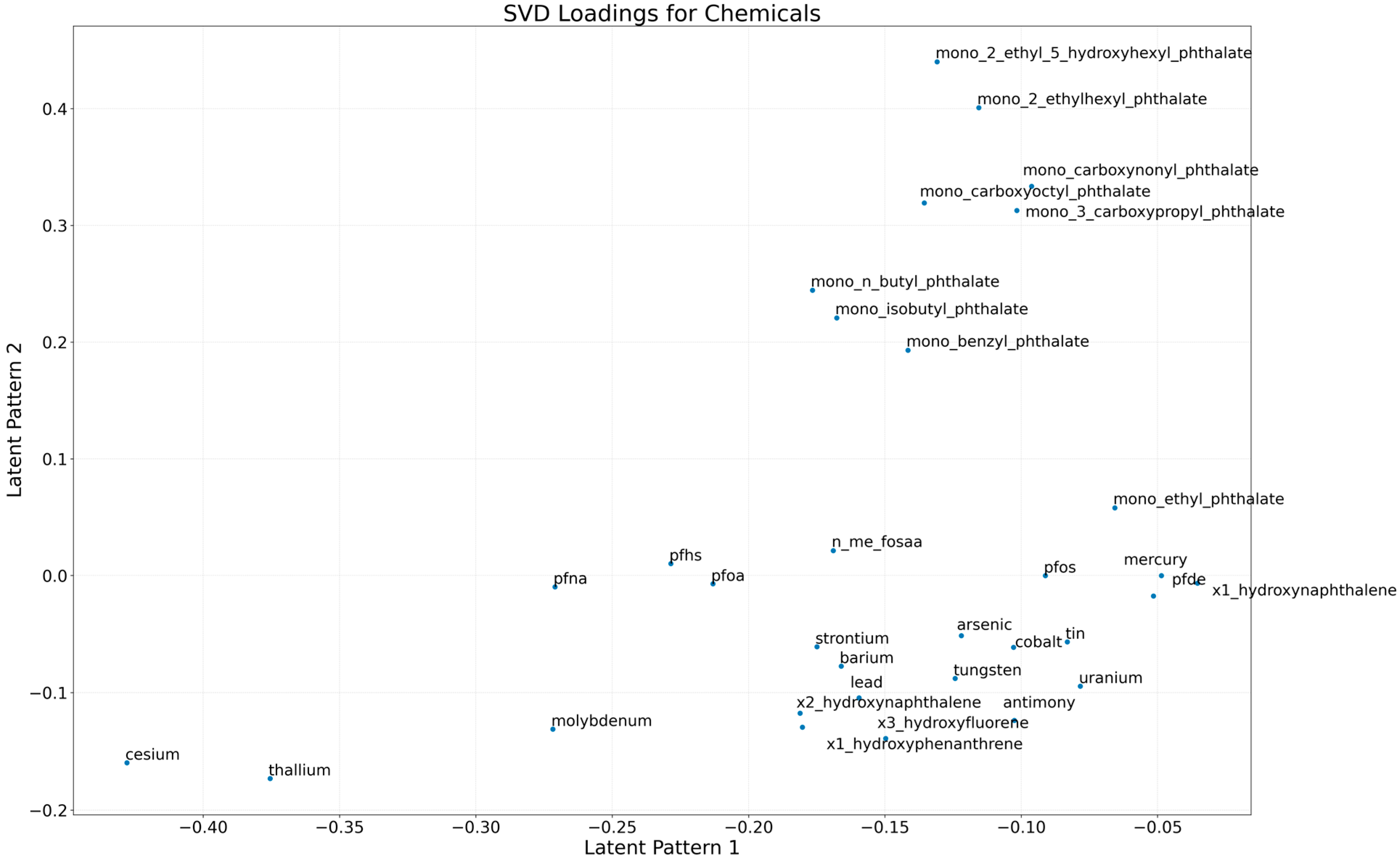
3.2.1. Latent Structure of Chemical Exposures
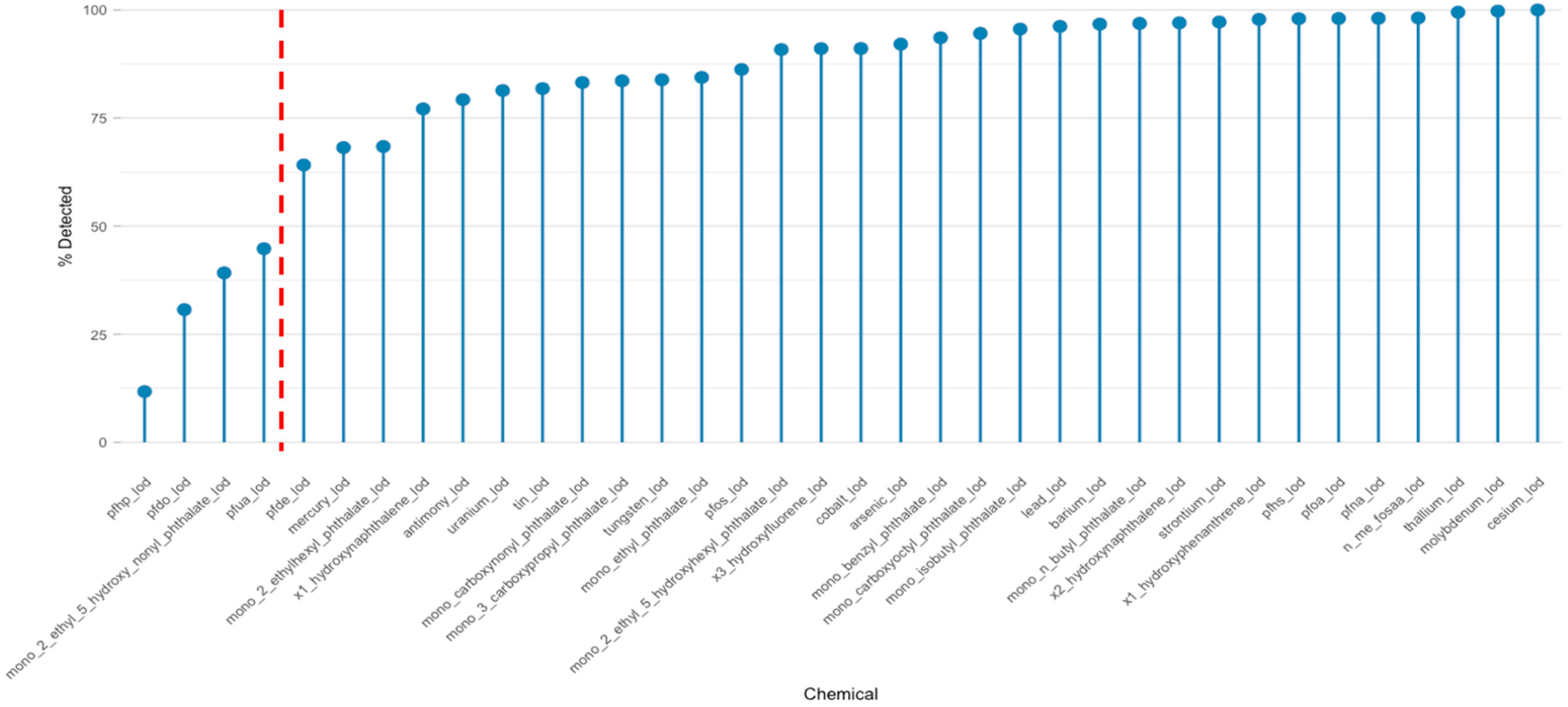
3.2.2. Detection Frequencies and Implications for Chemical Mixture Analysis
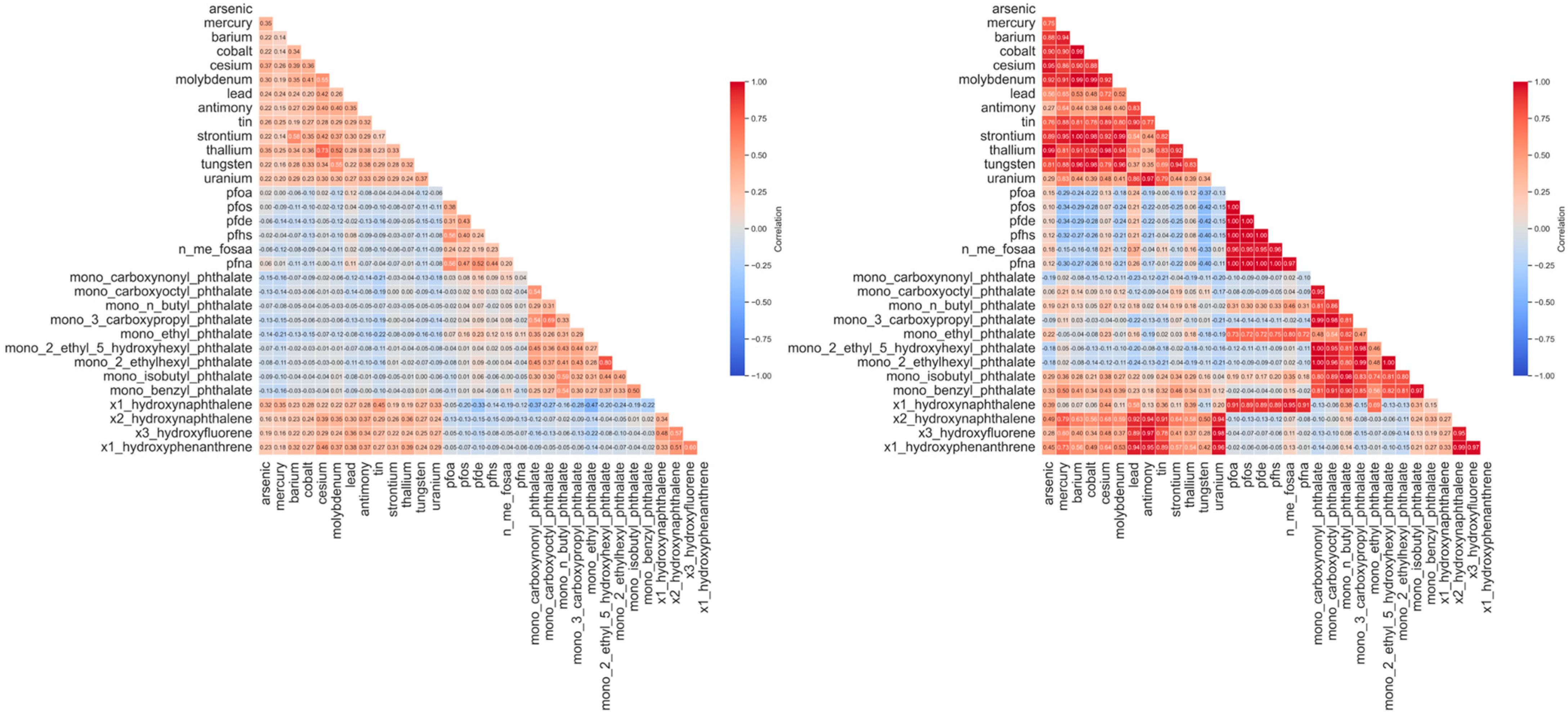
3.2.3. Revealing Latent Correlation Structures Through Low-Rank Decomposition
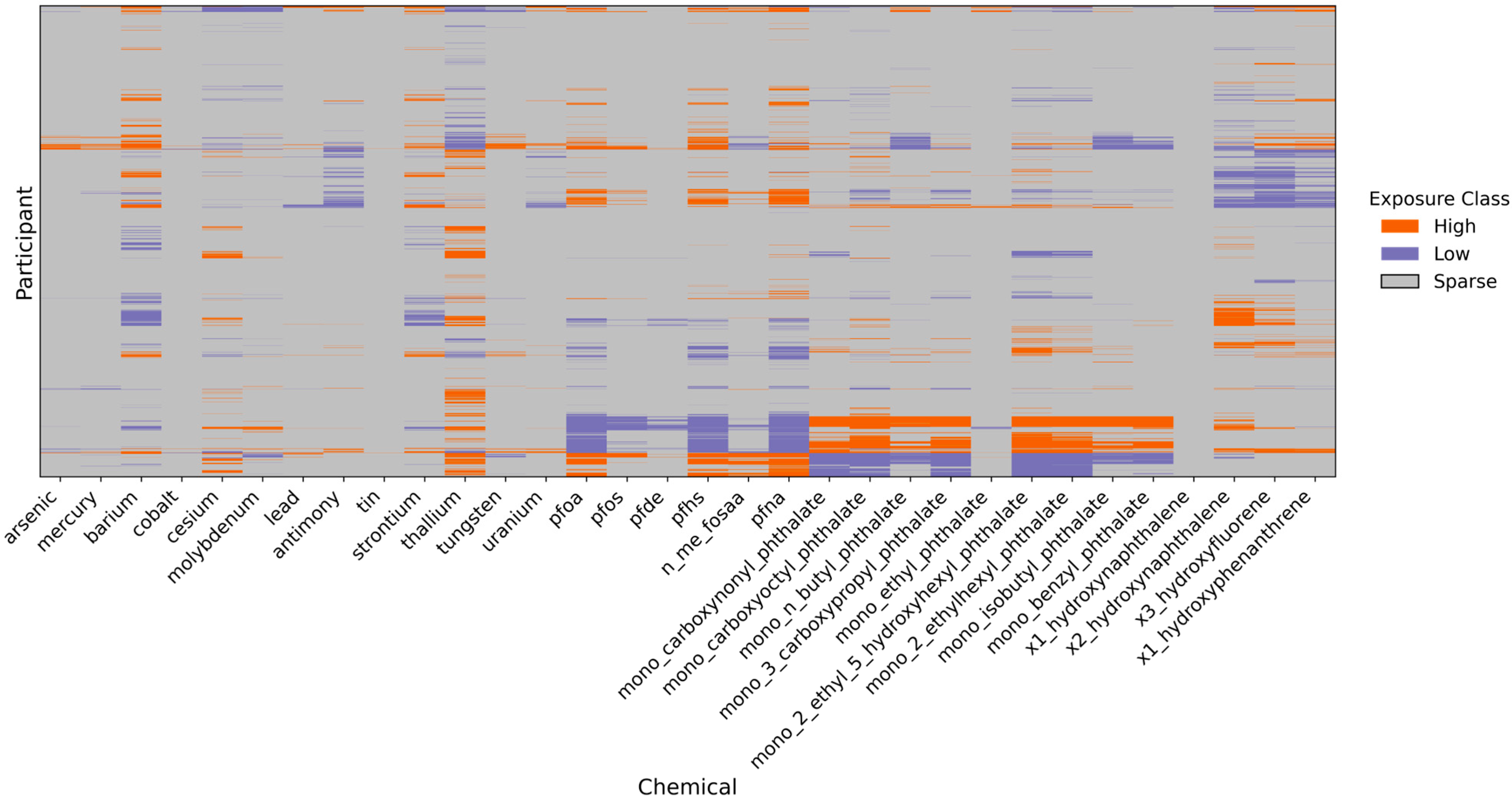
3.2.4. Characterization of Individual-Specific Chemical Anomalies via the Sparse Component
- Values above the 95th percentile were classified as High exposure events;
- Values below the 5th percentile were classified as Low exposure events;
- All other values were labeled as Sparse (representing the background or non-extreme cases).
3.2.5. Component Loadings Represent Dominant Chemical Co-Exposure Patterns
3.3. Associations Between Low-Rank Exposure Patterns and Hepatic Disease Risk
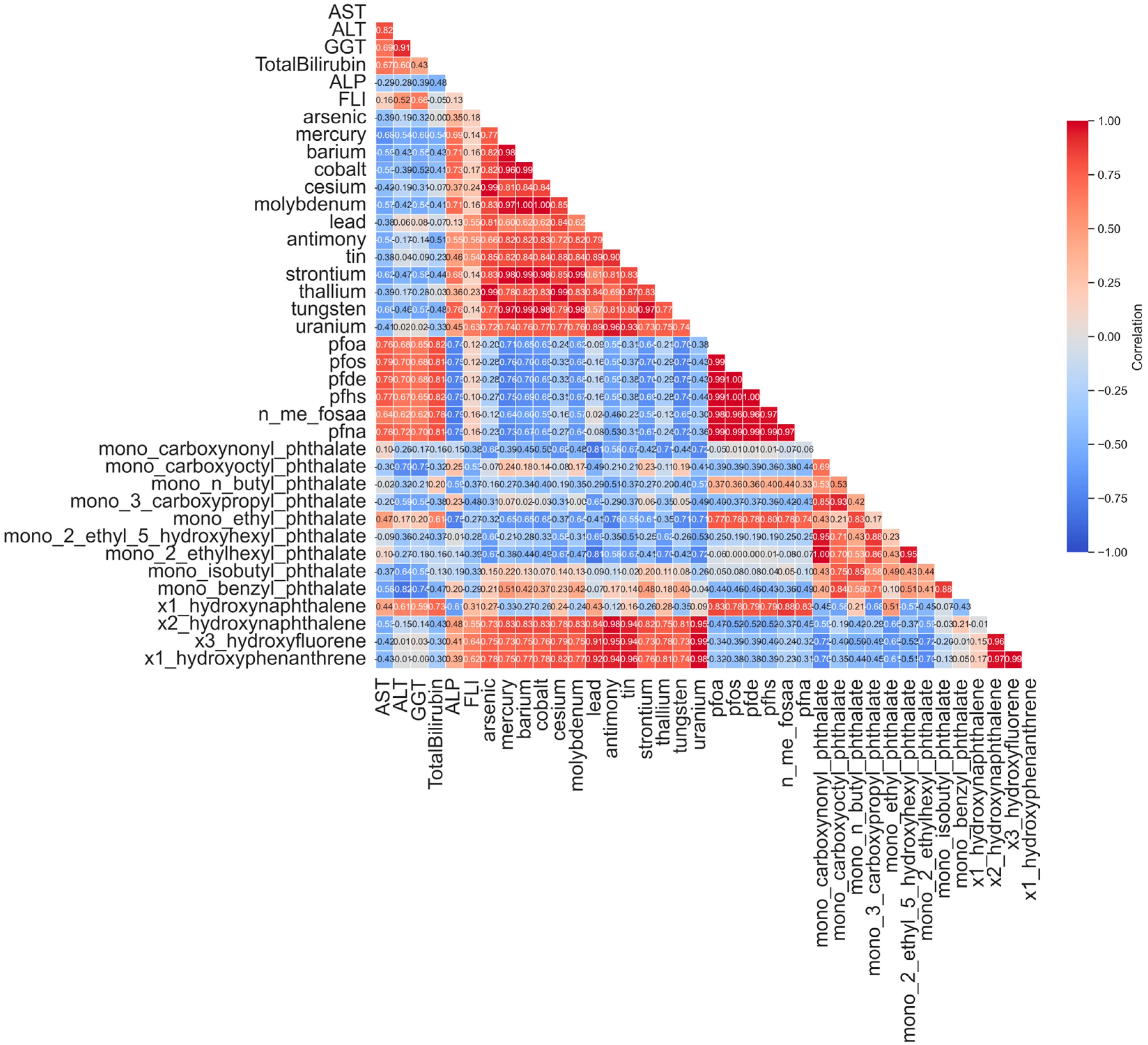
3.3.1. Spearman Correlation Matrix of Urinary Biomarkers
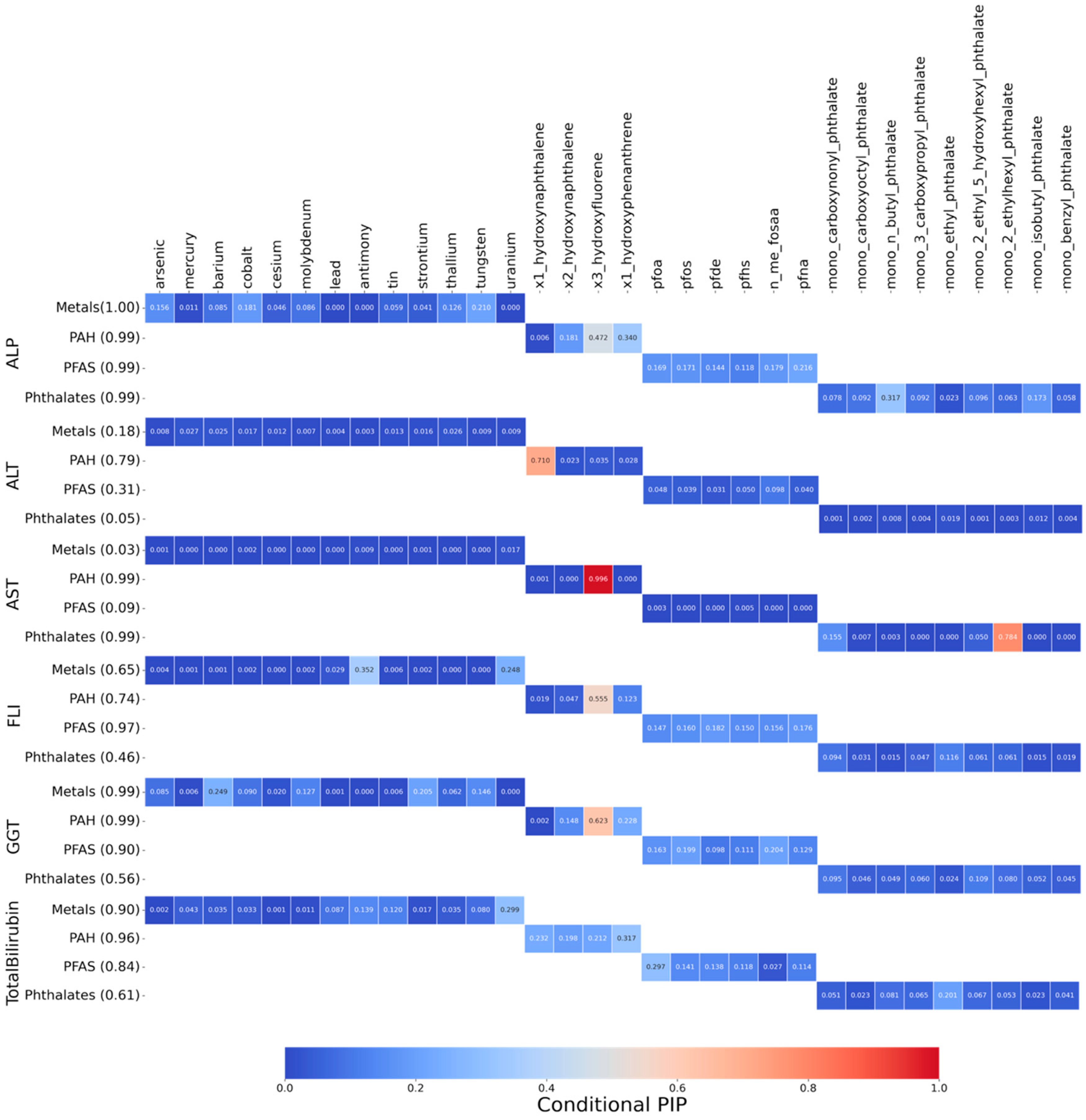
3.3.2. Posterior Inclusion Probabilities (PIPs) of Biomarkers for Hepatic Outcomes
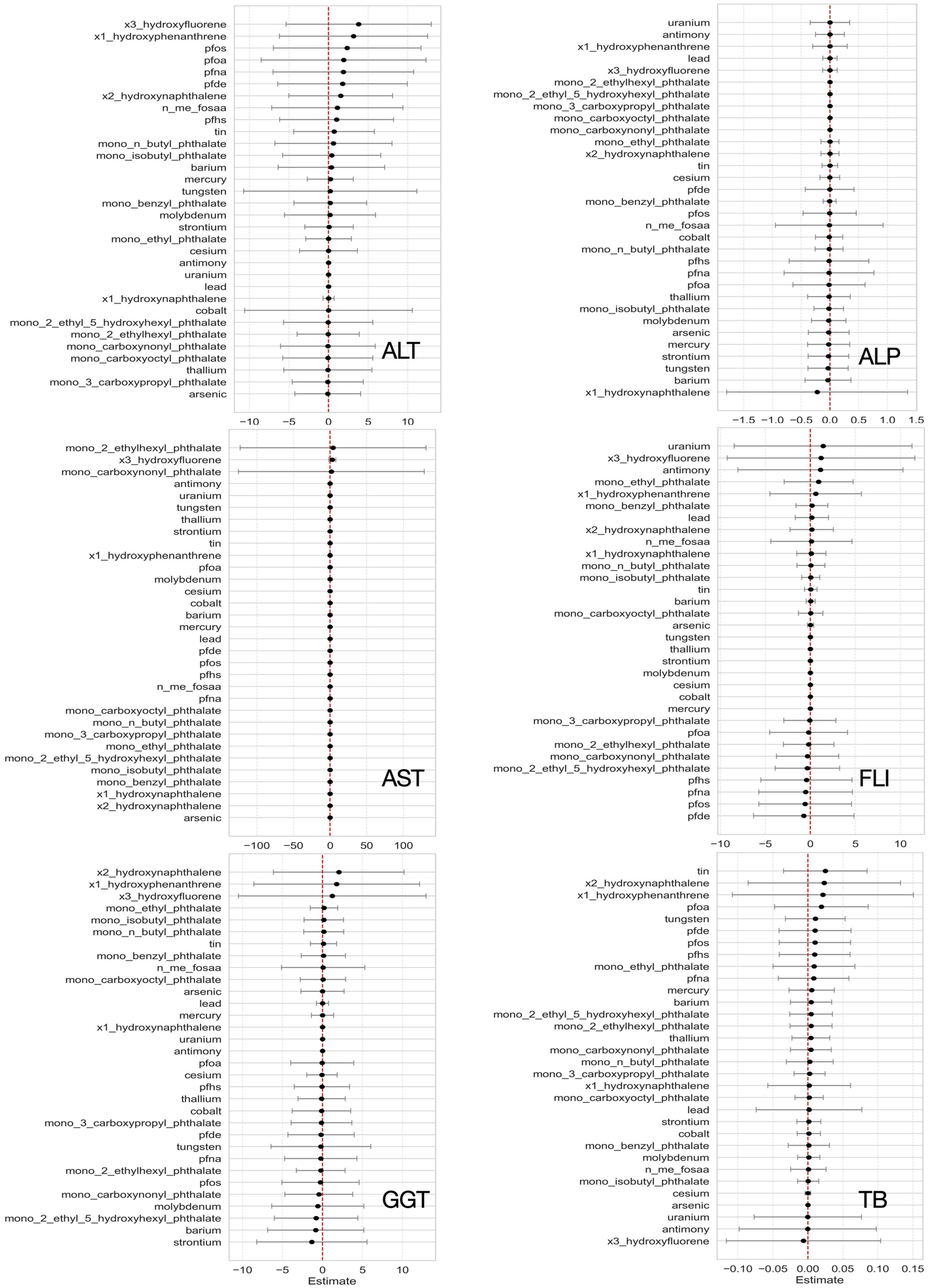
3.3.3. Single-Variable Exposure Effects on Hepatic Outcomes from BKMR
3.3.4. Individual Chemical Exposure Effects on Mixtures
3.3.5. Bivariate Exposure–Response Relationship
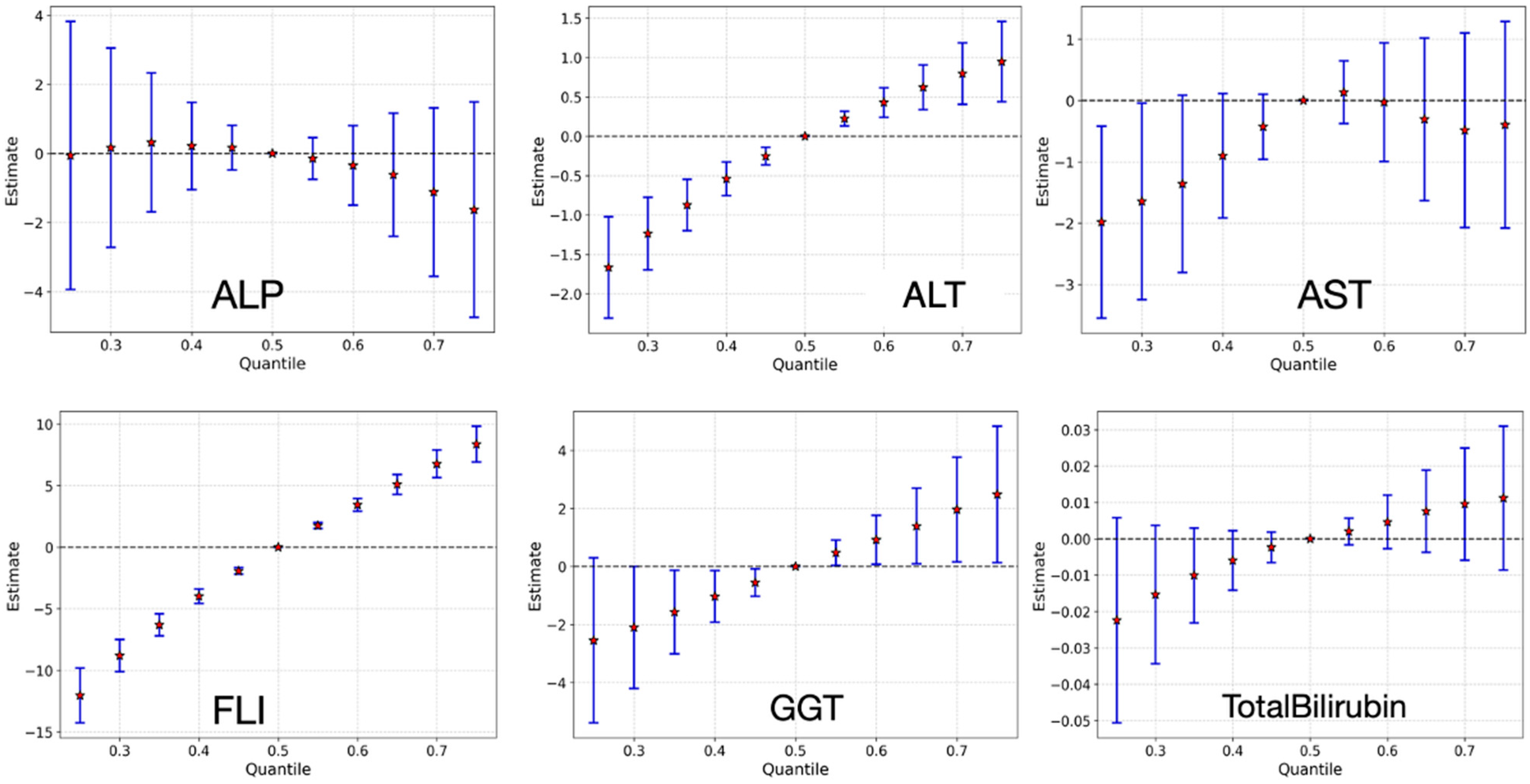
3.3.6. Overall Effect of Chemical Mixture on Liver Health Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Study design, datasets, and statistics | |
| NHANES | National Health and Nutrition Examination Survey |
| NCHS | National Center for Health Statistics |
| PCP | Principal Component Pursuit |
| PCP-LOD | Principal Component Pursuit with Limits of Detection |
| BKMR | Bayesian Kernel Machine Regression |
| PIP/PIPs | Posterior Inclusion Probability/Probabilities |
| PCA | Principal Component Analysis |
| FA | Factor Analysis |
| SVD | Singular Value Decomposition |
| LOD | Limit of Detection |
| Chemical classes and representative analytes | |
| PFAS | Per- and Polyfluoroalkyl Substances |
| PAH/PAHs | Polycyclic Aromatic Hydrocarbon(s) |
| PFOA | Perfluorooctanoic Acid |
| PFOS | Perfluorooctanesulfonic Acid |
| PFDE | Perfluorodecanoic Acid |
| PFNA | Perfluorononanoic Acid |
| PFHP | Perfluoroheptanoic Acid |
| PFUA | Perfluoroundecanoic Acid |
| PFDO | Perfluorododecanoic Acid |
| PFHS | Perfluorohexanesulfonic Acid (often written PFHxS in the literature) |
| PFHxA | Perfluorohexanoic Acid |
| PFBA | Perfluorobutanoic Acid |
| NMeFOSAA | N-Methyl Perfluorooctane Sulfonamidoacetic Acid |
| MEP | Mono-ethyl Phthalate |
| MnBP | Mono-n-butyl Phthalate |
| MiBP | Mono-isobutyl Phthalate |
| MBzP | Mono-benzyl Phthalate |
| MEHHP | Mono-(2-ethyl-5-hydroxyhexyl) Phthalate |
| MCPP | Mono-(3-carboxypropyl) Phthalate |
| (Other phthalate metabolite names expanded in Table 1 remain as written in the manuscript.) | |
| Liver outcomes and clinical indices | |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| GGT | Gamma-Glutamyl Transferase |
| ALP | Alkaline Phosphatase |
| FLI | Fatty Liver Index |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| TG | Triglycerides |
| BMI | Body Mass Index |
| Laboratory methods/instruments | |
| ICP-MS | Inductively Coupled Plasma–Mass Spectrometry |
| HPLC-MS/MS | High-Performance Liquid Chromatography–Tandem Mass Spectrometry |
| HPLC-ESI-MS/MS | High-Performance Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry |
| API (5500) | Atmospheric Pressure Ionization mass spectrometer platform (AB Sciex) |
References
- Carlin, D.J.; Rider, C.V.; Woychik, R.; Birnbaum, L.S. Unraveling the health effects of environmental mixtures: An NIEHS priority. Environ. Health Perspect. 2013, 121, A6–A8. [Google Scholar] [CrossRef]
- Pan, S.; Li, Z.; Rubbo, B.; Quon-Chow, V.; Chen, J.C.; Baumert, B.O.; Garcia, E.; Aung, M.T.; Conti, D.V.; Chatzi, L. Applications of mixture methods in epidemiological studies investigating the health impact of persistent organic pollutants exposures: A scoping review. J. Expo. Sci. Environ. Epidemiol. 2024, 35, 522–534. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Barr, C.D.; Bell, M.L. Protecting human health from air pollution: Shifting from a single-pollutant to a multipollutant approach. Epidemiology 2010, 21, 187–194. [Google Scholar] [CrossRef]
- Balogun, M.; Obeng-Gyasi, E. Association of Combined PFOA, PFOS, Metals and Allostatic Load on Hepatic Disease Risk. J. Xenobiot. 2024, 14, 516–536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, V.S.; Calafat, A.M.; Williams, P.L.; Chavarro, J.E.; Ford, J.B.; Souter, I.; Hauser, R.; Minguez-Alarcon, L. Temporal trends in urinary concentrations of phenols, phthalate metabolites and phthalate replacements between 2000 and 2017 in Boston, MA. Sci. Total Environ. 2023, 898, 165353. [Google Scholar] [CrossRef]
- Joubert, B.R.; Kioumourtzoglou, M.-A.; Chamberlain, T.; Chen, H.Y.; Gennings, C.; Turyk, M.E.; Miranda, M.L.; Webster, T.F.; Ensor, K.B.; Dunson, D.B. Powering research through innovative methods for mixtures in epidemiology (PRIME) program: Novel and expanded statistical methods. Int. J. Environ. Res. Public Health 2022, 19, 1378. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.A.; Zhang, J.; Yan, J.; Chillrud, L.; Benavides, J.; Nunez, Y.; Herbstman, J.B.; Goldsmith, J.; Wright, J.; Kioumourtzoglou, M.A. Principal Component Pursuit for Pattern Identification in Environmental Mixtures. Environ. Health Perspect. 2022, 130, 117008. [Google Scholar] [CrossRef]
- Braun, J.M.; Gennings, C.; Hauser, R.; Webster, T.F. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ. Health Perspect. 2016, 124, A6–A9. [Google Scholar] [CrossRef]
- Helsel, D.R. More than obvious: Better methods for interpreting nondetect data. Environ. Sci. Technol. 2005, 39, 419A–423A. [Google Scholar] [CrossRef] [PubMed]
- Helsel, D.R. Less than obvious: Statistical treatment of data below the detection limit. Environ. Sci. Technol. 1990, 24, 1766–1774. [Google Scholar] [CrossRef]
- Barr, D.B.; Landsittel, D.; Nishioka, M.; Thomas, K.; Curwin, B.; Raymer, J.; Donnelly, K.C.; McCauley, L.; Ryan, P.B. A survey of laboratory and statistical issues related to farmworker exposure studies. Environ. Health Perspect. 2006, 114, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Lubin, J.H.; Colt, J.S.; Camann, D.; Davis, S.; Cerhan, J.R.; Severson, R.K.; Bernstein, L.; Hartge, P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ. Health Perspect. 2004, 112, 1691–1696. [Google Scholar] [CrossRef]
- Carrico, C.; Gennings, C.; Wheeler, D.C.; Factor-Litvak, P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2015, 20, 100–120. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.H.; Chillrud, L.G.; Nunez, Y.; Rowland, S.T.; Boehme, A.K.; Yan, J.; Goldsmith, J.; Wright, J.; Kioumourtzoglou, M.A. Applying principal component pursuit to investigate the association between source-specific fine particulate matter and myocardial infarction hospitalizations in New York City. Environ. Epidemiol. 2023, 7, e243. [Google Scholar] [CrossRef]
- Candès, E.J.; Li, X.; Ma, Y.; Wright, J. Robust principal component analysis? J. ACM 2011, 58, 11. [Google Scholar] [CrossRef]
- Jehu-Appiah, D.; Obeng-Gyasi, E. Combined Effect of Metals, PFAS, Phthalates, and Plasticizers on Cardiovascular Disease Risk. Toxics 2025, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.S.; Makris, S.L.; Nietert, P.J.; Jinot, J. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ. Health Perspect. 2009, 117, 696–702. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Fourth National Report on Human Exposure to Environmental Chemicals—Updated Tables, January 2017; United States Department of Health and Human Services: Atlanta, GA, USA, 2017.
- Carroll, R.J.; Ruppert, D.; Stefanski, L.A.; Crainiceanu, C.M. Measurement Error in Nonlinear Models: A Modern Perspective, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Patel, C.J.; Bhattacharya, J.; Butte, A.J. An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS ONE 2010, 5, e10746. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Wright, J.; Ganesh, A.; Rao, S.; Peng, Y.; Ma, Y. Robust principal component analysis: Exact recovery of corrupted low-rank matrices via convex optimization. In Advances in Neural Information Processing Systems (NeurIPS) 22; Curran Associates, Inc.: Red Hook, NY, USA, 2009; pp. 2080–2088. [Google Scholar]
- Stanfield, Z.; Setzer, R.W.; Hull, V.; Sayre, R.R.; Isaacs, K.K.; Wambaugh, J.F. Characterizing Chemical Exposure Trends from NHANES Urinary Biomonitoring Data. Environ. Health Perspect. 2024, 132, 17009. [Google Scholar] [CrossRef]
- Lee, M.; Saha, A.; Sundaram, R.; Albert, P.S.; Zhao, S. Accommodating detection limits of multiple exposures in environmental mixture analyses: An overview of statistical approaches. Environ. Health 2024, 23, 48. [Google Scholar] [CrossRef]
- Helsel, D.R. Nondetects and data analysis. In Statistics for Censored Environmental Data; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Zhou, Z.; Li, X.; Wright, J.; Candès, E.J.; Ma, Y. Stable principal component pursuit. In Proceedings of the 2010 IEEE International Symposium on Information Theory (ISIT), Austin, TX, USA, 13–18 June 2010; pp. 1518–1522. [Google Scholar]
- Pratt, D.S.; Kaplan, M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N. Engl. J. Med. 2000, 342, 1266–1271. [Google Scholar] [CrossRef]
- Whitfield, J.B. Gamma glutamyl transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Fedchuk, L.; Nascimbeni, F.; Pais, R.; Charlotte, F.; Housset, C.; Ratziu, V. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2014, 40, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Alter, O.; Brown, P.O.; Botstein, D. Singular value decomposition for genome-wide expression data processing and modeling. Proc. Natl. Acad. Sci. USA 2000, 97, 10101–10106. [Google Scholar] [CrossRef] [PubMed]
- Eckart, C.; Young, G. The approximation of one matrix by another of lower rank. Psychometrika 1936, 1, 211–218. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef]
- Kazantzis, G. Thallium in the environment and health effects. Environ. Geochem. Health 2000, 22, 275–280. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Perfluoroalkyls (PFAS); U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2021. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed on 12 October 2025).
- National Health, Nutrition Examination Survey (US); National Center for Environmental Health (US); Division of Laboratory Sciences. National Report on Human Exposure to Environmental Chemicals; Department of Health and Human Services: Washington, DC, USA; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2005.
- Jain, R.B. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: Data from NHANES 2003–2008. Int. J. Hyg. Environ. Health 2013, 217, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Sobus, J.R.; Tan, Y.M.; Pleil, J.D.; Sheldon, L.S. A biomonitoring framework to support exposure and risk assessments. Sci. Total Environ. 2011, 409, 4875–4884. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Vexler, A.; Whitcomb, B.W.; Liu, A. The limitations due to exposure detection limits for regression models. Am. J. Epidemiol. 2006, 163, 374–383. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- McGinnis, D.P.; Brownstein, J.S.; Patel, C.J. Environment-Wide Association Study of Blood Pressure in the National Health and Nutrition Examination Survey (1999–2012). Sci. Rep. 2016, 6, 30373. [Google Scholar] [CrossRef] [PubMed]
- Babisch, W.; Wolf, K.; Petz, M.; Heinrich, J.; Cyrys, J.; Peters, A. Associations between traffic noise, particulate air pollution, hypertension, and isolated systolic hypertension in adults: The KORA study. Environ. Health Perspect. 2014, 122, 492–498. [Google Scholar] [CrossRef]
- Patel, C.J.; Manrai, A.K. Development of exposome correlation globes to map out environment-wide associations. Pac. Symp. Biocomput. 2015, 20, 231–242. [Google Scholar] [CrossRef]
- Palmer, G.; Herring, A.H.; Dunson, D.B. Low-Rank Longitudinal Factor Regression with Application to Chemical Mixtures. Ann. Appl. Stat. 2025, 19, 769–797. [Google Scholar] [CrossRef]
- Palmer, G.; Herring, A.H.; Dunson, D.B. Low-rank longitudinal factor regression. arXiv 2023, arXiv:2311.16470. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Wittassek, M.; Angerer, J. Phthalates: Metabolism and exposure. Int. J. Androl. 2008, 31, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A review of biomonitoring of phthalate exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Lampa, E.; Lind, L.; Birkholz, D.A.; Lind, P.M. Circulating levels of bisphenol A and phthalates in an elderly population in Sweden (PIVUS). Ecotoxicol. Environ. Saf. 2012, 75, 242–248. [Google Scholar] [CrossRef]
- Gomis, M.I.; Vestergren, R.; Borg, D.; Cousins, I.T. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ. Int. 2018, 113, 1–9. [Google Scholar] [CrossRef]
- Garcia-Sevillano, M.A.; Garcia-Barrera, T.; Navarro, F.; Gailer, J.; Gomez-Ariza, J.L. Use of elemental and molecular-mass spectrometry to assess the toxicological effects of inorganic mercury in the mouse Mus musculus. Anal. Bioanal. Chem. 2014, 406, 5853–5865. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs). 2021. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=122&tid=25 (accessed on 12 October 2025).
- Cave, M.; Appana, S.; Patel, M.; Falkner, K.C.; McClain, C.J.; Brock, G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ. Health Perspect. 2010, 118, 1735–1742. [Google Scholar] [CrossRef]
- Foulds, C.E.; Treviño, L.S.; York, B.; Walker, C.L. Endocrine-disrupting chemicals and fatty liver disease. Nat. Rev. Endocrinol. 2017, 13, 445–457. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M.K. Cadmium Inhibits the Electron Transfer Chain and Induces Reactive Oxygen Species. Free Radic. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef]
- Lovekamp-Swan, T.; Davis, B.J. Mechanisms of Phthalate Ester Toxicity in the Female Reproductive System. Environ. Health Perspect. 2003, 111, 139–145. [Google Scholar] [CrossRef]
- Vujic, E.; Ferguson, S.S.; Brouwer, K.L.R. Effects of PFAS on Human Liver Transporters: Implications for Health Outcomes. Toxicol. Sci. 2024, 200, 213–227. [Google Scholar] [CrossRef]
- Tamber, S.S.; Bansal, P.; Sharma, S.; Singh, R.B.; Sharma, R. Biomarkers of Liver Diseases. Mol. Biol. Rep. 2023, 50, 7815–7823. [Google Scholar] [CrossRef] [PubMed]
- Kalas, M.A.; Chavez, L.; Leon, M.; Taweesedt, P.T.; Surani, S. Abnormal Liver Enzymes: A Review for Clinicians. World J. Hepatol. 2021, 13, 1688. [Google Scholar] [CrossRef] [PubMed]
- Kaneva, A.M.; Bojko, E.R. Fatty Liver Index (FLI): More Than a Marker of Hepatic Steatosis. J. Physiol. Biochem. 2024, 80, 11–26. [Google Scholar] [CrossRef]
- Zota, A.R.; Shamasunder, B. The environmental injustice of beauty: Framing chemical exposures from beauty products as a health disparities concern. Am. J. Obstet. Gynecol. 2017, 217, 418.e1–418.e6. [Google Scholar] [CrossRef] [PubMed]



| Chemical Class (Analytes Included) | n | Mean (SD) |
|---|---|---|
| Metals | ||
| Arsenic (As, µg/L) | 4367.0 | 16 (31) |
| Mercury (Hg, µg/L) | 4367.0 | 1 (2) |
| Barium (Ba, µg/L) | 4367.0 | 2 (2) |
| Cobalt (Co, µg/L) | 4367.0 | 1 (2) |
| Cesium (Cs, µg/L) | 4367.0 | 5 (2) |
| Molybdenum (Mo, µg/L) | 4367.0 | 52 (41) |
| Lead (Pb, µg/L) | 4367.0 | 0.5 (1) |
| Antimony (Sb, µg/L) | 4367.0 | 0.07 (0.11) |
| Tin (Sn, µg/L) | 4367.0 | 1.33 (4) |
| Strontium (Sr, µg/L) | 4367.0 | 122.33 (152.25) |
| Thallium (TI, µg/L) | 4367.0 | 0.18 (0.10) |
| Tungsten (W, µg/L) | 4367.0 | 0.12 (0.20) |
| Uranium (U, µg/L) | 4367.0 | 0.01 (0.03) |
| PFAS | ||
| Perfluorooctanoic acid (PFOA) (ng/mL) | 4367.0 | 2.30 (2.19) |
| Perfluorooctanesulfonic acid (PFOS) (ng/mL) | 4367.0 | 8.15 (24.00) |
| Perfluorodecanoic acid (PFDE) (ng/mL) | 4367.0 | 0.30 (1.00) |
| Perfluorohexanesulfonic acid (PFHS) (ng/mL) | 4367.0 | 1.95 (1.70) |
| Perfluorononanoic acid (PFNA) (ng/mL) | 4367.0 | 0.87 (0.63) |
| N-Methyl perfluorooctane sulfonamidoacetic acid (NMeFOSAA) | 4367.0 | 0.18 (0.22) |
| Perfluoroheptanoic acid (PFHP) | 4367.0 | 0.08 (0.04) |
| Perfluoroundecanoic acid (PFUA) | 4367.0 | 0.22 (1.58) |
| Perfluorododecanoic acid (PFDO) | 4367.0 | 0.09 (0.13) |
| Phthalates | ||
| Mono-ethyl phthalate (MEP) (ng/mL) | 4367.0 | 204.46 (874.78) |
| Mono-n-butyl phthalate (MnBP) (ng/mL) | 4367.0 | 17.45 (20.07) |
| Mono-isobutyl phthalate (MiBP) (ng/mL) | 4367.0 | 14.06 (17.01) |
| Mono-benzyl phthalate (MBzP) (ng/mL) | 4367.0 | 9.93 (14.49) |
| Mono-(2-ethyl-5-carboxypentyl) phthalate (ng/mL) | 4367.0 | 0.11 (0.23) |
| Mono-(2-ethyl-5-hydroxyhexyl) phthalate (ng/mL) | 4367.0 | 12 (22) |
| Mono-(2-ethyl-5-hydroxynonyl) phthalate (ng/mL) | 4367.0 | 1 (3) |
| Mono-(3-carboxypropyl) phthalate (MCPP) (ng/mL) | 4367.0 | 5 (12) |
| Mono (carboxynonyl) phthalate (ng/mL) | 4367.0 | 6 (21) |
| Mono (carboxyoctyl) phthalate (ng/mL) | 4367.0 | 53 (85) |
| PAH | ||
| 1-Hydroxynaphthalene (ng/L) | 4367.0 | 29,726 (581,208) |
| 2-Hydroxynaphthalene (ng/L) | 4367.0 | 9109 (11,183) |
| 2-Hydroxyfluorene (ng/L) | 4367.0 | 425 (605) |
| 3-Hydroxyfluorene (ng/L) | 4367.0 | 241 (394) |
| 1-Hydroxyphenanthrene (ng/L) | 4367.0 | 149 (180) |
| 1-Hydroxyphenol (ng/L) | 4367.0 | 197 (238) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jehu-Appiah, D.; Obeng-Gyasi, E. Uncovering Exposure Patterns of Metals, PFAS, Phthalates, and PAHs and Their Combined Effect on Liver Injury Markers. J. Xenobiot. 2025, 15, 178. https://doi.org/10.3390/jox15060178
Jehu-Appiah D, Obeng-Gyasi E. Uncovering Exposure Patterns of Metals, PFAS, Phthalates, and PAHs and Their Combined Effect on Liver Injury Markers. Journal of Xenobiotics. 2025; 15(6):178. https://doi.org/10.3390/jox15060178
Chicago/Turabian StyleJehu-Appiah, Doreen, and Emmanuel Obeng-Gyasi. 2025. "Uncovering Exposure Patterns of Metals, PFAS, Phthalates, and PAHs and Their Combined Effect on Liver Injury Markers" Journal of Xenobiotics 15, no. 6: 178. https://doi.org/10.3390/jox15060178
APA StyleJehu-Appiah, D., & Obeng-Gyasi, E. (2025). Uncovering Exposure Patterns of Metals, PFAS, Phthalates, and PAHs and Their Combined Effect on Liver Injury Markers. Journal of Xenobiotics, 15(6), 178. https://doi.org/10.3390/jox15060178







