Silica Nanoparticles Induced Epithelial–Mesenchymal Transition in BEAS-2B Cells via ER Stress and SIRT1/HSF1/HSPs Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Preparation and Characterization of SiNPs
2.3. Cell Culture, Treatment, and Transfection
2.4. Observation of Endoplasmic Reticulum by TEM
2.5. BEAS-2B Cells Viability Analysis
2.6. Immunofluorescence Analysis
2.7. Western Blot Analysis
2.8. Immunoprecipitation Analysis
2.9. RNA Extraction and Real-Time PCR
2.10. Data Analysis
3. Results
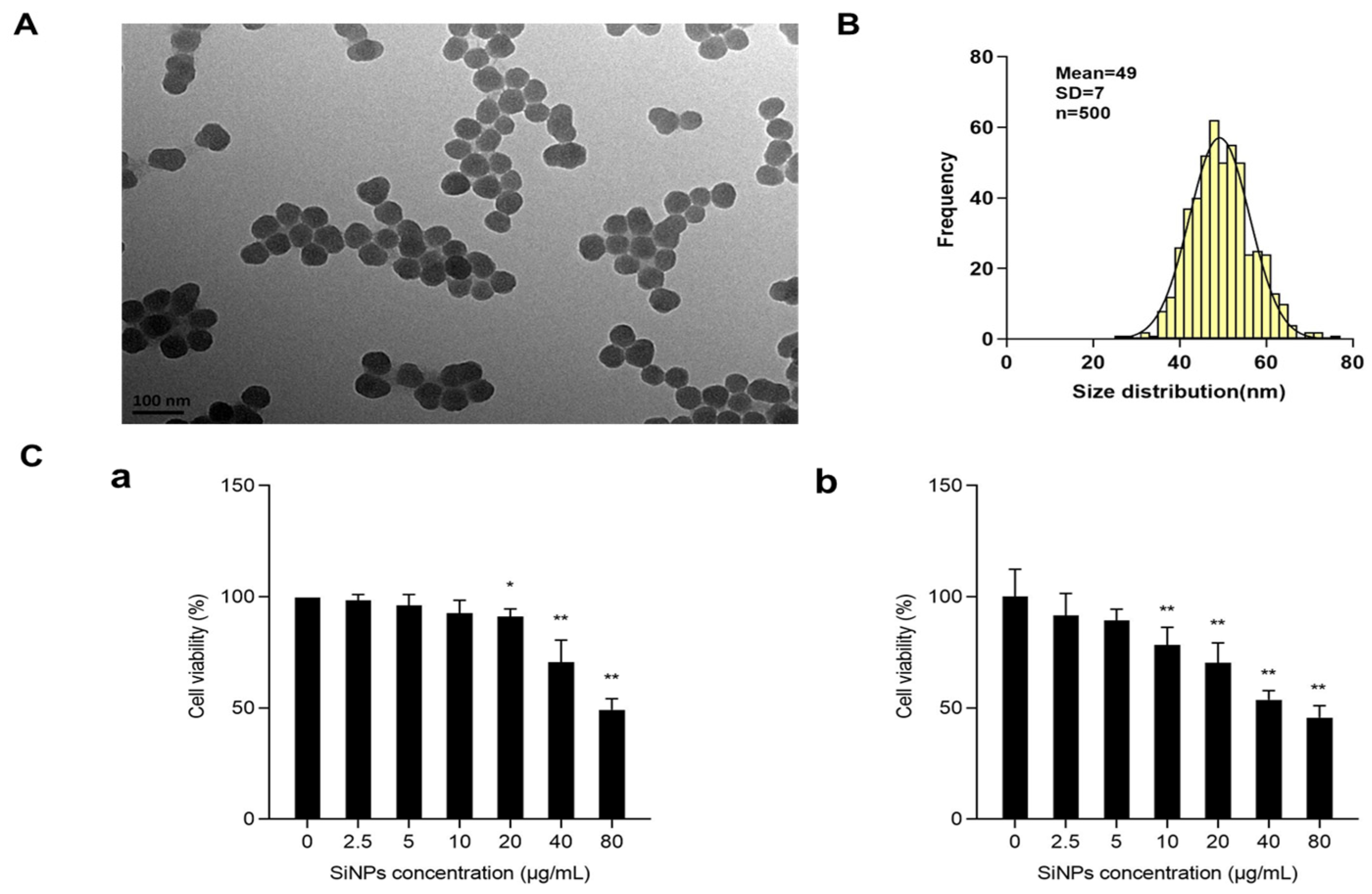
3.1. Characterization and Cytotoxicity of SiNPs
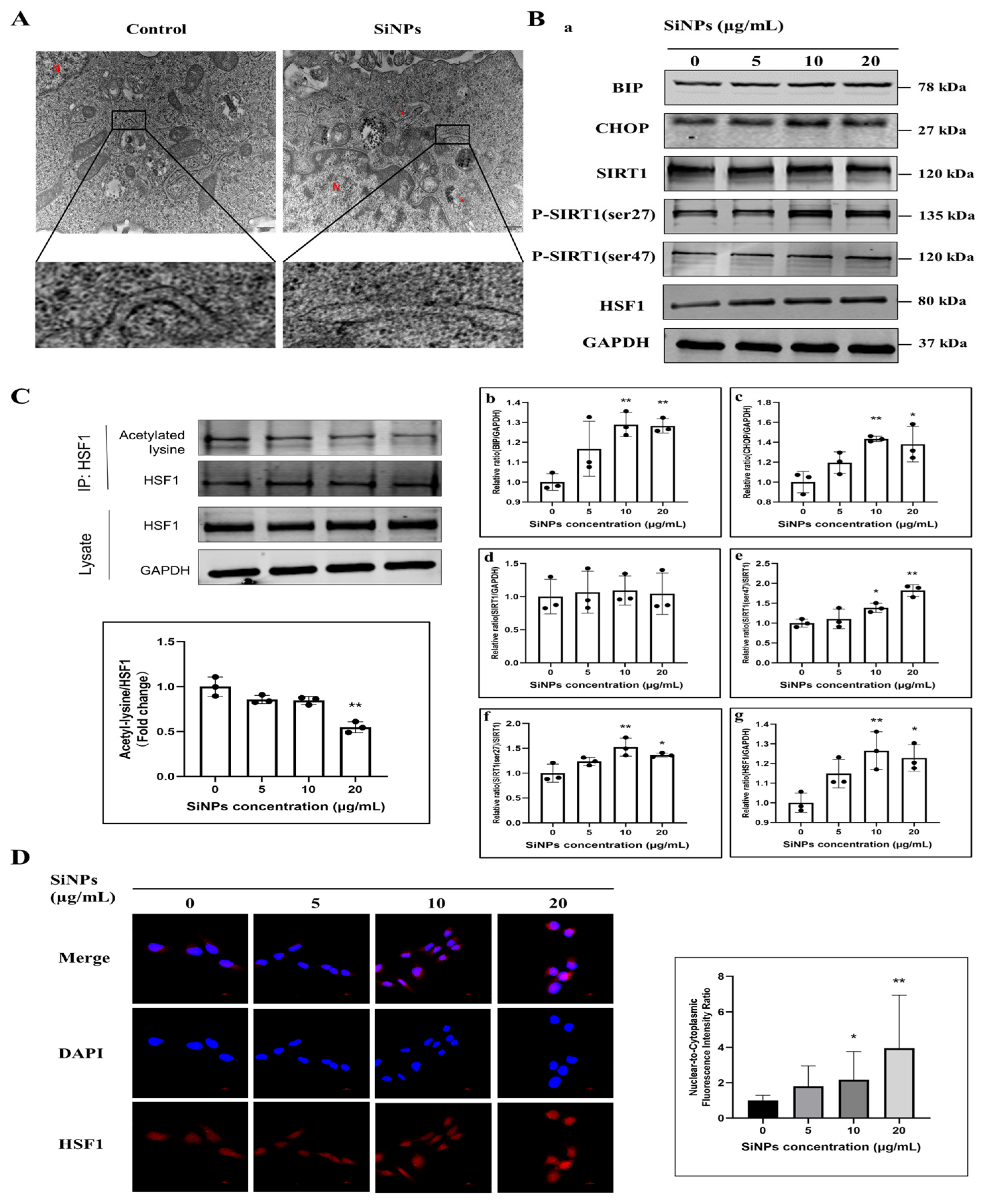
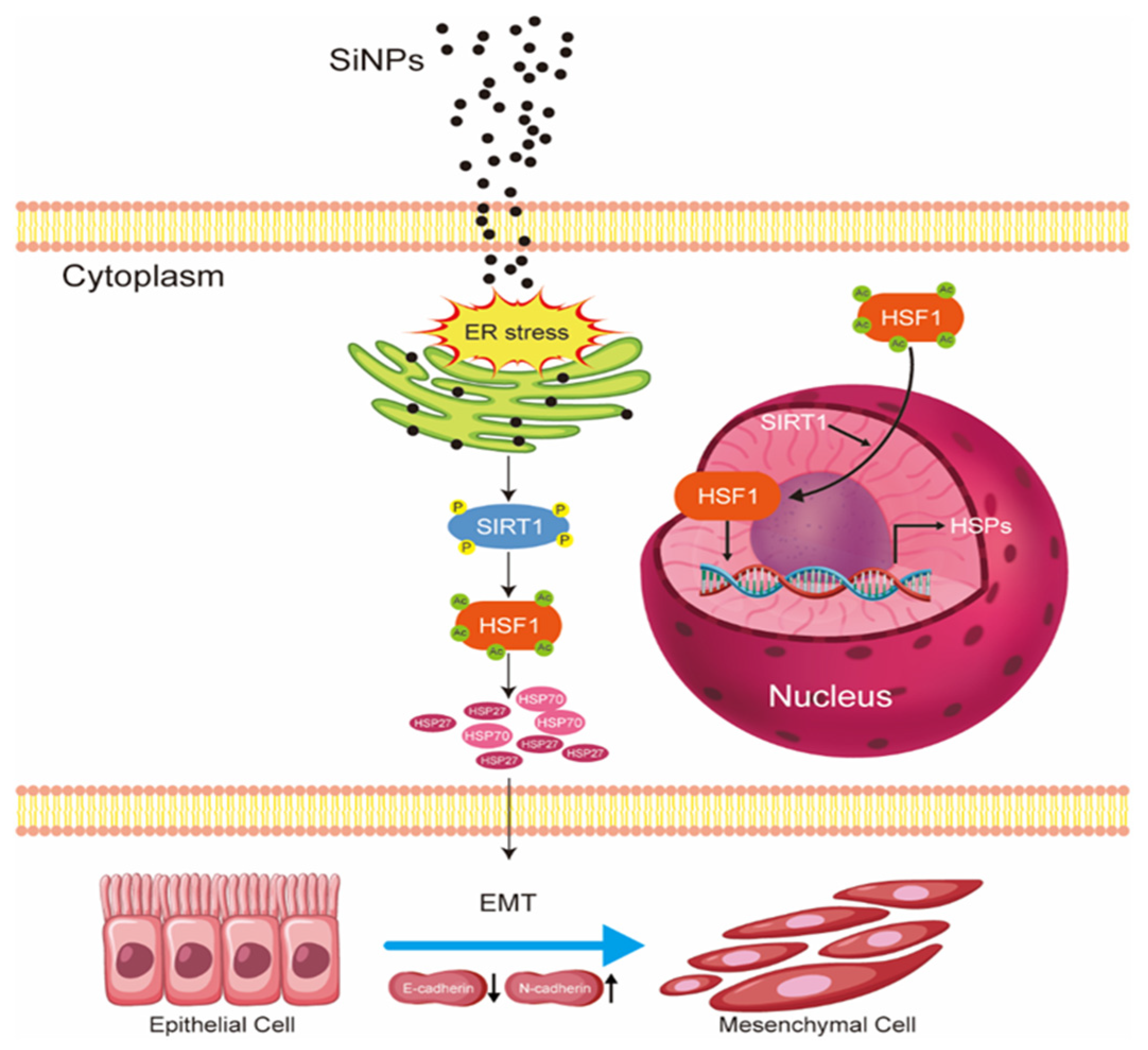
3.2. SiNPs Induced ER Stress and Activated the SIRT1/HSF1 Signaling Pathway
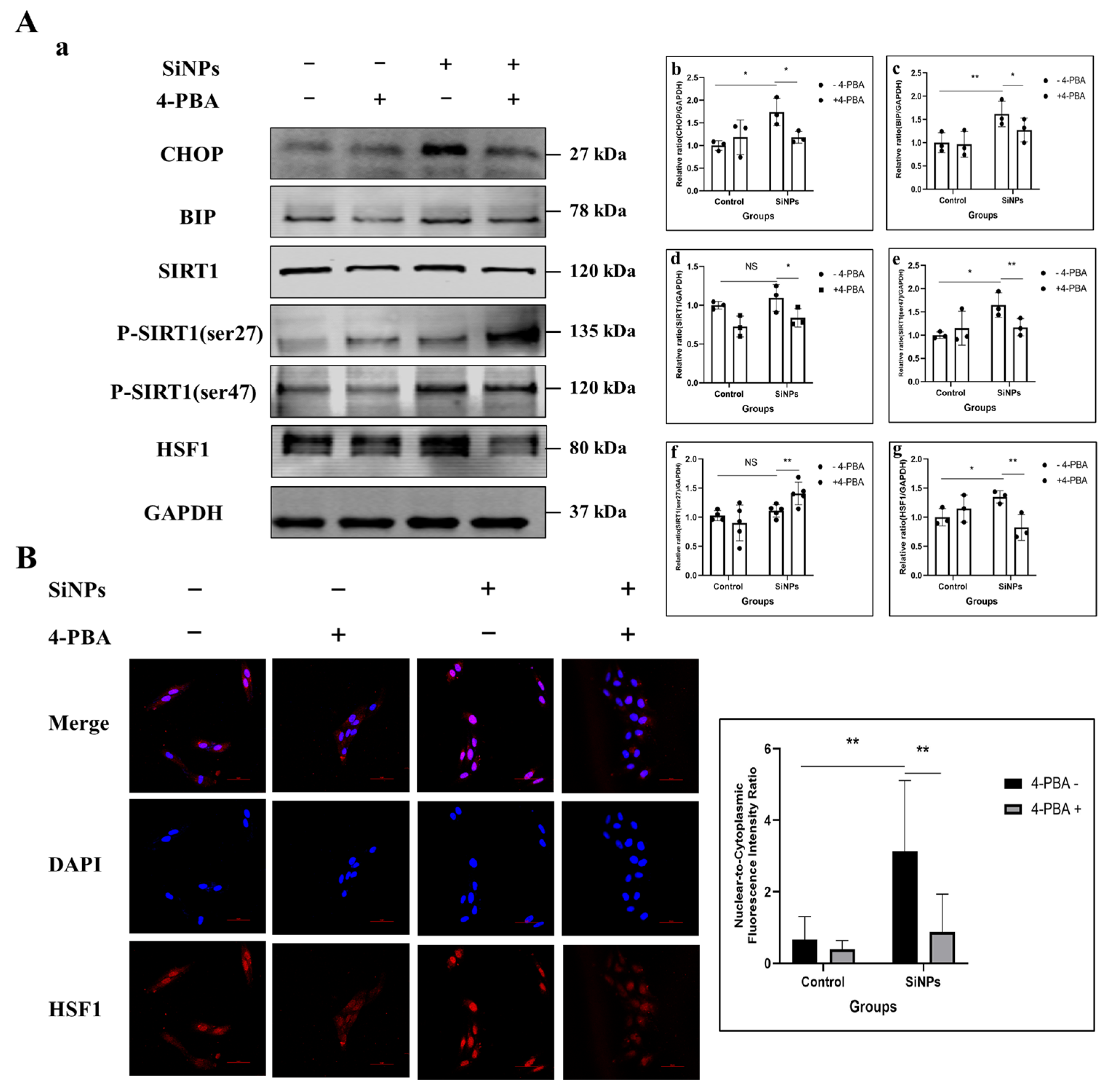
3.3. 4-PBA Inhibitied the ER Stress and Further SIRT1/HSF1 Signaling Pathway Activation of BEAS-2B Cells Induced by SiNPs
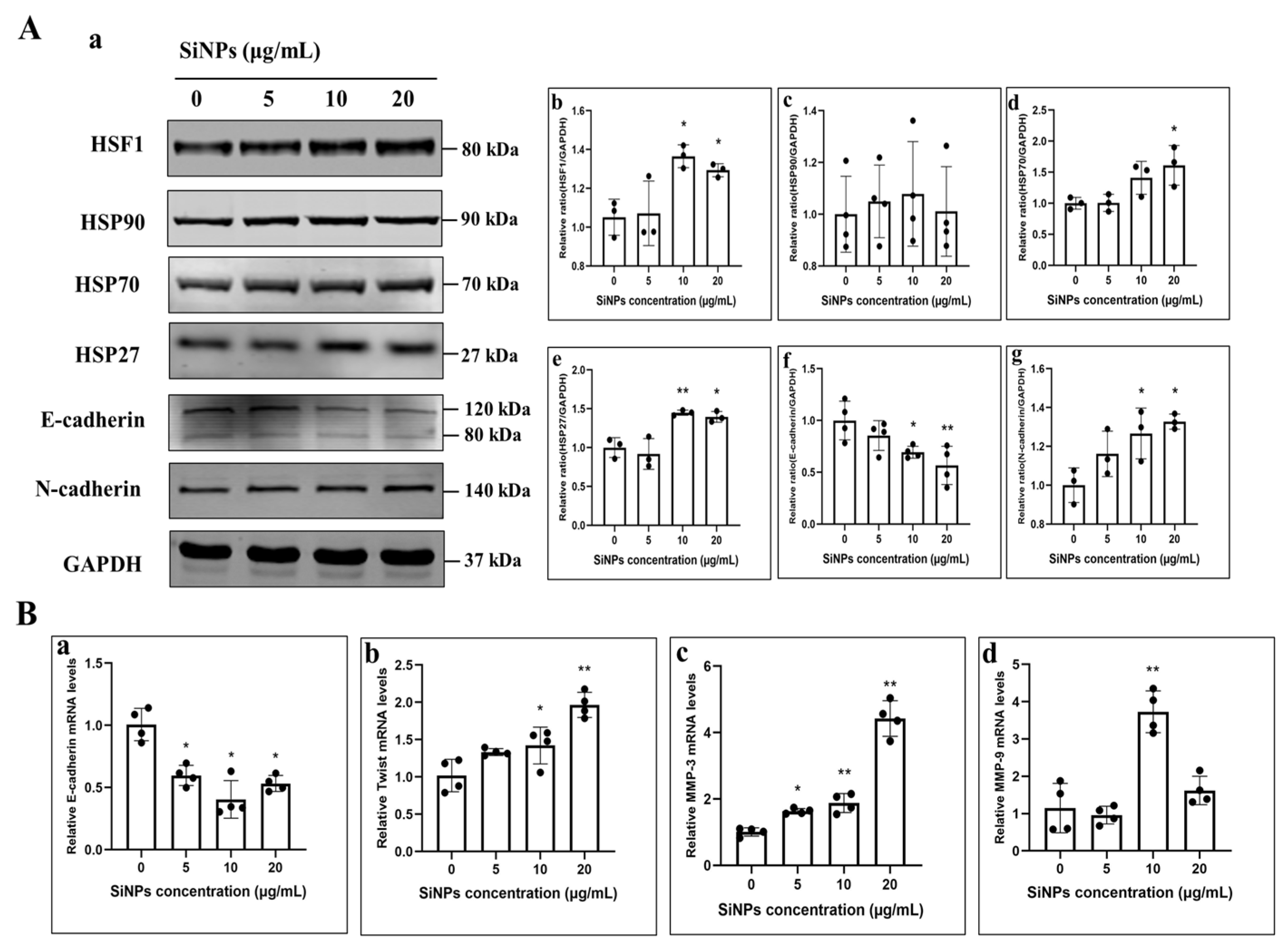
3.4. SiNPs Activated the HSF1/HSPs Signaling Pathway and Induced EMT in BEAS-2B Cells
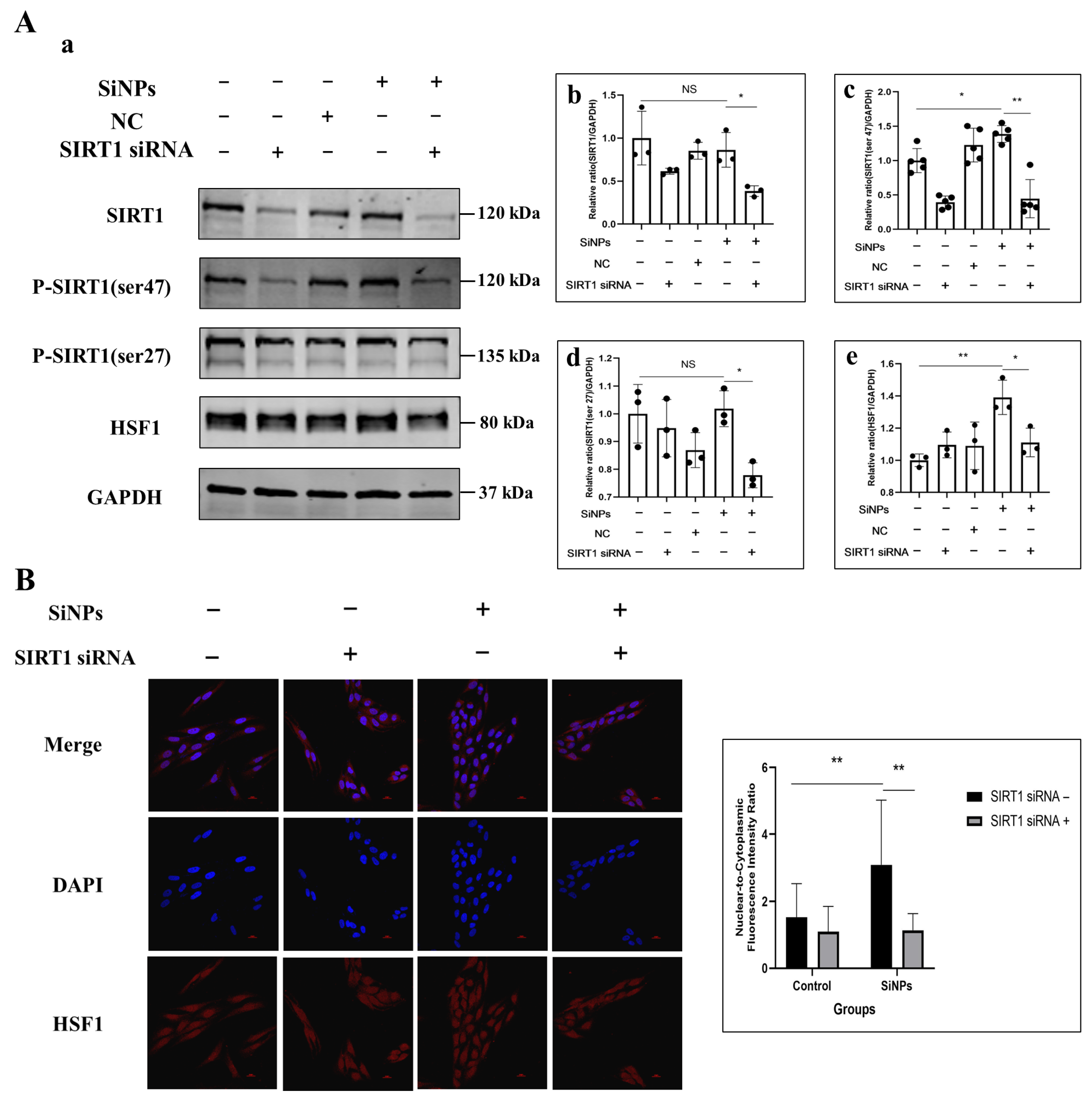
3.5. SIRT1 siRNA Inhibited SIRT1 Phosphorylation and Further Suppressed HSF1 Protein Activity Induced by SiNPs
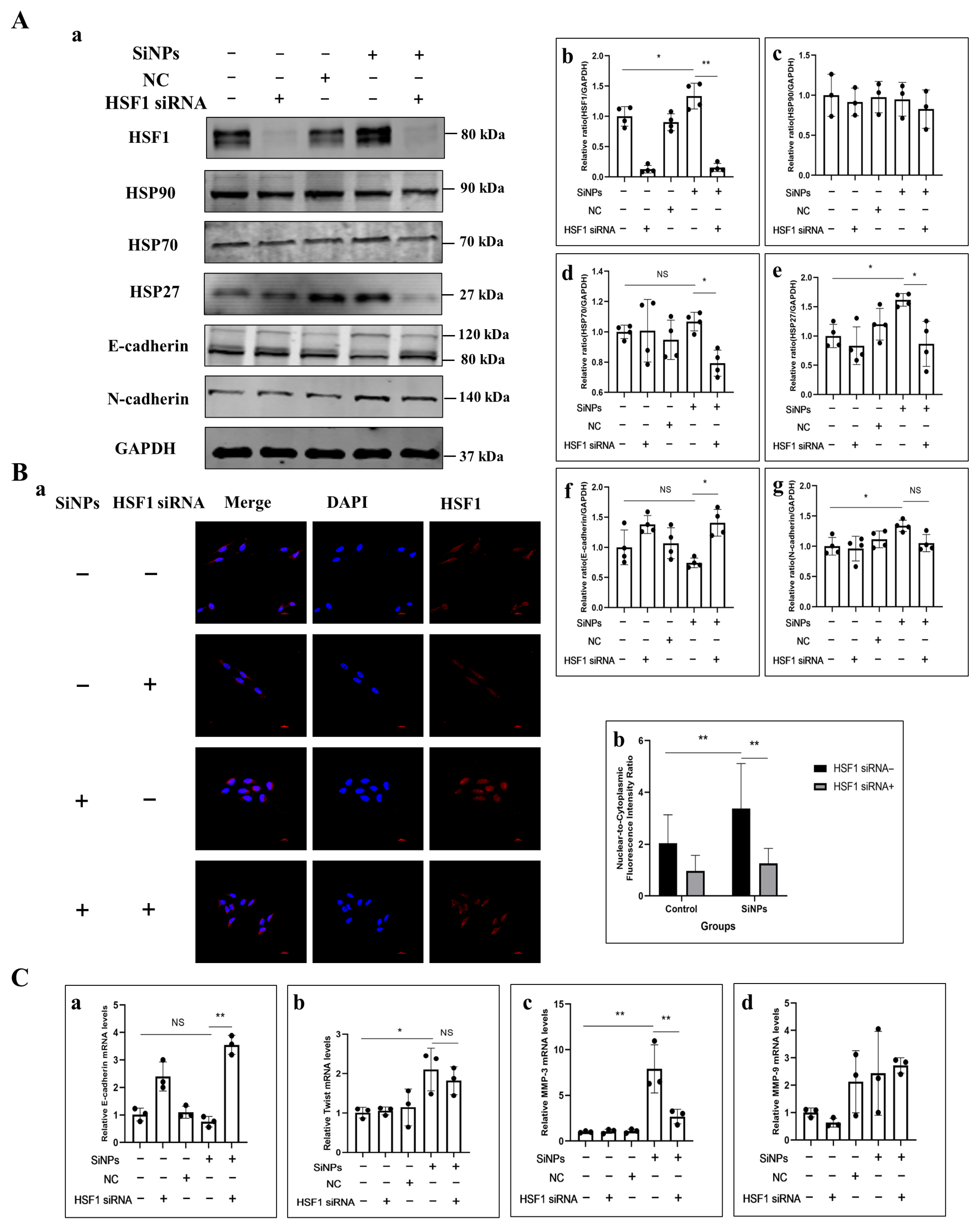
3.6. HSF1 siRNA Alleviated EMT Induced by SiNPs in BEAS-2B Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SiNPs | Amorphous silica nanoparticles |
| OECD | Organization for Economic Co-operation and Development |
| SiO2 | Silicon dioxide |
| EMT | Epithelial–mesenchymal transition |
| HSPs | Heat shock proteins |
| ER | Endoplasmic reticulum |
| UPR | Unfolded protein response |
| HSF1 | Heat shock factor 1 |
| siRNA | small interfering RNA |
| TGF-β | Transforming growth factor-β |
| TEM | Transmission Electron Microscope |
| 4-PBA | 4-Phenylbutyric acid |
References
- Ding, R.; Li, Y.; Yu, Y.; Sun, Z.; Duan, J. Prospects and hazards of silica nanoparticles: Biological impacts and implicated mechanisms. Biotechnol. Adv. 2023, 69, 108277. [Google Scholar] [CrossRef]
- Li, Z.; Mu, Y.; Peng, C.; Lavin, M.F.; Shao, H.; Du, Z. Understanding the mechanisms of silica nanoparticles for nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1658. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, Y.; Zhu, L.; Wang, D.; Lv, S.; Li, X.; Guo, C.; Li, Y. Warning on the inhalation of silica nanoparticles: Experimental evidence for its easy passage through the air-blood barrier, resulting in systemic distribution and pathological injuries. Chem.-Biol. Interact. 2025, 409, 111423. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Lv, S.; Xu, H.; Ma, R.; Sun, Z.; Li, Y.; Guo, C. Long-term respiratory exposure to amorphous silica nanoparticles promoted systemic inflammation and progression of fibrosis in a susceptible mouse model. Chemosphere 2022, 300, 134633. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, S.; Cui, G.; Yang, Y.; Zhang, E.; Wang, Q.; Lavin, M.F.; Yeo, A.J.; Bo, C.; Zhang, Y.; et al. Silica nanoparticles induce cardiomyocyte apoptosis via the mitochondrial pathway in rats following intratracheal instillation. Int. J. Mol. Med. 2019, 43, 1229–1240. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Zhao, X.; Li, Y.; Lv, S.; Zhou, W.; Wang, J.; Sun, Z.; Li, Y.; Guo, C. Ferroptosis contributing to cardiomyocyte injury induced by silica nanoparticles via miR-125b-2-3p/HO-1 signaling. Part. Fibre Toxicol. 2024, 21, 17. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A. Influence of silica nanoparticles on cadmium-induced cytotoxicity, oxidative stress, and apoptosis in human liver HepG2 cells. Environ. Toxicol. 2020, 35, 599–608. [Google Scholar] [CrossRef]
- Azouz, R.A.; Korany, R.M.S. Toxic Impacts of Amorphous Silica Nanoparticles on Liver and Kidney of Male Adult Rats: An In Vivo Study. Biol. Trace Elem. Res. 2021, 199, 2653–2662. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, J.; Duan, J.; Guo, C.; Zhang, J.; Ren, L.; Liu, J.; Li, Y.; Sun, Z.; Zhou, X. Silica nanoparticles exacerbates reproductive toxicity development in high-fat diet-treated Wistar rats. J. Hazard. Mater. 2020, 384, 121361. [Google Scholar] [CrossRef]
- Wei, J.; Liu, J.; Liang, S.; Sun, M.; Duan, J. Low-Dose Exposure of Silica Nanoparticles Induces Neurotoxicity via Neuroactive Ligand-Receptor Interaction Signaling Pathway in Zebrafish Embryos. Int. J. Nanomed. 2020, 15, 4407–4415. [Google Scholar] [CrossRef]
- Ao, L.H.; Wei, Y.G.; Tian, H.R.; Zhao, H.; Li, J.; Ban, J.Q. Advances in the study of silica nanoparticles in lung diseases. Sci. Total Environ. 2024, 912, 169352. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, S.; Liang, X.; He, K.; Wei, T.; Wang, Y.; Zou, L.; Zhang, T.; Xue, Y.; Tang, M. The apoptosis induced by silica nanoparticle through endoplasmic reticulum stress response in human pulmonary alveolar epithelial cells. Toxicol. In Vitro 2019, 56, 126–132. [Google Scholar] [CrossRef]
- Zhao, X.; Abulikemu, A.; Lv, S.; Qi, Y.; Duan, J.; Zhang, J.; Chen, R.; Guo, C.; Li, Y.; Sun, Z. Oxidative stress- and mitochondrial dysfunction-mediated cytotoxicity by silica nanoparticle in lung epithelial cells from metabolomic perspective. Chemosphere 2021, 275, 129969. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Duan, Y.; Cai, X.; You, D.; Zhou, F.; Yang, C.; Tuo, X.; Liu, Z. Blockage of TGF-α Induced by Spherical Silica Nanoparticles Inhibits Epithelial-Mesenchymal Transition and Proliferation of Human Lung Epithelial Cells. BioMed Res. Int. 2019, 2019, 8231267. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liang, Q.; Wang, F.; Yan, K.; Sun, M.; Lin, L.; Li, T.; Duan, J.; Sun, Z. Silica nanoparticles induce pulmonary autophagy dysfunction and epithelial-to-mesenchymal transition via p62/NF-κB signaling pathway. Ecotoxicol. Environ. Saf. 2022, 232, 113303. [Google Scholar] [CrossRef]
- Lachat, C.; Peixoto, P.; Hervouet, E. Epithelial to Mesenchymal Transition History: From Embryonic Development to Cancers. Biomolecules 2021, 11, 782. [Google Scholar] [CrossRef]
- Hill, C.; Li, J.; Liu, D.; Conforti, F.; Brereton, C.J.; Yao, L.; Zhou, Y.; Alzetani, A.; Chee, S.J.; Marshall, B.G.; et al. Autophagy inhibition-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in pulmonary fibrosis. Cell Death Dis. 2019, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Jinesh, G.G.; Brohl, A.S. Classical epithelial-mesenchymal transition (EMT) and alternative cell death process-driven blebbishield metastatic-witch (BMW) pathways to cancer metastasis. Signal Transduct. Target. Ther. 2022, 7, 296. [Google Scholar] [CrossRef]
- Li, Y.; Duan, J.; Chai, X.; Yang, M.; Wang, J.; Chen, R.; Sun, Z. Microarray-assisted size-effect study of amorphous silica nanoparticles on human bronchial epithelial cells. Nanoscale 2019, 11, 22907–22923. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Duan, J.; Yang, M.; Yu, Y.; Feng, L.; Yang, X.; Zhou, X.; Zhao, Z.; Sun, Z. Silica nanoparticles induce autophagosome accumulation via activation of the EIF2AK3 and ATF6 UPR pathways in hepatocytes. Autophagy 2018, 14, 1185–1200. [Google Scholar] [CrossRef]
- Pires Da Silva, J.; Monceaux, K.; Guilbert, A.; Gressette, M.; Piquereau, J.; Novotova, M.; Ventura-Clapier, R.; Garnier, A.; Lemaire, C. SIRT1 Protects the Heart from ER Stress-Induced Injury by Promoting eEF2K/eEF2-Dependent Autophagy. Cells 2020, 9, 426. [Google Scholar] [CrossRef]
- Prola, A.; Pires Da Silva, J.; Guilbert, A.; Lecru, L.; Piquereau, J.; Ribeiro, M.; Mateo, P.; Gressette, M.; Fortin, D.; Boursier, C.; et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ. 2017, 24, 343–356. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, F.; Liang, H.; Cao, H.; Cai, M.; Xu, W.; Weng, J. SIRT1/HSF1/HSP pathway is essential for exenatide-alleviated, lipid-induced hepatic endoplasmic reticulum stress. Hepatology 2017, 66, 809–824. [Google Scholar] [CrossRef]
- Huang, C.; Wu, J.; Xu, L.; Wang, J.; Chen, Z.; Yang, R. Regulation of HSF1 protein stabilization: An updated review. Eur. J. Pharmacol. 2018, 822, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Wang, B.; Sastry, N.; Masliah, E.; Nelson, P.T.; Cai, H.; Liao, F.F. NEDD4-mediated HSF1 degradation underlies α-synucleinopathy. Hum. Mol. Genet. 2016, 25, 211–222. [Google Scholar] [CrossRef]
- Devi, K.; Singh, N.; Jaggi, A.S. Dual role of sirtuin 1 in inflammatory bowel disease. Immunopharmacol. Immunotoxicol. 2020, 42, 385–391. [Google Scholar] [CrossRef]
- Fiser, O.; Muller, P. Role of HSF1 in cell division, tumorigenesis and therapy: A literature review. Cell Div. 2025, 20, 11. [Google Scholar] [CrossRef]
- Pariollaud, M.; Ibrahim, L.H.; Irizarry, E.; Mello, R.M.; Chan, A.B.; Altman, B.J.; Shaw, R.J.; Bollong, M.J.; Wiseman, R.L.; Lamia, K.A. Circadian disruption enhances HSF1 signaling and tumorigenesis in Kras-driven lung cancer. Sci. Adv. 2022, 8, eabo1123. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Hu, Y.; Buckhaults, P.; Moskophidis, D.; Mivechi, N.F. Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. J. Biol. Chem. 2012, 287, 35646–35657. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Chang, R.; Yang, L. Heat shock factor 1 promotes invasion and metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer 2012, 118, 1782–1794. [Google Scholar] [CrossRef]
- Toma-Jonik, A.; Widlak, W.; Korfanty, J.; Cichon, T.; Smolarczyk, R.; Gogler-Piglowska, A.; Widlak, P.; Vydra, N. Active heat shock transcription factor 1 supports migration of the melanoma cells via vinculin down-regulation. Cell. Signal. 2015, 27, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.D.; Paullin, T.R.; Aoisa, C.; Menzie, C.J.; Ubaldini, A.; Westerheide, S.D. The Heat Shock Transcription Factor HSF1 Induces Ovarian Cancer Epithelial-Mesenchymal Transition in a 3D Spheroid Growth Model. PLoS ONE 2016, 11, e0168389. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yang, X.; Liang, S.; Xu, Q.; Miller, M.R.; Duan, J.; Sun, Z. Silica nanoparticles trigger the vascular endothelial dysfunction and prethrombotic state via miR-451 directly regulating the IL6R signaling pathway. Part. Fibre Toxicol. 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, F.; Liu, H.; Shen, Y.; Zhang, J.; Hu, L.; Zhu, L. Suppressing Endoplasmic Reticulum Stress Alleviates LPS-Induced Acute Lung Injury via Inhibiting Inflammation and Ferroptosis. Inflammation 2024, 47, 1067–1082. [Google Scholar] [CrossRef]
- Shi, M.; Liu, K.; Li, X.; Zeng, X.L.; Liu, X.J. Melatonin ameliorates PM2.5-induced airway inflammation and apoptosis by PERK/eIF2α/ATF4/CHOP in chronic obstructive pulmonary disease mice. Toxicol. Appl. Pharmacol. 2025, 499, 117314. [Google Scholar] [CrossRef]
- Guo, C.; Ma, R.; Liu, X.; Chen, T.; Li, Y.; Yu, Y.; Duan, J.; Zhou, X.; Li, Y.; Sun, Z. Silica nanoparticles promote oxLDL-induced macrophage lipid accumulation and apoptosis via endoplasmic reticulum stress signaling. Sci. Total Environ. 2018, 631–632, 570–579. [Google Scholar] [CrossRef]
- Liang, Q.; Sun, M.; Ma, Y.; Wang, F.; Sun, Z.; Duan, J. Adverse effects and underlying mechanism of amorphous silica nanoparticles in liver. Chemosphere 2023, 311, 136955. [Google Scholar] [CrossRef]
- Miao, C.; Jia, P.; Luo, C.; Pang, J.; Xiao, L.; Zhang, T.; Duan, J.; Li, Y.; Sun, Z. The size-dependent in vivo toxicity of amorphous silica nanoparticles: A systematic review. Ecotoxicol. Environ. Saf. 2024, 271, 115910. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Jin, M.; Du, Z.; Liu, X.; Guo, C.; Li, Y.; Huang, P.; Sun, Z. Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cells. Toxicol. In Vitro 2011, 25, 1343–1352. [Google Scholar] [CrossRef]
- Kwon, D.; Koh, J.; Kim, S.; Go, H.; Min, H.S.; Kim, Y.A.; Kim, D.K.; Jeon, Y.K.; Chung, D.H. Overexpression of endoplasmic reticulum stress-related proteins, XBP1s and GRP78, predicts poor prognosis in pulmonary adenocarcinoma. Lung Cancer 2018, 122, 131–137. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 2014, 14, 581–597. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Gao, H.; Zeng, Y. Profiling of metabolites, proteins, and protein phosphorylation in silica-exposed BEAS-2B epithelial cells. PLoS ONE 2023, 18, e0273034. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, H. Identification of differentially expressed genes and pathways in BEAS-2B cells upon long-term exposure to particulate matter (PM(2.5)) from biomass combustion using bioinformatics analysis. Environ. Health Prev. Med. 2023, 28, 51. [Google Scholar] [CrossRef]
- Grilli, A.; Bengalli, R.; Longhin, E.; Capasso, L.; Proverbio, M.C.; Forcato, M.; Bicciato, S.; Gualtieri, M.; Battaglia, C.; Camatini, M. Transcriptional profiling of human bronchial epithelial cell BEAS-2B exposed to diesel and biomass ultrafine particles. BMC Genom. 2018, 19, 302. [Google Scholar] [CrossRef]
- Ye, G.; Gao, H.; Zhang, X.; Liu, X.; Chen, J.; Liao, X.; Zhang, H.; Huang, Q. Aryl hydrocarbon receptor mediates benzo[a]pyrene-induced metabolic reprogramming in human lung epithelial BEAS-2B cells. Sci. Total Environ. 2021, 756, 144130. [Google Scholar] [CrossRef]
- Wang, N.; Chen, H.-Q.; Zeng, Y.; Shi, Y.; Zhang, Z.; Li, J.-Y.; Zhou, S.-M.; Li, Y.-W.; Deng, S.-W.; Han, X.; et al. Benzo(a)pyrene promotes the malignant progression of malignant-transformed BEAS-2B cells by regulating YTH N6-methyladenosine RNA binding protein 1 to inhibit ferroptosis. Toxicology 2024, 507, 153886. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, R.; Liu, X.; Xia, Y.; Niu, P.; Ma, J.; Zhou, X.; Li, Y.; Sun, Z. Silica nanoparticles induced endothelial apoptosis via endoplasmic reticulum stress-mitochondrial apoptotic signaling pathway. Chemosphere 2018, 210, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, R.L.; Mesgarzadeh, J.S.; Hendershot, L.M. Reshaping endoplasmic reticulum quality control through the unfolded protein response. Mol. Cell 2022, 82, 1477–1491. [Google Scholar] [CrossRef]
- Lam, T.Y.W.; Nguyen, N.; Peh, H.Y.; Shanmugasundaram, M.; Chandna, R.; Tee, J.H.; Ong, C.B.; Hossain, M.Z.; Venugopal, S.; Zhang, T.; et al. ISM1 protects lung homeostasis via cell-surface GRP78-mediated alveolar macrophage apoptosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2019161119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, S.; Huang, Y.; Wei, X.; Liu, L.; Huo, F.; Huang, P.; Wu, Y.; Tian, W. Inhibition of TRPA1 Ameliorates Periodontitis by Reducing Periodontal Ligament Cell Oxidative Stress and Apoptosis via PERK/eIF2α/ATF-4/CHOP Signal Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 4107915. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, C.; Liu, D.; Wang, Y.; Qi, H.; Liu, X.; Zhang, Y.; Chen, H.; Zeng, Y.; Li, J. Polystyrene nanoplastics-induced lung apoptosis and ferroptosis via ROS-dependent endoplasmic reticulum stress. Sci. Total Environ. 2024, 912, 169260. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Qiao, M.; Sun, X.; Li, G. Resveratrol Alleviates Inflammation and ER Stress Through SIRT1/NRF2 to Delay Ovarian Aging in a Short-Lived Fish. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2023, 78, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Deng, L.; Qian, R.; Huang, X.; Liu, W.; Tang, S. Curculigoside Attenuates Endoplasmic Reticulum Stress-Induced Epithelial Cell and Fibroblast Senescence by Regulating the SIRT1-P300 Signaling Pathway. Antioxidants 2024, 13, 420. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Flick, F.; Lüscher, B. Regulation of sirtuin function by posttranslational modifications. Front. Pharmacol. 2012, 3, 29. [Google Scholar] [CrossRef]
- Sasaki, T.; Maier, B.; Koclega, K.D.; Chruszcz, M.; Gluba, W.; Stukenberg, P.T.; Minor, W.; Scrable, H. Phosphorylation regulates SIRT1 function. PLoS ONE 2008, 3, e4020. [Google Scholar] [CrossRef]
- Liu, S.; Shan, L.; Li, Y.; Xiang, J.; Zhang, H. Phosphorylation-driven regulation of SIRT1 in muscle senescence: Insights from molecular dynamics simulation and experimental validation. Int. J. Biol. Macromol. 2025, 306, 141734. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, J.; Zhan, L.; Wang, M.; Shi, R.; Yuan, X.; Gao, X.; Liu, X.; Zang, J.; Liu, W.; et al. Resveratrol-induced Sirt1 phosphorylation by LKB1 mediates mitochondrial metabolism. J. Biol. Chem. 2021, 297, 100929. [Google Scholar] [CrossRef]
- Wen, L.; Chen, Z.; Zhang, F.; Cui, X.; Sun, W.; Geary, G.G.; Wang, Y.; Johnson, D.A.; Zhu, Y.; Chien, S.; et al. Ca2+/calmodulin-dependent protein kinase kinase β phosphorylation of Sirtuin 1 in endothelium is atheroprotective. Proc. Natl. Acad. Sci. USA 2013, 110, E2420–E2427. [Google Scholar] [CrossRef]
- Nasrin, N.; Kaushik, V.K.; Fortier, E.; Wall, D.; Pearson, K.J.; de Cabo, R.; Bordone, L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS ONE 2009, 4, e8414. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, S.J.; Fang, X.; Song, N.Y.; Kim, D.H.; Suh, J.; Na, H.K.; Kim, K.O.; Baek, J.H.; Surh, Y.J. JNK-mediated Ser27 phosphorylation and stabilization of SIRT1 promote growth and progression of colon cancer through deacetylation-dependent activation of Snail. Mol. Oncol. 2022, 16, 1555–1571. [Google Scholar] [CrossRef]
- Koga, T.; Suico, M.A.; Shimasaki, S.; Watanabe, E.; Kai, Y.; Koyama, K.; Omachi, K.; Morino-Koga, S.; Sato, T.; Shuto, T.; et al. Endoplasmic Reticulum (ER) Stress Induces Sirtuin 1 (SIRT1) Expression via the PI3K-Akt-GSK3β Signaling Pathway and Promotes Hepatocellular Injury. J. Biol. Chem. 2015, 290, 30366–30374. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on its biological functions. Cell Commun. Signal. CCS 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M., Jr.; Sistonen, L.; Morimoto, R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef]
- Huang, M.; Dong, W.; Xie, R.; Wu, J.; Su, Q.; Li, W.; Yao, K.; Chen, Y.; Zhou, Q.; Zhang, Q.; et al. HSF1 facilitates the multistep process of lymphatic metastasis in bladder cancer via a novel PRMT5-WDR5-dependent transcriptional program. Cancer Commun. 2022, 42, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tu, K.; Fu, Q.; Schmitt, D.C.; Zhou, L.; Lu, N.; Zhao, Y. Multifaceted roles of HSF1 in cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 4923–4931. [Google Scholar] [CrossRef]
- Kovács, D.; Sigmond, T.; Hotzi, B.; Bohár, B.; Fazekas, D.; Deák, V.; Vellai, T.; Barna, J. HSF1Base: A Comprehensive Database of HSF1 (Heat Shock Factor 1) Target Genes. Int. J. Mol. Sci. 2019, 20, 5815. [Google Scholar] [CrossRef]
- Akerfelt, M.; Trouillet, D.; Mezger, V.; Sistonen, L. Heat shock factors at a crossroad between stress and development. Ann. N. Y. Acad. Sci. 2007, 1113, 15–27. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin. Ther. Targets 2009, 13, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, M.; Zhou, J.; Zhang, X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). Int. J. Oncol. 2014, 45, 18–30. [Google Scholar] [CrossRef]
- Nikotina, A.D.; Vladimirova, S.A.; Komarova, E.Y.; Alexeev, D.; Efremov, S.; Leonova, E.; Pavlov, R.; Kartsev, V.G.; Polonik, S.G.; Margulis, B.A.; et al. Prevention of High Glucose-Mediated EMT by Inhibition of Hsp70 Chaperone. Int. J. Mol. Sci. 2021, 22, 6902. [Google Scholar] [CrossRef]
- Qi, Z.; Tang, T.; Sheng, L.; Ma, Y.; Liu, Y.; Yan, L.; Qi, S.; Ling, L.; Zhang, Y. Salidroside inhibits the proliferation and migration of gastric cancer cells via suppression of Src-associated signaling pathway activation and heat shock protein 70 expression. Mol. Med. Rep. 2018, 18, 147–156. [Google Scholar] [CrossRef]
- Kasioumi, P.; Vrazeli, P.; Vezyraki, P.; Zerikiotis, S.; Katsouras, C.; Damalas, A.; Angelidis, C. Hsp70 (HSP70A1A) downregulation enhances the metastatic ability of cancer cells. Int. J. Oncol. 2019, 54, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Bishop, J.L.; Nip, K.M.; Zardan, A.; Takeuchi, A.; Cordonnier, T.; Beraldi, E.; Bazov, J.; Fazli, L.; Chi, K.; et al. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res. 2013, 73, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Liang, W.; Luo, L. HSP27 promotes epithelial-mesenchymal transition through activation of the β-catenin/MMP3 pathway in pancreatic ductal adenocarcinoma cells. Transl. Cancer Res. 2019, 8, 1268–1278. [Google Scholar] [CrossRef]
| Gene Name | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| MMP-3 | TGGATTGGAGGTGACGGGGAAG | ATGCCAGGAAAGGTTCTGAAGTGAC |
| MMP-9 | AGTCCACCCTTGTGCTCTTCCC | TCTCTGCCACCCGAGTGTAACC |
| Twist | CCTCGGACAAGCTGAGCAAGATTC | GTCGCTCTGGAGGACCTGGTAG |
| E-cadherin | AGTCACTGACACCAACGATAAT | ATCGTTGTTCACTGGATTTGTG |
| GAPDH | CAGGAGGCATTGCTGATGAT | GAAGGCTGGGGCTCATTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, J.; Xiao, L.; Xiong, Z.; Zhang, K.; Yang, M.; Wang, J.; Li, Y.; Li, Y. Silica Nanoparticles Induced Epithelial–Mesenchymal Transition in BEAS-2B Cells via ER Stress and SIRT1/HSF1/HSPs Signaling Pathway. J. Xenobiot. 2025, 15, 137. https://doi.org/10.3390/jox15050137
Pang J, Xiao L, Xiong Z, Zhang K, Yang M, Wang J, Li Y, Li Y. Silica Nanoparticles Induced Epithelial–Mesenchymal Transition in BEAS-2B Cells via ER Stress and SIRT1/HSF1/HSPs Signaling Pathway. Journal of Xenobiotics. 2025; 15(5):137. https://doi.org/10.3390/jox15050137
Chicago/Turabian StylePang, Jinyan, Liyan Xiao, Zhiqin Xiong, Kexin Zhang, Man Yang, Ji Wang, Yanbo Li, and Yang Li. 2025. "Silica Nanoparticles Induced Epithelial–Mesenchymal Transition in BEAS-2B Cells via ER Stress and SIRT1/HSF1/HSPs Signaling Pathway" Journal of Xenobiotics 15, no. 5: 137. https://doi.org/10.3390/jox15050137
APA StylePang, J., Xiao, L., Xiong, Z., Zhang, K., Yang, M., Wang, J., Li, Y., & Li, Y. (2025). Silica Nanoparticles Induced Epithelial–Mesenchymal Transition in BEAS-2B Cells via ER Stress and SIRT1/HSF1/HSPs Signaling Pathway. Journal of Xenobiotics, 15(5), 137. https://doi.org/10.3390/jox15050137





