Separation and Detection of Microplastics in Human Exposure Pathways: Challenges, Analytical Techniques, and Emerging Solutions
Abstract
1. Introduction
1.1. The Emerging Risk of Microplastic Contamination
1.2. Scope and Structure of the Review
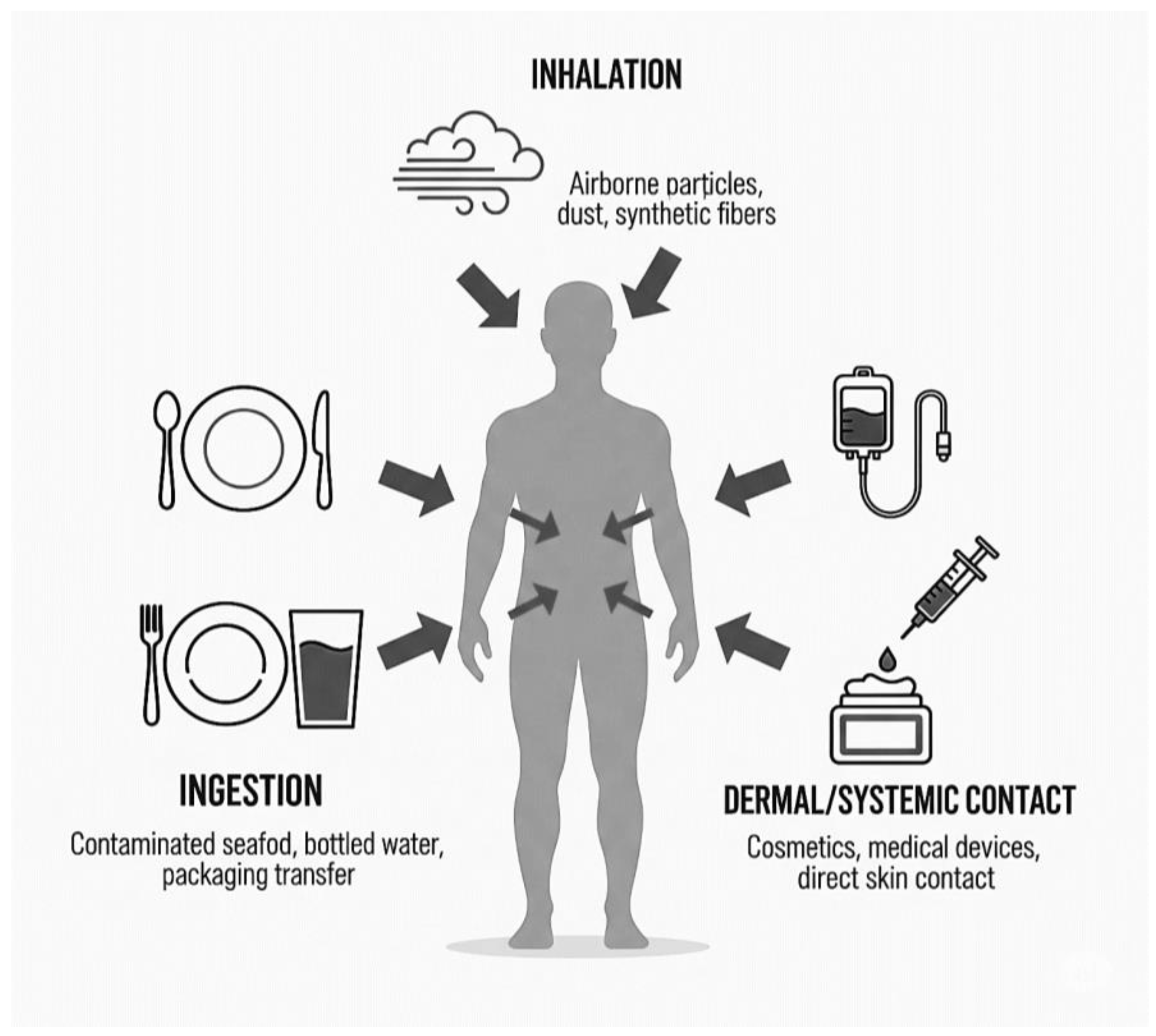
2. Microplastic Exposure Pathways and Characteristics
2.1. The Gastrointestinal Tract: Ingestion as a Primary Route
2.2. The Respiratory System: Inhalation of Airborne Particulates
2.3. Dermal and Other Systemic Exposure Routes
2.4. A Profile of Human-Relevant Microplastics: Polymer Types, Morphologies, and Size Distributions
3. Foundational Challenges in Microplastic Separation from Biological Samples
3.1. The Complexity of Biological MatriceS
3.2. Challenges of Trace-Level Detection Due to Low Microplastic Concentrations
3.3. Interference from Biogenic Organic and Inorganic Matter
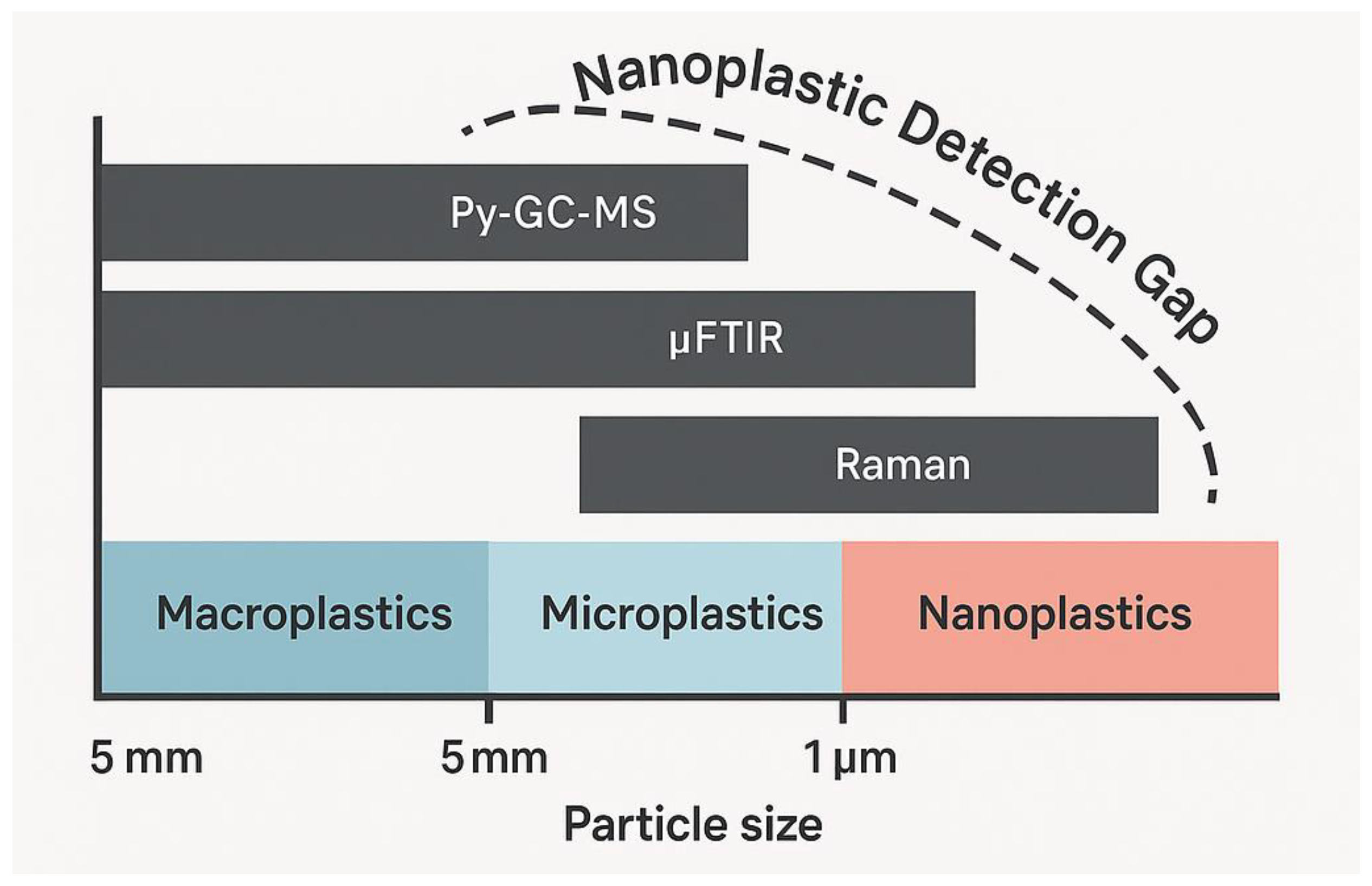
3.4. Detection Challenges at the Nanoscale: Addressing the Analytical Limitations of Nanoplastics
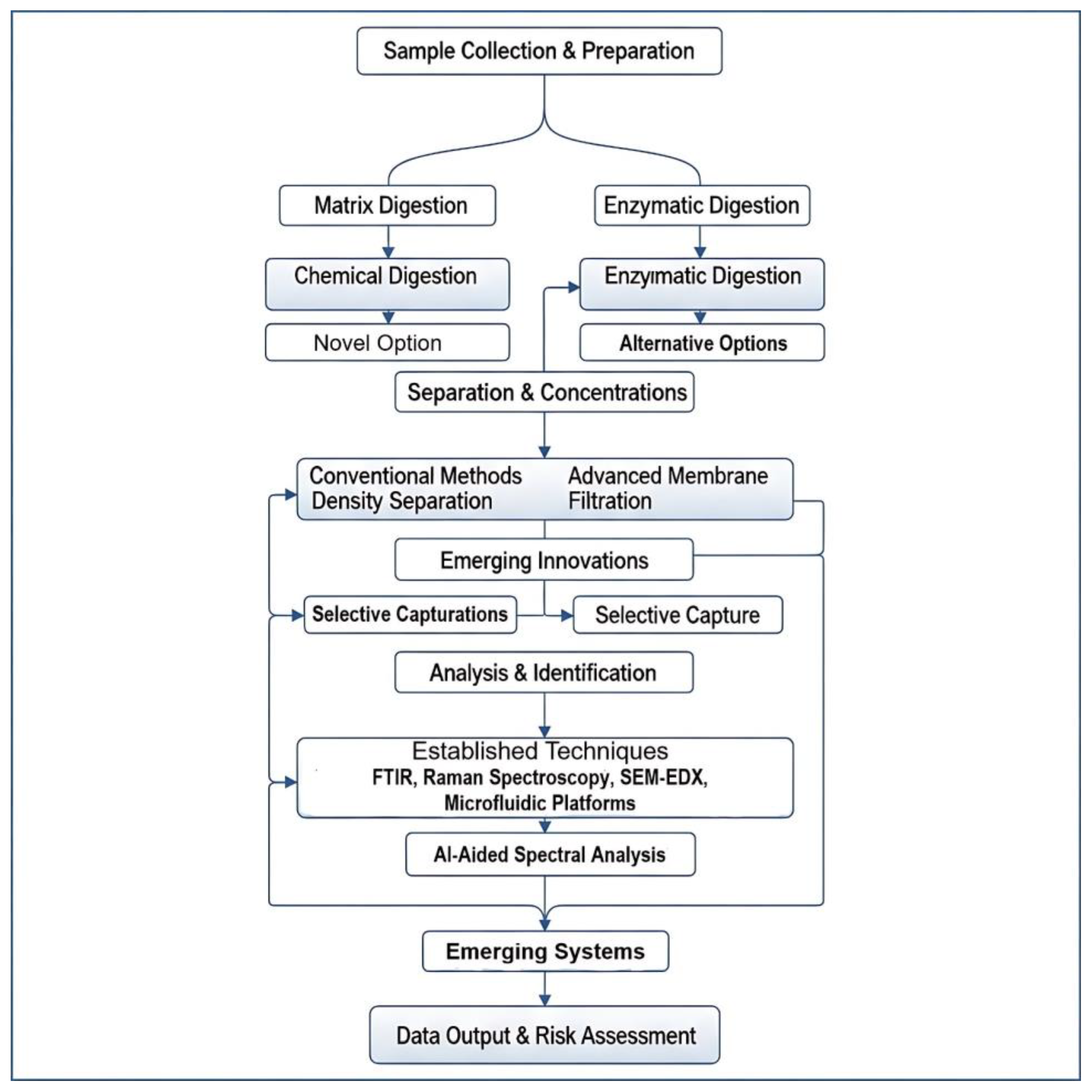
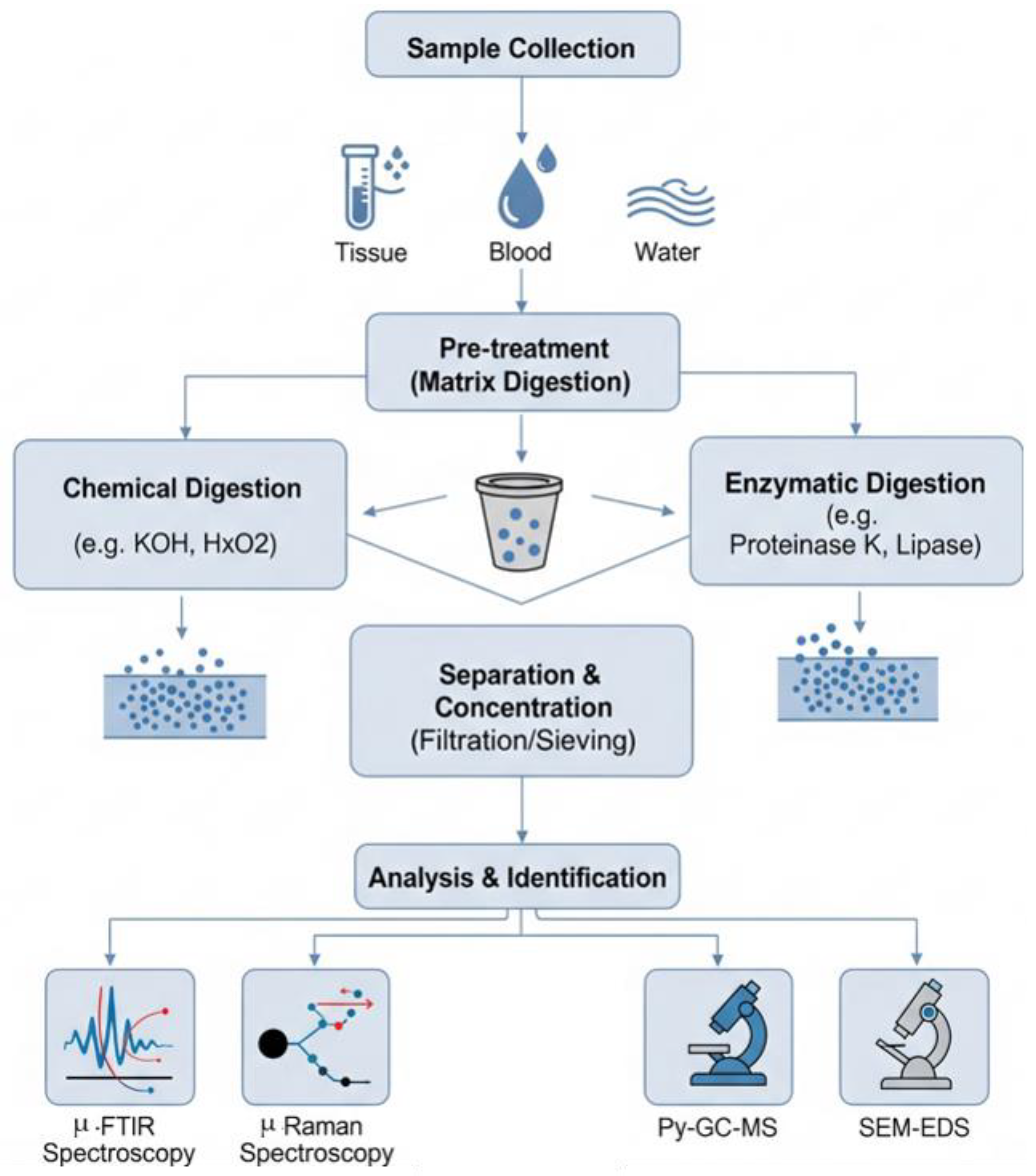
4. Current Separation and Pre-Treatment Techniques
4.1. Physical Separation: Filtration and Sieving
4.2. Density-Based Separation: Principles and Limitations
4.3. Chemical and Oxidative Digestion for Matrix Removal
4.4. Enzymatic Digestion: As a Selective Approach to Preserving Polymer Integrity
4.5. Quality Assurance and Quality Control (QA/QC) in Pre-Treatment
5. Analytical Techniques Post-Separation
5.1. Vibrational Spectroscopy: FTIR and Raman Microspectroscopy
5.2. Thermal Analysis: Pyrolysis–Gas Chromatography–Mass Spectrometry (Py-GC-MS)
5.3. Microscopic and Elemental Analysis: SEM-EDS
5.4. Emerging and Portable Detection Methods
6. Limitations, Trade-Offs, and Critical Gaps
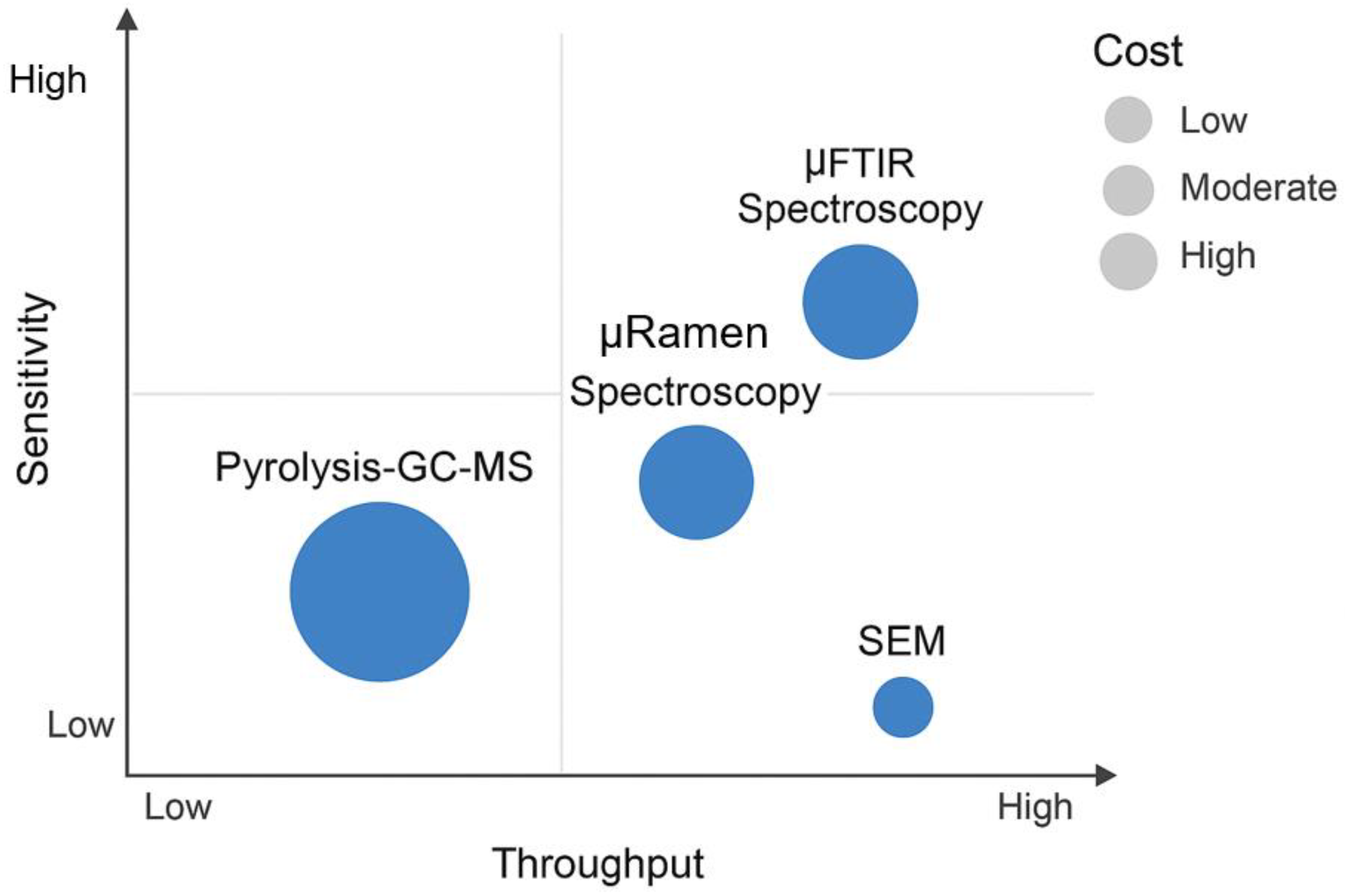
6.1. Balancing Sensitivity, Throughput and Cost in Microplastic Analysis
6.2. The Lack of Standardization: A Barrier to Comparative Science
6.3. The Nanoplastic Detection Gap
6.4. Contamination Control and Environmental Relevance
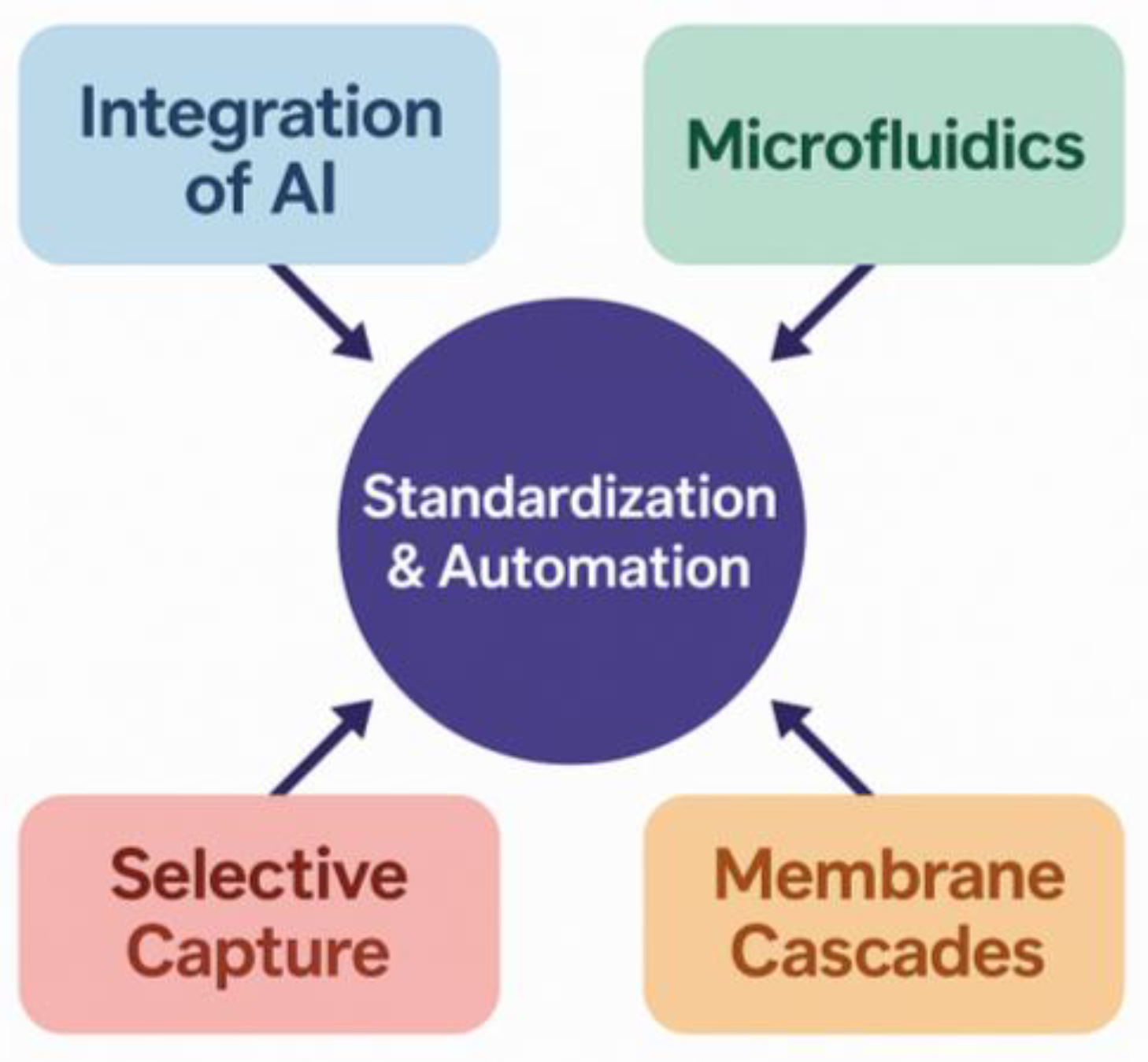
7. Innovations and Future Directions
7.1. Advanced Nanofiltration Based Membrane Technologies
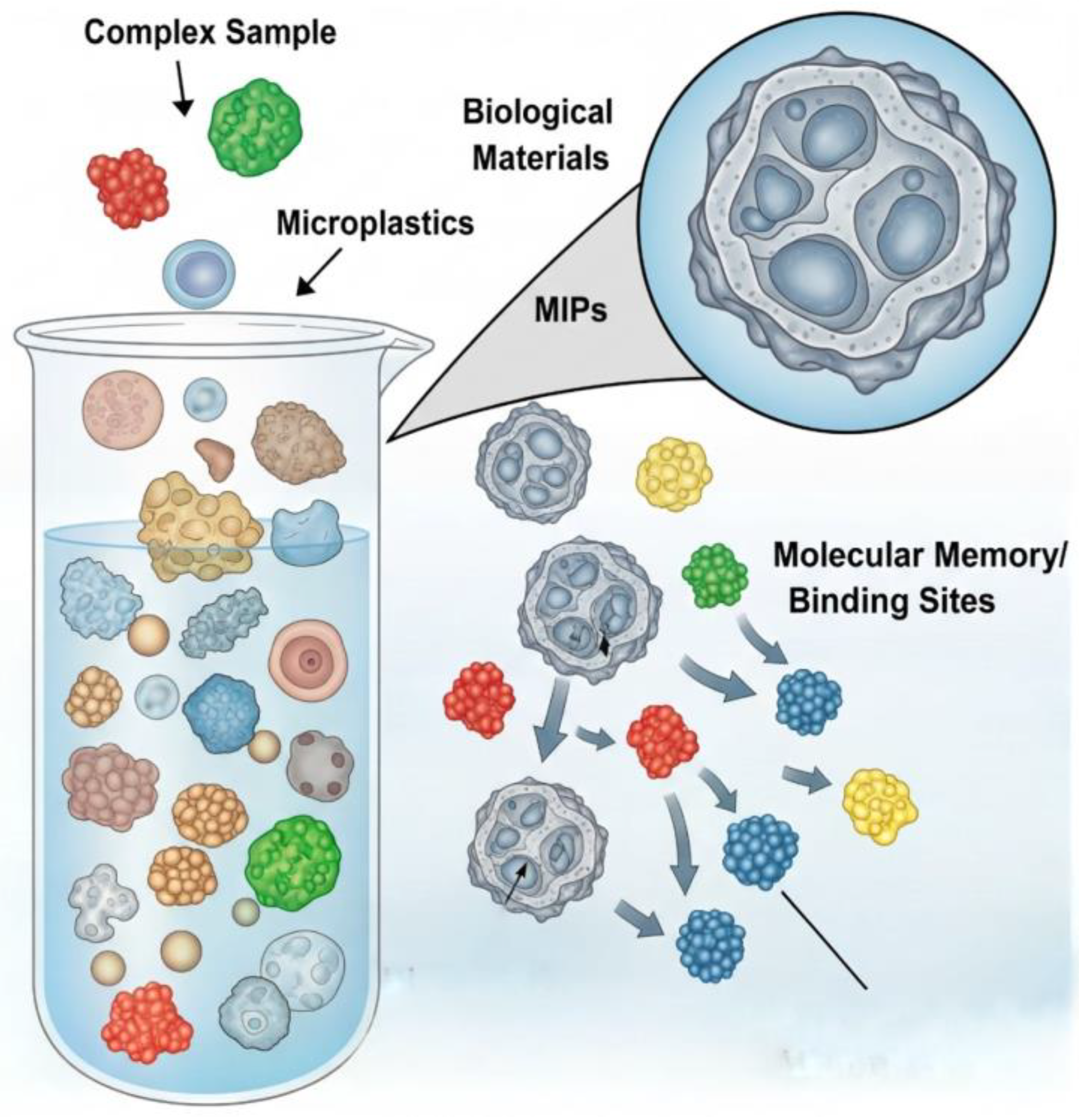
7.2. Smart Materials: Selective Binding with Molecularly Imprinted Polymers and Functionalized Adsorbents
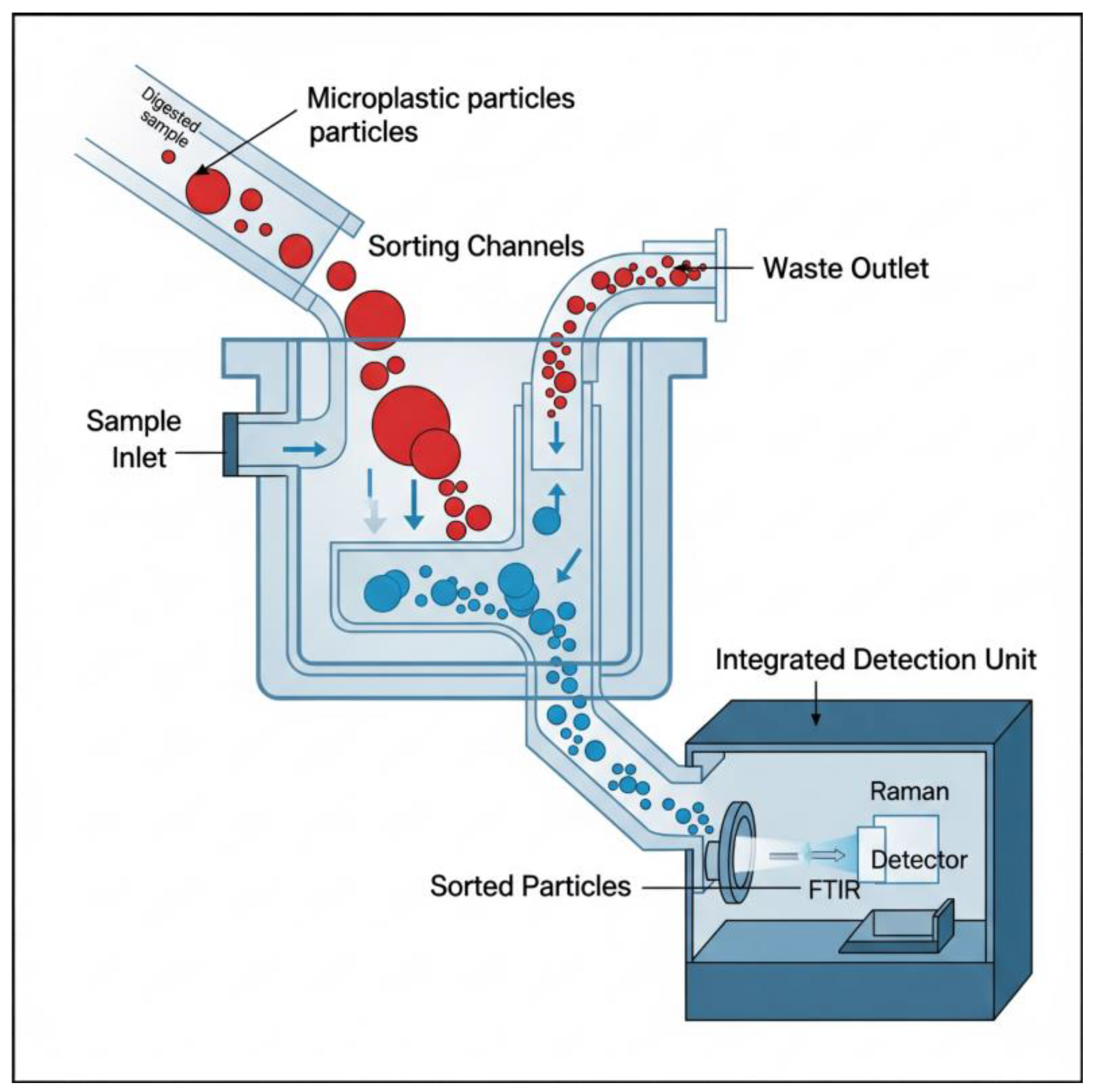
7.3. Process Intensification: The Role of Microfluidic Separation and Analysis Platforms
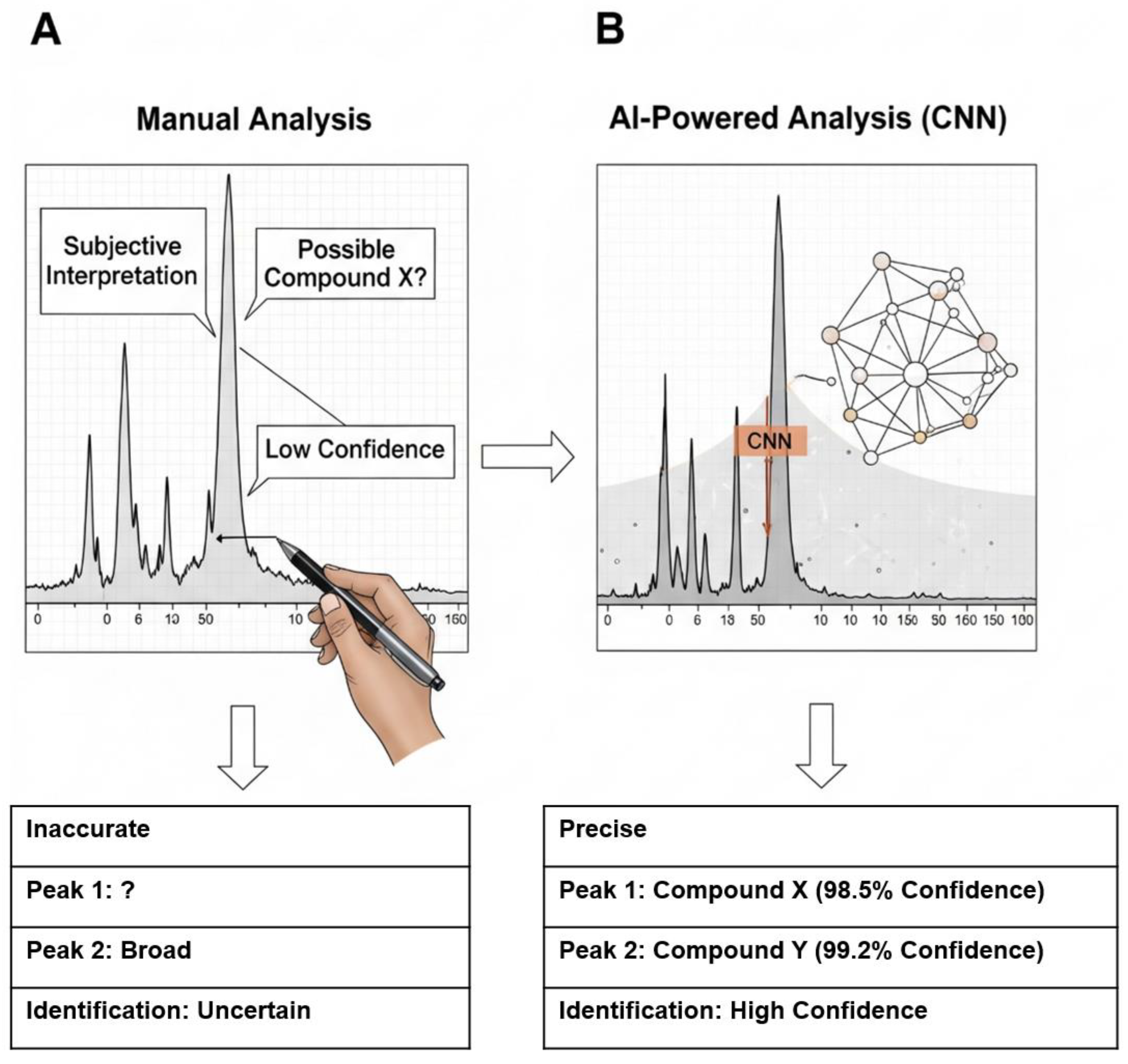
7.4. AI-Aided Spectral Classification and High-Throughput Analysis
7.5. Development of Integrated and Scalable Workflows for Advanced Microplastic Detection
8. Conclusion and Outlook
8.1. Synthesis of the Current State-of-the-Art
8.2. Advancing Microplastic Analysis Through Integrated Separation and Analytical Strategies
8.3. A Call for Multidisciplinary Research and Standardization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutkar, P.; Dhulap, V.; Girigosavi, S.; Deshmukh, S.; Desai, M. Microplastics in the Human Body: A Comprehensive Review of Exposure, Detection, and Health Risks. Wastewater Purif. 2025, 1, 20250317163738. [Google Scholar]
- Zhang, X.; Yu, C.; Wang, P.; Yang, C. Microplastics and Human Health: Unraveling the Toxicological Pathways and Implications for Public Health. Front. Public Health 2025, 13, 1567200. [Google Scholar] [CrossRef]
- Hoang, H.G.; Nguyen, N.S.H.; Zhang, T.; Tran, H.-T.; Mukherjee, S.; Naidu, R. A Review of Microplastic Pollution and Human Health Risk Assessment: Current Knowledge and Future Outlook. Front. Environ. Sci. 2025, 13, 1606332. [Google Scholar] [CrossRef]
- Rashid, S.; Majeed, L.R.; Mehta, N.; Radu, T.; Martín-Fabiani, I.; Bhat, M.A. Microplastics in Terrestrial Ecosystems: Sources, Transport, Fate, Mitigation, and Remediation Strategies. Euro-Mediterr. J. Environ. Integr. 2025, 10, 2633–2659. [Google Scholar] [CrossRef]
- Méndez Rodríguez, K.B.; Jiménez Avalos, J.A.; Fernández Macias, J.C.; González Palomo, A.K. Microplastics: Challenges of Assessment in Biological Samples and Their Implication for in Vitro and in Vivo Effects. Environ. Sci. Pollut. Res. 2023, 30, 119733–119749. [Google Scholar] [CrossRef] [PubMed]
- de Ruijter, V.N.; Redondo-Hasselerharm, P.E.; Gouin, T.; Koelmans, A.A. Quality Criteria for Microplastic Effect Studies in the Context of Risk Assessment: A Critical Review. Environ. Sci. Technol. 2020, 54, 11692–11705. [Google Scholar] [CrossRef]
- Gao, W.; Deng, X.-J.; Zhang, J.; Qi, L.; Zhao, X.-Q.; Zhang, P.-Y. Assessment of Quality Control Measures in the Monitoring of Microplastic: A Critical Review. Environ. Pollut. Bioavailab. 2023, 35, 2203349. [Google Scholar] [CrossRef]
- Roslan, N.S.; Lee, Y.Y.; Ibrahim, Y.S.; Anuar, S.T.; Lai, L.A.; Brentnall, T. Detection of Microplastics in Human Tissues and Organs: A Scoping Review. J. Glob. Health 2024, 14, 04179. [Google Scholar] [CrossRef]
- Bhat, M.A.; Janaszek, A. Delving into River Health: Unveiling Microplastic Intrusion and Heavy Metal Contamination in Freshwater. Discov. Environ. 2024, 2, 61. [Google Scholar] [CrossRef]
- Kim, E.-H.; Baek, S.M.; Park, H.J.; Bian, Y.; Chung, H.Y.; Bae, O.-N. Polystyrene Nanoplastics Promote the Blood-Brain Barrier Dysfunction through Autophagy Pathway and Excessive Erythrophagocytosis. Ecotoxicol. Environ. Saf. 2025, 289, 117471. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, H.; Niu, S.; Guo, M.; Xue, Y. Mechanisms of Micro- and Nanoplastics on Blood-Brain Barrier Crossing and Neurotoxicity: Current Evidence and Future Perspectives. Neurotoxicology 2025, 109, 92–107. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, S.J.; Hwang, D.Y.; Seo, S. Recent Purification Technologies and Human Health Risk Assessment of Microplastics. Materials 2020, 13, 5196. [Google Scholar] [CrossRef]
- Singh, B.; Srivastava, G.; Kala, D.; Gill, M.; Kaushal, A.; Gupta, S. Microplastics as an Emerging Threat to Human Health: Challenges and Advancements in Their Detection. Appl. Chem. Eng. 2023, 6, 2103. [Google Scholar] [CrossRef]
- Huang, M.; Si, C.; Qiu, C.; Wang, G. Microplastics Analysis: From Qualitative to Quantitative. Environ. Sci. Adv. 2024, 3, 1652–1668. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Jahedi, F.; Jaafarzadeh Haghighi Fard, N. Micro- and Nanoplastic Toxicity in Humans: Exposure Pathways, Cellular Effects, and Mitigation Strategies. Toxicol. Rep. 2025, 14, 102043. [Google Scholar] [CrossRef]
- Bhat, M.A. Indoor Microplastics and Microfibers Sources and Impacts on Human Health. In Microfibre Pollution from Textiles; CRC Press: Boca Raton, FL, USA, 2024; ISBN 9781003331995. [Google Scholar]
- Huang, T.; Liu, Y.; Wang, L.; Ruan, X.; Ge, Q.; Ma, M.; Wang, W.; You, W.; Zhang, L.; Valev, V.K.; et al. MPs Entering Human Circulation through Infusions: A Significant Pathway and Health Concern. Environ. Heal. 2025, 3, 551–559. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Devi, A.; Kadono, H.; Wang, Q.; Rabin, M.H. The Plastic Within: Microplastics Invading Human Organs and Bodily Fluids Systems. Environments 2023, 10, 194. [Google Scholar] [CrossRef]
- Dzierżyński, E.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Jóźwik, K.; Teresiński, G.; Forma, A.; Wdowiak, P.; Baj, J.; Flieger, J. Microplastics in the Human Body: Exposure, Detection, and Risk of Carcinogenesis: A State-of-the-Art Review. Cancers 2024, 16, 3703. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Vimalkumar, K. A Review of Human Exposure to Microplastics and Insights Into Microplastics as Obesogens. Front. Endocrinol. 2021, 12, 724989. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Fischer, E.K. Various Digestion Protocols Within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Carrillo-Barragan, P.; Sugden, H.; Scott, C.L.; Fitzsimmons, C. Enzymatic Digestion Method Development for Long-Term Stored Chitinaceous Planktonic Samples. Mar. Pollut. Bull. 2022, 179, 113691. [Google Scholar] [CrossRef]
- Shoopman, C.H.; Pan, X. Microplastics: A Review of Methodology for Sampling and Characterizing Environmental and Biological Samples BT. In Environmental Toxicology and Toxicogenomics: Principles, Methods, and Applications; Pan, X., Zhang, B., Eds.; Springer US: New York, NY, USA, 2021; pp. 339–359. ISBN 978-1-0716-1514-0. [Google Scholar]
- Zhao, S.; Zhu, L.; Gao, L.; Li, D. Chapter 2—Limitations for Microplastic Quantification in the Ocean and Recommendations for Improvement and Standardization. In Microplastic Contamination in Aquatic Environments; Zeng, E.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–49. ISBN 978-0-12-813747-5. [Google Scholar]
- Zhang, W.; Wang, Q.; Chen, H. Challenges in Characterization of Nanoplastics in the Environment. Front. Environ. Sci. Eng. 2021, 16, 11. [Google Scholar] [CrossRef]
- Wirnkor, V.; Ebere, E.; Evelyn Ngozi, V. Microplastics, an Emerging Concern: A Review of Analytical Techniques for Detecting and Quantifying Microplatics. Anal. Methods Environ. Chem. J. 2019, 2, 13–30. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Intercomparison Study on Commonly Used Methods to Determine Microplastics in Wastewater and Sludge Samples. Environ. Sci. Pollut. Res. 2019, 26, 12109–12122. [Google Scholar] [CrossRef]
- Philipp, M.; Bucheli, T.D.; Kaegi, R. The Use of Surrogate Standards as a QA/QC Tool for Routine Analysis of Microplastics in Sewage Sludge. Sci. Total Environ. 2022, 835, 155485. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in Environment: Global Concern, Challenges, and Controlling Measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. 9—Microplastic Separation Techniques. In Microplastic Pollutants; Crawford, C.B., Quinn, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–218. ISBN 978-0-12-809406-8. [Google Scholar]
- Rani, M.; Ducoli, S.; Depero, L.E.; Prica, M.; Tubić, A.; Ademovic, Z.; Morrison, L.; Federici, S. A Complete Guide to Extraction Methods of Microplastics from Complex Environmental Matrices. Molecules 2023, 28, 5710. [Google Scholar] [CrossRef]
- Prihandari, R.; Karnpanit, W.; Kittibunchakul, S.; Kemsawasd, V. Development of Optimal Digesting Conditions for Microplastic Analysis in Dried Seaweed Gracilaria Fisheri. Foods 2021, 10, 2118. [Google Scholar] [CrossRef]
- Courtene-Jones, W.; Quinn, B.; Murphy, F.; Gary, S.F.; Narayanaswamy, B.E. Optimisation of Enzymatic Digestion and Validation of Specimen Preservation Methods for the Analysis of Ingested Microplastics. Anal. Methods 2017, 9, 1437–1445. [Google Scholar] [CrossRef]
- Horton, A.A.; Cross, R.K.; Read, D.S.; Jürgens, M.D.; Ball, H.L.; Svendsen, C.; Vollertsen, J.; Johnson, A.C. Semi-Automated Analysis of Microplastics in Complex Wastewater Samples. Environ. Pollut. 2021, 268, 115841. [Google Scholar] [CrossRef]
- Li, K.; Gao, Y.; Zhang, Y.; Zheng, Y.; Li, G.; Zhang, L.; Wu, J.; Shi, Y.; Huo, M.; Wang, X. Establishment and Application of Standard Analysis Methods for Microplastic Samples: Urban Sewage and Sewage Sludge as a Source of Microplastics in the Environment. Environ. Res. 2025, 273, 121237. [Google Scholar] [CrossRef]
- ITRC Microplastics. Available online: https://mp-1.itrcweb.org/ (accessed on 8 August 2025).
- Geppner, L.; Karaca, J.; Wegner, W.; Rados, M.; Gutwald, T.; Werth, P.; Henjakovic, M. Testing of Different Digestion Solutions on Tissue Samples and the Effects of Used Potassium Hydroxide Solution on Polystyrene Microspheres. Toxics 2023, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Löder, M.G.J.; Imhof, H.K.; Ladehoff, M.; Löschel, L.A.; Lorenz, C.; Mintenig, S.; Piehl, S.; Primpke, S.; Schrank, I.; Laforsch, C.; et al. Enzymatic Purification of Microplastics in Environmental Samples. Environ. Sci. Technol. 2017, 51, 14283–14292. [Google Scholar] [CrossRef] [PubMed]
- Adelugba, A.; Emenike, C. Comparative Review of Instrumental Techniques and Methods for the Analysis of Microplastics in Agricultural Matrices. Microplastics 2024, 3, 1–21. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, J.; Song, D.; Jiang, J.; Jiang, Y. Recent Applications in Analytical Techniques of Microplastics. Crit. Rev. Anal. Chem. 2025, 1–21. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Insa, S.; Arxé, M.; Buttiglieri, G.; Rodríguez-Mozaz, S.; Barceló, D. Analysis of Microplastics in the Environment: Identification and Quantification of Trace Levels of Common Types of Plastic Polymers Using Pyrolysis-GC/MS. MethodsX 2023, 10, 102143. [Google Scholar] [CrossRef]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous Trace Identification and Quantification of Common Types of Microplastics in Environmental Samples by Pyrolysis-Gas Chromatography–Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef]
- Peel, B. Development and Application of a Py-GC/MS Method for the Quantification Of. Environ. Sci. Pollut. Res. 2017, 26, 292–298. [Google Scholar]
- Gregoris, E.; Gallo, G.; Rosso, B.; Piazza, R.; Corami, F.; Gambaro, A. Microplastics Analysis: Can We Carry out a Polymeric Characterisation of Atmospheric Aerosol Using Direct Inlet Py-GC/MS? J. Anal. Appl. Pyrolysis 2023, 170, 105903. [Google Scholar] [CrossRef]
- Mattonai, M.; Nardella, F.; Filomena, M.; Gesti, S.; Medaglia, G.; Ribechini, E. Quantification of Polyethylene in Biodegradable Plastics by Analytical Pyrolysis-Based Methods with GC Split Modulation. J. Anal. Appl. Pyrolysis 2025, 191, 107172. [Google Scholar] [CrossRef]
- Wenzel, M.; Schoettl, J.; Pruin, L.; Fischer, B.; Wolf, C.; Kube, C.; Renner, G.; Schram, J.; Schmidt, T.C.; Tuerk, J. Determination of Atmospherically Deposited Microplastics in Moss: Method Development and Performance Evaluation. Green Anal. Chem. 2023, 7, 100078. [Google Scholar] [CrossRef]
- Lou, F.; Wang, J.; Sima, J.; Lei, J.; Huang, Q. Mass Concentration and Distribution Characteristics of Microplastics in Landfill Mineralized Refuse Using Efficient Quantitative Detection Based on Py-GC/MS. J. Hazard. Mater. 2023, 459, 132098. [Google Scholar] [CrossRef] [PubMed]
- Makhtar, S.N.N.M.; Hamed, N.K.A.; Hamdan, M.A.H. bin 10—Morphological Analysis of Photocatalytic Membrane (SEM, FESEM, TEM). In Elsevier Series on Advanced Ceramic Materials; Othman, M.H.D., Rahman, M.A., Matsuura, T., Adam, M.R., Mohd Makhtar, S.N.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 221–238. ISBN 978-0-323-95418-1. [Google Scholar]
- Slusarski-Santana, V.; Panatto, M.L.; dos Passos, F.R.; Maestre, K.L.; Triques, C.C.; Fiorentin-Ferrari, L.D.; Fiorese, M.L. Methods for Micro- and Nanoplastics Analysis. In Toxic Effects of Micro- and Nanoplastics; Wiley: Hoboken, NJ, USA, 2024; pp. 415–469. ISBN 9781394238163. [Google Scholar]
- Williams, W.A.; Aravamudhan, S. Micro-Nanoparticle Characterization: Establishing Underpinnings for Proper Identification and Nanotechnology-Enabled Remediation. Polymers 2024, 16, 2837. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Wang, H. Analytical Methods for Microplastics in the Environment: A Review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef]
- Vazquez, Y.V.; Barbosa, S.E. Recycling of Polymer Nanocomposites. Plastic e-Waste Case Study. In Chemical Physics of Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2024; pp. 759–774. ISBN 9783527837021. [Google Scholar]
- Sargadillos, R.; Endoma Jr, L.; Nillos, M.G.; Yap, E.E. Nile Red Staining Technique for Microplastics Detection in Sardines (Sardinella Lemuru) Using Fluorescence Stereomicroscopy. Philipp. J. Sci. 2025, 154, 475–488. [Google Scholar]
- Rermborirak, K.; Nanuan, P.; Komonpan, P.; Sukpancharoen, S. Low-Cost Portable Microplastic Detection System Integrating Nile Red Fluorescence Staining with YOLOv8-Based Deep Learning. J. Hazard. Mater. Adv. 2025, 19, 100787. [Google Scholar] [CrossRef]
- Peinador, R.I.; H.P., P.T.; Calvo, J.I. Innovative Application of Nile Red (NR)-Based Dye for Direct Detection of Micro and Nanoplastics (MNPs) in Diverse Aquatic Environments. Chemosphere 2024, 362, 142609. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef]
- Rivera-Rivera, D.M.; Quintanilla-Villanueva, G.E.; Luna-Moreno, D.; Sánchez-Álvarez, A.; Rodríguez-Delgado, J.M.; Cedillo-González, E.I.; Kaushik, G.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M.M. Exploring Innovative Approaches for the Analysis of Micro- and Nanoplastics: Breakthroughs in (Bio)Sensing Techniques. Biosensors 2025, 15, 44. [Google Scholar] [CrossRef]
- Lin, L.L.; Alvarez-Puebla, R.; Liz-Marzán, L.M.; Trau, M.; Wang, J.; Fabris, L.; Wang, X.; Liu, G.; Xu, S.; Han, X.X.; et al. Surface-Enhanced Raman Spectroscopy for Biomedical Applications: Recent Advances and Future Challenges. ACS Appl. Mater. Interfaces 2025, 17, 16287–16379. [Google Scholar] [CrossRef] [PubMed]
- Khonina, S.N.; Kazanskiy, N.L. Trends and Advances in Wearable Plasmonic Sensors Utilizing Surface-Enhanced Raman Spectroscopy (SERS): A Comprehensive Review. Sensors 2025, 25, 1367. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, A. Advances in Microplastics Detection: A Comprehensive Review of Methodologies and Their Effectiveness. TrAC Trends Anal. Chem. 2024, 170, 117440. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, X.; Li, L.; Song, W.; Dong, D.; Yue, X.; Chen, S.; Zeng, Q. Research on Rapid and Non-Destructive Detection of Coffee Powder Adulteration Based on Portable Near-Infrared Spectroscopy Technology. Foods 2025, 14, 536. [Google Scholar] [CrossRef]
- di Luca, C.; Garcia, J.; Munoz, M.; Hernando-Pérez, M.; de Pedro, Z.M.; Casas, J.A. Strategies for the Quantification and Characterization of Nanoplastics in AOPs Research. Chem. Eng. J. 2024, 493, 152490. [Google Scholar] [CrossRef]
- Vohl, S.; Kristl, M.; Stergar, J. Harnessing Magnetic Nanoparticles for the Effective Removal of Micro- and Nanoplastics: A Critical Review. Nanomaterials 2024, 14, 1179. [Google Scholar] [CrossRef]
- Kılıç, B.I.; Üstün, G.E.; Can, T. Characteristics, and Seasonal Change of Microplastics in Organized Industrial Zone Wastewater Treatment Plant. J. Environ. Chem. Eng. 2025, 13, 115516. [Google Scholar] [CrossRef]
- Sarvalkar, P.D.; Prasad, N.; Prasad, S.R.; Prasad, R.B.; Desai, C.B.; Vaidya, A.K.; Teli, S.B.; Saxena, M.; Kale, V.B.; Pandey, R.S.; et al. A Review on Spectroscopic Techniques for Analysis of Nanomaterials and Biomaterials. ES Energy Environ. 2025, 27, 1264. [Google Scholar]
- Jani, V.; Wu, S.; Venkiteshwaran, K. Advancements and Regulatory Situation in Microplastics Removal from Wastewater and Drinking Water: A Comprehensive Review. Microplastics 2024, 3, 98–123. [Google Scholar] [CrossRef]
- Tan, S. Convex Optimization of Environmental Processes; University of Toronto (Canada): Toronto, ON, Canada; Ontario, CA, USA, 2024. [Google Scholar]
- Belz, S.; Cella, C.; Geiss, O.; Gilliland, D.; La Spina, R.; Mėhn, D.; Sokull-Kluettgen, B. Analytical Methods to Measure Microplastics in Drinking Water; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- Wenzel, M. Development of Sustainable Sample Preparation Methods for Microplastic Analysis. Doctoral Dissertation, Universität Duisburg-Essen, Duisburg, Germany, 2025. [Google Scholar]
- Laycock, A.; Clark, N.J.; Clough, R.; Smith, R.; Handy, R.D. Determination of Metallic Nanoparticles in Biological Samples by Single Particle ICP-MS: A Systematic Review from Sample Collection to Analysis. Environ. Sci. Nano 2022, 9, 420–453. [Google Scholar] [CrossRef]
- Lv, L.; Yan, X.; Feng, L.; Jiang, S.; Lu, Z.; Xie, H.; Sun, S.; Chen, J.; Li, C. Challenge for the Detection of Microplastics in the Environment. Water Environ. Res. 2021, 93, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Seifert, S.; Gärber, F. SERS Microscopy as a Tool for Comprehensive Biochemical Characterization in Complex Samples. Chem. Soc. Rev. 2024, 53, 7641–7656. [Google Scholar] [CrossRef]
- Chandra, A.; Kumar, V.; Garnaik, U.C.; Dada, R.; Qamar, I.; Goel, V.K.; Agarwal, S. Unveiling the Molecular Secrets: A Comprehensive Review of Raman Spectroscopy in Biological Research. ACS Omega 2024, 9, 50049–50063. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Hartmann, N.B.; Drobne, D.; Potthoff, A.; Kühnel, D. Quality of Nanoplastics and Microplastics Ecotoxicity Studies: Refining Quality Criteria for Nanomaterial Studies. J. Hazard. Mater. 2021, 415, 125751. [Google Scholar] [CrossRef]
- Gong, B.; Qiu, H.; Romero-Freire, A.; Van Gestel, C.A.M.; He, E. Incorporation of Chemical and Toxicological Availability into Metal Mixture Toxicity Modeling: State of the Art and Future Perspectives. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1730–1772. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Gaga, E.O.; Gedik, K. How Can Contamination Be Prevented during Laboratory Analysis of Atmospheric Samples for Microplastics? Environ. Monit. Assess. 2024, 196, 159. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.C.; Kutralam-Muniasamy, G. Blanks and Bias in Microplastic Research: Implications for Future Quality Assurance. Trends Environ. Anal. Chem. 2023, 38, e00203. [Google Scholar] [CrossRef]
- Wong, C.S.; Weisberg, S.B. Development of an Accreditation Process for Analytical Methods to Measure Microplastics in Drinking Water for Regulatory Monitoring. Chemosphere 2024, 353, 141568. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kong, F.; Li, X.; Shen, J. Artificial Intelligence in Microplastic Detection and Pollution Control. Environ. Res. 2024, 262, 119812. [Google Scholar] [CrossRef]
- Azizi, A.; Bottaro, C.S. A Critical Review of Molecularly Imprinted Polymers for the Analysis of Organic Pollutants in Environmental Water Samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Wang, D.; Yang, Y.; Duan, Y.; Ma, L.; Lin, T.; Liu, H. A Review on Molecularly Imprinted Polymers Preparation by Computational Simulation-Aided Methods. Polymers 2021, 13, 2657. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly Imprinted Polymer Micro- and Nano-Particles: A Review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef]
- Kaya, S.I.; Cetinkaya, A.; Ozkan, S.A. Molecularly Imprinted Polymers as Highly Selective Sorbents in Sample Preparation Techniques and Their Applications in Environmental Water Analysis. Trends Environ. Anal. Chem. 2023, 37, e00193. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhou, Y.; Zhang, X.; Liu, X. Artificial Intelligence-Based Microfluidic Platform for Detecting Contaminants in Water: A Review. Sensors 2024, 24, 4350. [Google Scholar] [CrossRef]
- Daoutakou, M.; Kintzios, S. Biosensors for Micro- and Nanoplastics Detection: A Review. Chemosensors 2025, 13, 143. [Google Scholar] [CrossRef]
- Khanam, M.M.; Uddin, M.K.; Kazi, J.U. Advances in Machine Learning for the Detection and Characterization of Microplastics in the Environment. Front. Environ. Sci. 2025, 13, 1573579. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, G.-W.; Jeong, C.W.; Kim, H.T.; Choi, M.J.; Park, S.B.; Lee, T.-J.; Kim, H.J. Raman Spectroscopy and One-Dimensional Convolutional Neural Networks for the Forensic Identification of Red Stamp Inks in Questioned Documents. J. Raman Spectrosc. 2025. [Google Scholar] [CrossRef]
- Villegas-Camacho, O.; Francisco-Valencia, I.; Alejo-Eleuterio, R.; Granda-Gutiérrez, E.E.; Martínez-Gallegos, S.; Villanueva-Vásquez, D. FTIR-Based Microplastic Classification: A Comprehensive Study on Normalization and ML Techniques. Recycling 2025, 10, 46. [Google Scholar] [CrossRef]
- Hufnagl, B.; Stibi, M.; Martirosyan, H.; Wilczek, U.; Möller, J.N.; Löder, M.G.J.; Laforsch, C.; Lohninger, H. Computer-Assisted Analysis of Microplastics in Environmental Samples Based on ΜFTIR Imaging in Combination with Machine Learning. Environ. Sci. Technol. Lett. 2022, 9, 90–95. [Google Scholar] [CrossRef]
- Brandt, J.; Mattsson, K.; Hassellöv, M. Deep Learning for Reconstructing Low-Quality FTIR and Raman Spectra─A Case Study in Microplastic Analyses. Anal. Chem. 2021, 93, 16360–16368. [Google Scholar] [CrossRef]
- Bang, R.S.; Bergman, M.; Li, T.; Mukherjee, F.; Alshehri, A.S.; Abbott, N.L.; Crook, N.C.; Velev, O.D.; Hall, C.K.; You, F. An Integrated Chemical Engineering Approach to Understanding Microplastics. AIChE J. 2023, 69, e18020. [Google Scholar] [CrossRef]
- Bacha, A.-U.-R.; Nabi, I.; Zhang, L. Mechanisms and the Engineering Approaches for the Degradation of Microplastics. ACS EST Eng. 2021, 1, 1481–1501. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Zhang, Z. A Critical Review on Artificial Intelligence—Based Microplastics Imaging Technology: Recent Advances, Hot-Spots and Challenges. Int. J. Environ. Res. Public Health 2023, 20, 1150. [Google Scholar] [CrossRef] [PubMed]
| Exposure Pathway | Common Polymer Types | Typical Particle Sizes and Morphologies | Primary Sources |
|---|---|---|---|
| Ingestion | Polyethylene (PE), Polypropylene (PP), Polystyrene (PS), Polyethylene terephthalate (PET), Polyvinyl chloride (PVC) [12]. | Wide range (10 µm–5 mm) [21]. Fragments and spheres are common. Particles ≤10–20 µm are most likely to translocate [22]. | Contaminated drinking/bottled water, seafood, salt, sugar, food packaging, take-out containers, baby bottles, agricultural soil contamination [3]. |
| Inhalation | Polyester (PET), Nylon (PA), Acrylic, Polyvinyl chloride (PVC), Styrene–butadiene rubber (from tires) [21]. | Primarily fibers and small fragments. Aerodynamic diameter determines deposition; smaller particles (<10 µm) reach deep lung tissue [17]. | Synthetic textiles, clothing, carpets, tire and road wear particles, industrial emissions, waste incineration [13]. |
| Dermal/Systemic | Varied, including PE, PVC, PET from medical devices. | Varied. Particles from 1 µm to 62 µm detected in IV fluids [19]. | Cosmetics, synthetic clothing (dermal). Intravenous (IV) bags, catheters, and other medical devices (systemic) [19]. |
| Method | Principle of Action | Typical Digestion Efficiency | Polymer Compatibility/Integrity | Pros | Cons |
|---|---|---|---|---|---|
| Alkaline Digestion (e.g., 10% KOH) | Saponification of lipids and hydrolysis of proteins. | High (>90%) for many tissues [40]. | Poor. Known to degrade or damage sensitive polymers like PET, PC, and cellulose acetate [24]. | Fast, low-cost, highly effective for fatty tissues [40]. | Destructive to certain key polymers, leading to biased results [24]. |
| Oxidative Digestion (e.g., 30% H2O2, Fenton) | Radical oxidation of organic matter. | High (93–96%) [35]. | Moderate. Generally safer than strong acids/bases, but can damage polymers like PA at elevated temperatures [24]. | Effective on a wide range of organic materials; relatively inexpensive [35]. | Can be aggressive; requires careful temperature control; safety concerns with concentrated reagents [24]. |
| Acid Digestion (e.g., HNO3) | Strong acid hydrolysis and oxidation of all organic matter. | Very High (>95%) [24]. | Poor. Highly destructive to many polymers, particularly PA [24]. | Extremely effective at removing organic matrix. | Highly corrosive and hazardous; significant risk of polymer degradation [24]. |
| Enzymatic Digestion (e.g., Proteinase K, Trypsin, Lipase) | Specific catalytic hydrolysis of target macromolecules (proteins, lipids). | Moderate to High (60–97%) depending on enzyme cocktail and matrix [35]. | Excellent. Generally non-destructive and preserves the integrity of most synthetic polymers [35]. | Highly specific and gentle on polymers; minimizes analytical artifacts [41]. | Slow (hours to days); expensive; may require multiple enzymes for complex matrices; digestion may be incomplete [25]. |
| Technique | Principle | Information Provided | Lower Size Limit | Strengths | Weaknesses |
|---|---|---|---|---|---|
| µ-FTIR Spectroscopy | Infrared light absorption by molecular bonds. | Polymer type, size, shape, chemical imaging. | ~10–20 µm (Diffraction-limited) [63]. | Robust, fast mapping with FPA detectors, extensive spectral libraries, non-destructive [64]. | Poor spatial resolution for small MPs/NPs; water interference; sample must be dry [65]. |
| µ-Raman Spectroscopy | Inelastic scattering of laser light by molecular bonds. | Polymer type, size, shape, chemical imaging. | ~1 µm (can be <500 nm with advanced methods) [29]. | High spatial resolution for small MPs/NPs; non-destructive; minimal water interference [66]. | Fluorescence interference from biological residues/dyes; can be slower for large area mapping; potential for sample heating [27]. |
| Py-GC-MS | Thermal decomposition followed by chromatographic separation and mass analysis. | Polymer type (mass concentration), additives. | No particle size limit (mass-based). | High chemical specificity; excellent for quantification of polymer mass; can analyze complex mixtures [67]. | Destructive (loses all morphological data); low throughput for particle analysis; potential for overlapping pyrolysis products [29]. |
| SEM-EDS | Electron beam imaging and characteristic X-ray emission. | High-resolution morphology, surface texture, size, elemental composition. | Nanometer-scale imaging. | Unparalleled imaging resolution; provides detailed physical characterization and degradation state; identifies inorganic additives [68]. | Does not identify polymer structure; requires conductive sample (may need coating); quantitative analysis is complex [54]. |
| Emerging Technology | Core Principle | Key Limitation Addressed | Potential Impact |
|---|---|---|---|
| AI-Aided Spectroscopy | Automated Process Control/Data Science | Low throughput, human error, and subjectivity in spectral analysis. | Fully automated, objective, and high-throughput polymer identification with high accuracy; analysis of weathered/complex particles [90]. |
| Microfluidic Platforms | Process Intensification/Transport Phenomena | Large sample/reagent volumes, low throughput, high risk of contamination from manual handling. | Miniaturized, integrated “sample-to-answer” systems that automate separation, staining, and detection, increasing speed and reducing costs [88]. |
| Smart Adsorbents (e.g., MIPs) | Materials Science/Surface Chemistry | Poor selectivity of separation; persistent interference from complex biological matrices. | Highly selective pre-concentration of target polymers, enabling cleaner samples for analysis and reducing matrix effects [84]. |
| Advanced Mass Spectrometry (e.g., SRS, ToF-SIMS) | Advanced Analytical Instrumentation/Optics | Inability to detect and chemically identify individual nanoplastics in complex samples. | Routine chemical imaging and identification of particles < 1 µm, closing the critical nanoplastic detection gap [83]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.L.; Zaidi, A.A. Separation and Detection of Microplastics in Human Exposure Pathways: Challenges, Analytical Techniques, and Emerging Solutions. J. Xenobiot. 2025, 15, 154. https://doi.org/10.3390/jox15050154
Khan AL, Zaidi AA. Separation and Detection of Microplastics in Human Exposure Pathways: Challenges, Analytical Techniques, and Emerging Solutions. Journal of Xenobiotics. 2025; 15(5):154. https://doi.org/10.3390/jox15050154
Chicago/Turabian StyleKhan, Asim Laeeq, and Asad A. Zaidi. 2025. "Separation and Detection of Microplastics in Human Exposure Pathways: Challenges, Analytical Techniques, and Emerging Solutions" Journal of Xenobiotics 15, no. 5: 154. https://doi.org/10.3390/jox15050154
APA StyleKhan, A. L., & Zaidi, A. A. (2025). Separation and Detection of Microplastics in Human Exposure Pathways: Challenges, Analytical Techniques, and Emerging Solutions. Journal of Xenobiotics, 15(5), 154. https://doi.org/10.3390/jox15050154







