1. Introduction
Since the beginning of modern agriculture, the use of pesticides has become the norm for pest elimination. Despite the multiple advantages they offer, there are several problems posed by their use. Many of these substances have high mobility, so the vast majority end up elsewhere [
1,
2]. Another main characteristic of pesticides that threatens the environment is their persistence [
3,
4]. Most pass into the soil or volatilize and, depending on their characteristics, can reach groundwater or other environmental compartments, remaining there for a long time [
5,
6]. The long-term behavior of pesticides depends on soil properties, compound characteristics, and environmental factors, leading to volatilization, degradation, leaching, or accumulation [
7]. Moreover, pesticides can negatively affect soil microbiota, disrupting microbial communities essential for soil health and nutrient cycling [
8]. Some pesticides, such as those with xenoestrogenic activity, have been associated with risks to human health, including potential carcinogenic effects and endocrine disruptions, which increases the urgency for their control and monitoring [
9].
One of the fundamental aspects in the determination of xenobiotics in soils is the choice of an appropriate extraction method, due to the structural and compositional complexity of soils, which often contain organic matter, clay minerals, and microbial communities that can interfere with analyte recovery [
10,
11,
12]. The extraction of pesticides in different environment matrices has traditionally been carried out using the QuEChERS method known by its acronym (quick, easy, cheap, effective, rugged, and safe) [
13], which has been used by numerous authors for the quantification of many contaminants in various matrices, Ex. [
14,
15,
16,
17]. Modifications of this method for contaminant extraction in a wide variety of matrices are continuously being validated [
18,
19,
20,
21,
22]. Furthermore, in 2021, a new QuEChERS mega-method was published by one of the authors of the QuEChERS method: QuEChERSER (more than QuEChERS, adding “Efficient and Robust” to its acronym) [
23], which captures a wider polarity range than QuEChERS and has already been validated in a variety of matrices for environmental contaminants, veterinary drugs, and pesticides [
24,
25,
26]. However, at the time of writing this article, we have not found any reference to its application in soils. The study of the persistence of xenobiotics in soils and their interactions with the constituents of the edaphic environment continue to be topics of great interest and relevance [
5,
27,
28,
29] since they can be retained in the soil exchange complex, in organic matter, in the clay fraction or precipitates in the soil matrix, which can decrease their ecosystem functions and present risks to the health of plants, animals and humans [
30,
31,
32].
Three widely used pesticides today—pendimethalin (PMDT) and oxyfluorfen (OXF), both herbicides, and the fungicide trifloxystrobin (TFXT) [
33]—were selected for this study based on their extensive agricultural use, along with their documented toxicity and persistence in the environment. PMDT or 3,4-dimethyl-2,6-dinitro-N-pentan-3-ylaniline (CAS: 40487-42-1) is an herbicide that belongs to the group of dinitroanilines; it has a wide spectrum of application and is used in pre- and post-emergence grass and weed control in crops. It acts by inhibiting cell division and elongation [
34]. It is considered to be irritating to the skin, eyes, and respiratory tract [
35]. It is also considered to have negative effects on reproduction, and some studies suggest that it is associated with a higher incidence of pancreatic cancer [
36]. Although it is not classified as persistent, its half-lives range from 76 to 98 days, which is relatively high compared to other compounds [
37,
38]. OXF or 2-chloro-1-(3-ethoxy-4-nitrophenoxy)-4-(trifluoromethyl) benzene (CAS: 42874-03-3) belongs to the chemical group of diphenyl ethers. It is a broad-spectrum herbicide used in both pre-emergence and post-emergence weed control. It is considered to be a persistent and immobile pesticide in soil since it binds to soil organic carbon [
39]. In humans, its main toxic effects include liver dysfunction and impaired red blood cell production, which may lead to anemia [
40]. TFXT, according to IUPAC: (2E)-2-methoxyimino-2-[2-[[(E)-1-[3-(trifluoromethyl) phenyl] ethylideneamino] oxymethyl] phenyl] acetate (CAS: 141517-21-7), is a fungicide of the strobilurin group, which acts by externally inhibiting quinone in complex 3 of the respiratory chain [
41]. It is a foliar-applied compound for the control of certain foliar, stem, and root diseases. It is a widely used broad-spectrum fungicide, with very low water solubility and volatility [
42]. It has low toxicity in mammals, but there is evidence that it is toxic to birds, fish, and aquatic invertebrates, causing negative effects on their fertility [
43]. In some cases, it has been observed to decompose rapidly in soils, and its residues have been shown to dissipate below the detection limit in 15 days [
44].
Protecting and preserving soil health is essential for the maintenance of its ecosystem functions. Its main functions are climate control, water quality and quantity maintenance, nutrient cycling, purification capacity, and providing habitat for a vast array of biodiversity [
45,
46]. As indicated above, the use of pesticides can inhibit these functions, so strategies are needed to remove these xenobiotics from contaminated soils. In this regard, bioremediation is becoming one of the most commonly used techniques, with a large number of bioremediation methodologies being used satisfactorily [
47,
48,
49]. Some of the interesting technologies for the removal of these contaminants in soils are biostimulation and/or bioenhancement provided during the application of organic amendments to contaminated soils; these bioremediation strategies have proven to be effective under certain conditions [
50,
51,
52,
53,
54]. In addition, they are sustainable, low-cost, non-invasive, and can be carried out in situ. Some ways in which the pesticide concentration in contaminated soils has been reduced have been through the application of compost [
55,
56,
57,
58,
59] or manure [
60].
For all the above reasons, the objectives of this study were: (i) to compare and evaluate the effectiveness of the QuEChERS and QuEChERSER extraction methods on 13 pesticides, which represent a wide range of functional groups and polarities, in loamy-clayed soil; (ii) to determine the persistence of the herbicides PDMT and OXF and the fungicide TFXT in the selected soil using a microcosm test under laboratory conditions; and (iii) to evaluate the effect of the application of biowaste compost and cow manure on the natural persistence in the soil of the three selected pesticides.
2. Materials and Methods
2.1. Chemicals, Reagents, Soil, and Organic Amendments
Pure standards (>99%) from Dr. Ehrenstofer (Augsburg, Germany) were used. The following were used: methanol (MeOH) and acetonitrile (ACN) of LC-MS grade, Scharlab S.L. (Barcelona, Spain); ultrapure water HPLC, Milli-Q grade (Millipore, Bedford, MA, USA); formic acid (>98%, ACS reagent grade) and ammonium formate (>99%, LiChropur LC-MS grade) from Sigma-Aldrich (Darmstadt, Germany); QuEChERS EN ExtraBond extractive kit and dispersive kit (Scharlab, Barcelona, Spain); and pesticide mixture 301 × 10
5 µg L
−1 in can from LGC Standards Ltd., reference DRE-GA09000301AL, Dr. Ehrenstorfer (Augsburg, Germany). The following commercial products were used: Scorpio from Bayer (Leverkusen, Germany), Goal from Dow AgroSciences (Indianapolis, IN, USA), and StompLE from BASF (Ludwigshafen, Germany), which contained 330 g L
−1 of PDMT, 240 g L
−1 of OXF, and 500 g kg
−1 of TFXT, respectively. Stock solutions of 1000 µg ml
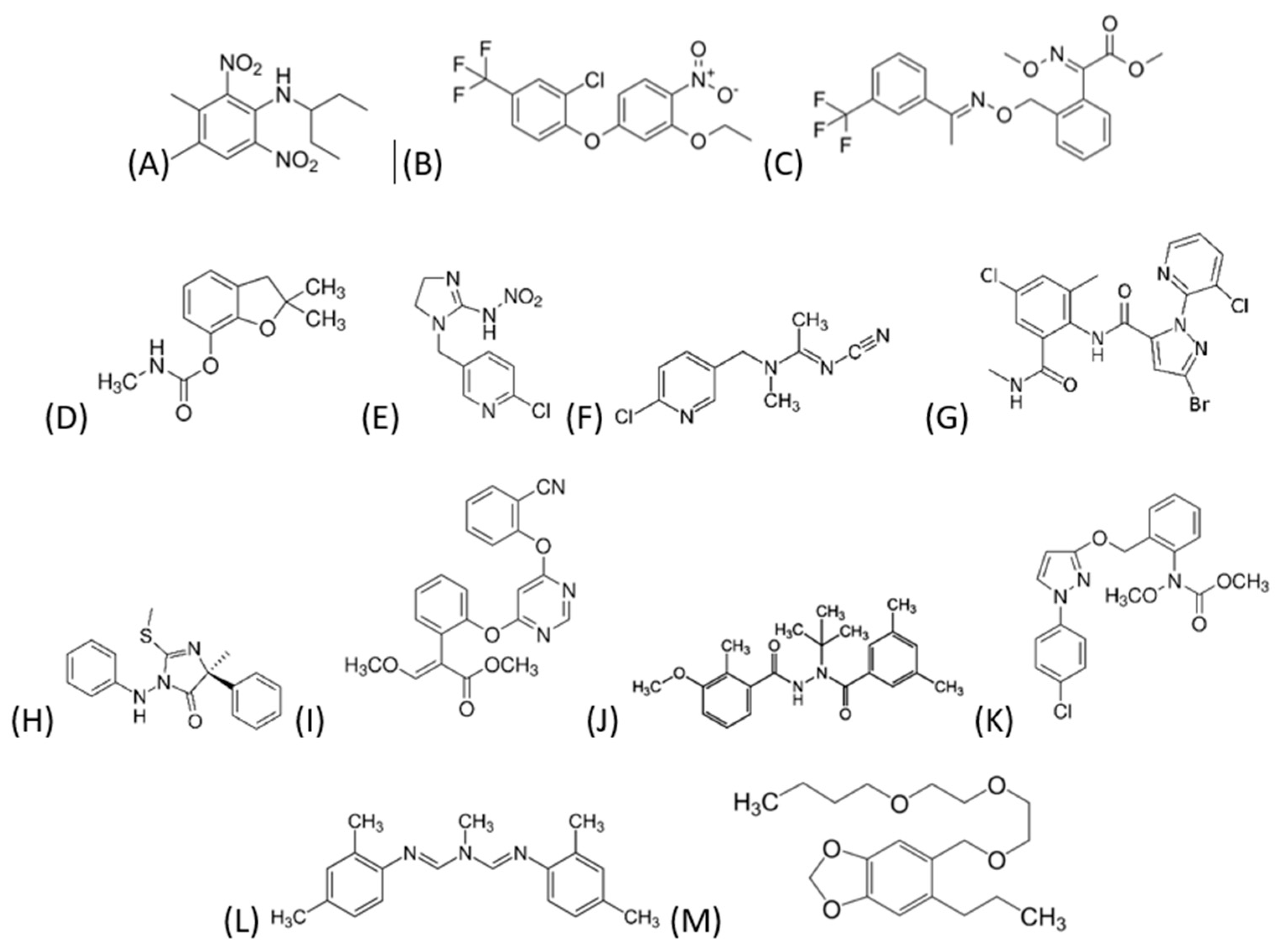
−1 were prepared by dissolving a sufficient amount in ACN and stored in amber glass bottles at a maximum temperature of 4 °C. The pesticides studied were: carbofuran-3OH (CB-3OH), imidacloprid (IMDCPD), acetamiprid (ACMP), chlorantraniliprole (CHLMP), fenamidone (FNMD), azoxystrobin (AZXB), methoxyfenozide (MTXFZD), pyraclostrobin (PCTB), amitraz (AMTZ), piperonyl-butoxide (PIP-BTX), PDMT, OXF, and TFXT.
Figure 1 shows the chemical structure of each one. Their water solubility, Log K
ow, and vapor pressure are shown in
Table A1. The ones that are more soluble are ACMP and IMDCP. The most lipophilic are AMTZ and PDMT, and the most volatile are IMDCP and AMTZ. For quality control during ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) analysis,
13C-phenacetin was used as an internal standard, prepared at a concentration of 0.7 ng μL
−1 in water. A mixture of the 13 pesticides containing 10 µg mL
−1 was prepared. The standards of the prepared calibration curve contained the following concentrations: 0, 10, 20, 50, 100, and 200 ng mL
−1 in the mobile phase. It is important to note that this mixture of compounds is representative of a variety of functional groups.
The soil sample used in the experiment corresponds to a Bt horizon of a calcium Luvisol. This soil was taken from the Mondúver mountain range (La Safor, Valencia, Spain). The sample was duly taken using the procedures described in the FAO Soil Description Guide [
61]. The soil coordinates are: 39°02′24.3″ N 0°21′14.5″ W. The pre-treatment of the samples was conducted according to the procedure described by the Spanish Standard UNE-EN 16179 [
62], which provides guidance for sample preparation of sludge, treated biowaste, and soil. Moisture content was determined by gravimetry following the Spanish Standard UNE-EN 16586 [
63]. The pH was measured in a 1:5 (
v/
v) soil–water suspension according to the Spanish Standard UNE-EN 10390 [
64]. Electrical conductivity (EC) was determined in the aqueous extract in a 1:5 (
w/
v) ratio using a Crison conductometer, following the Spanish Standard UNE-EN 77308 [
65]. Granulometric analysis was performed using the Bouyoucos densimeter method as described by the Colombian Technical Standard NTC 6299 [
66]. Carbonate content was determined by gas volumetry with a Scheibler apparatus, according to the Spanish Standard UNE-EN 10693 [
67]. Oxidizable organic carbon was determined following the potassium permanganate oxidation method described in UNE 103204 [
68], and total organic matter content was calculated by calcination according to the Spanish Standard UNE-EN 13039 [
69].
Two organic amendments were used: Class A compost (C), made from biowaste and pruning waste, and cow manure (E) frequently used in agriculture. Their fundamental properties are shown in
Table 1.
2.2. Extraction Methods Used
As indicated above, this study compares two extraction methods, QuEChERS (Q) and QuEChERSER (QS), in order to investigate which is more effective. The method of extracting pesticides from soils has traditionally been Q [
70]. This is a type of dispersive solid phase extraction (d-SPE) used for sample preparation consisting of an initial extraction phase with 10 g of sample and 10 mL of ACN mixed with MgSO
4 and NaCl in a 50 mL Falcon tube. After vortexing for 1 min and centrifugation at 5000 rpm for 5 min, the solid dispersive phase is performed, where 1 mL of the supernatant from the previous step is transferred to another 15 mL Falcon tube with primary secondary amine (PSA) and anhydrous MgSO
4. Then it was stirred again and centrifuged. The supernatant was filtered with a 0.45 µm pore size nylon filter and vitalized. Quantification of the compounds in the extracts was carried out by UHPLC-MS/MS [
13].
In 2021, a new method, QS, was published. This method is faster and requires the use of less material [
23]. It consists of adding 2 g of the sample to a 15 mL Falcon tube together with 10 mL of a 4/1 solution of ACN/H2O. After vortexing for 10 min, it was centrifuged at 3500 rpm for 5 min. Then, 204 µL of the supernatant was taken and transferred to a 2 mL Eppendorf centrifuge mini-tube, and 25 µL of an internal standard (
13C-phenacetin at 0.7 ng µL in water
−1) and 571 µL of ultrapure water were added. It was then centrifuged at 13,000 rpm for 5 min. Finally, 500 µL of the supernatant was placed in a vial for analysis by UHPLC-MS/Ms.
For extraction by method Q, 40 µL of a solution containing 10 µg mL
−1 of the 13 pesticides to be tested was added to the soil, while for QS, 112 µL of this 10 µg mL
−1 solution of the pure standards was added. Five replicates were made with their corresponding targets in order to validate both methods. The comparison process of both extraction procedures is shown schematically in
Figure 2.
2.3. Quantification by UHPLC-MS/MS
The concentration of the analytes in all the extracts placed in vials was quantified by UHPLC-MS/MS liquid chromatography, with an ABSciex 6500 QTRAP mass spectrometer equipped with an electrospray ionization interface (ESI), using the following instrumental conditions: column C18, BEH waters 2.1 × 50 mm, 1.7 µm; temperature 35 °C; flow 0.35 mL min
−1; and mobile phase, A: H2O (0.5% Formic acid) and B: ACN. The gradient was: %B: 5, 50, 60, 78, 88, 92, 100, 100, 5 with the following times respectively: 0, 1, 1.5, 2.5, 4, 5, 6, 7, and 8 min. For the quantification and confirmation of each pesticide, two product ions were monitored. For the identification and confirmation of the compounds, the relative response (ion ratio) was used together with their retention times (
Table 2), as well as their quantification and confirmation transitions in a soil extract. The recovery tests were conducted on a control sample corresponding to the target soil type of the study at a concentration level of 28.6 µg L
−1 of the pesticides analyzed. Linearity was evaluated by injecting soil extract in the concentration range between 0–100 µg L
−1. The calibration curves were linear with 1/X adjustment in the concentration range with regression coefficients > 0.99, except for methoxyfenozide, which was 0.98. The limits of detection (LOD) and quantification (LOQ) were determined based on chromatographic peak area criteria following the US Food and Drug Administration’s Bioanalytical Method Validation Guidance (2018) [
71]. The data are shown in
Table 2.
2.4. Persistence Assay
A microcosm assay was conducted to study the persistence of PDMT, OXF, and TFX in the selected soil and the effect of the addition of the two organic amendments (C and E). Three treatments were performed in parallel: soil with pesticides (S+P), soil with pesticides and compost (S+P+C), and soil with pesticides and cow manure (S+P+E). Each experiment was conducted in triplicate. Samples were taken at 0, 3, 5, 7, 14, and 21 days (
Figure 3). To confirm the results of the extraction method, both extraction methods tested were applied to the sample at the beginning of the experiment (t = 0) for the S+P treatment.
For the preparation of the experiment, 4.5 kg of soil were weighed and distributed in equal parts of 1.5 kg in three plastic trays. In the compost tray, 30 g of compost was added since the agronomic application dose is between 20 t ha−1 and 40 t ha−1. Furthermore, 21.5 g were added to the manure tray, taking into account that the agronomic application dose is 20 t ha−1. The soil was completely homogenized, and the organic amendments were applied to ensure that there was contact between the two. Of the commercial pesticide products, 10 times the manufacturer’s recommended application dose for rice cultivation was placed in each soil tray, simulating conditions of high contamination in the soil. For Scorpio (PDMT), this dose was 0.25 kg ha−1, for Goal (OXF) it was 1 L ha−1, and for StompLE (TFXT) it was 3 L ha−1. Thus, in our case, the dose to be applied was calculated based on the amount of soil, and it was obtained that 260 µg of Scorpio, 30 µL of StompLE, and 10 µL of Goal had to be applied. As the amount for Scorpio was so small, a solution of 100 mg L−1 of commercial product was made, and 2.6 mL of this solution was applied. These quantities of commercial products were diluted in 315 mL of Milli-Q water, which was distributed in each of the trays. This amount of water was used because it was the amount corresponding to 70% of the soil’s water holding capacity.
Each dose of pesticide was added to a spray bottle together with 315 mL of water and distributed over the soil surface in the tray while mixing, spraying, and washing the walls of the bottle with the different applications of water. Thus, the theoretical initial application dose of the pesticides per soil mass was 6600 µg kg−1 of PDMT, 1600 µg kg−1 of OXF, and 86.6 µg kg−1 of TFXT. Subsequently, the soil was distributed in sterile 100 mL containers. Each control point of the study was performed in triplicate. A total of 80 g of their respective mixture was placed in each. Each container was correctly labeled, indicating the experiment, day, and repetition. All containers were incubated with the unclosed lids in a dark chamber at room temperature (25 ± 2 °C) for the 21-day duration of the experiment. The only ones that were not incubated were those on day 0, which were directly frozen. For each day of extraction, only the 9 soil samples corresponding to that day were frozen to perform the extraction together. Every 2 to 3 days, both temperature and humidity were monitored by weight.
To interpret the dissipation of the applied pesticides, in each case, first-order regression models were fitted (Equation (1)). Pesticide dissipation in soil is often assumed to be a first-order reaction [
72,
73,
74,
75,
76,
77].
where
- -
C is the concentration after a given time (t)
- -
Co is the initial concentration
- -
k is the dissipation constant
The half-life time was calculated with the regression equation using Equation (2):
All calculations were performed using Microsoft Office Excel (version 2021 18.0). In order to evaluate the effect of the treatments on the degradation time of the pesticides, a one-factor ANOVA and Tukey’s Post Hoc test were performed. Previously, the homogeneity of the variances was checked with Levene’s statistic, and a test for the normality of the variables (Kolmogorov-Smirnov test) was performed. A t-test for equality of means was also performed, in which the Levene test was previously carried out, as well as the normality test to determine the differences between the recoveries in the extraction methods. The program used was IBM’s SPSS Statistics (version 28).
4. Conclusions
In this study, the QS mega-method was compared with the classical Q method for the analysis of 13 pesticides in a Mediterranean calcic Luvisol. Additionally, the persistence of three of these pesticides (PDMT, OXF, and TFXT) was evaluated in this soil under three conditions: without amendment (S+P), with biowaste compost (S+P+C), and with cow manure (S+P+E).
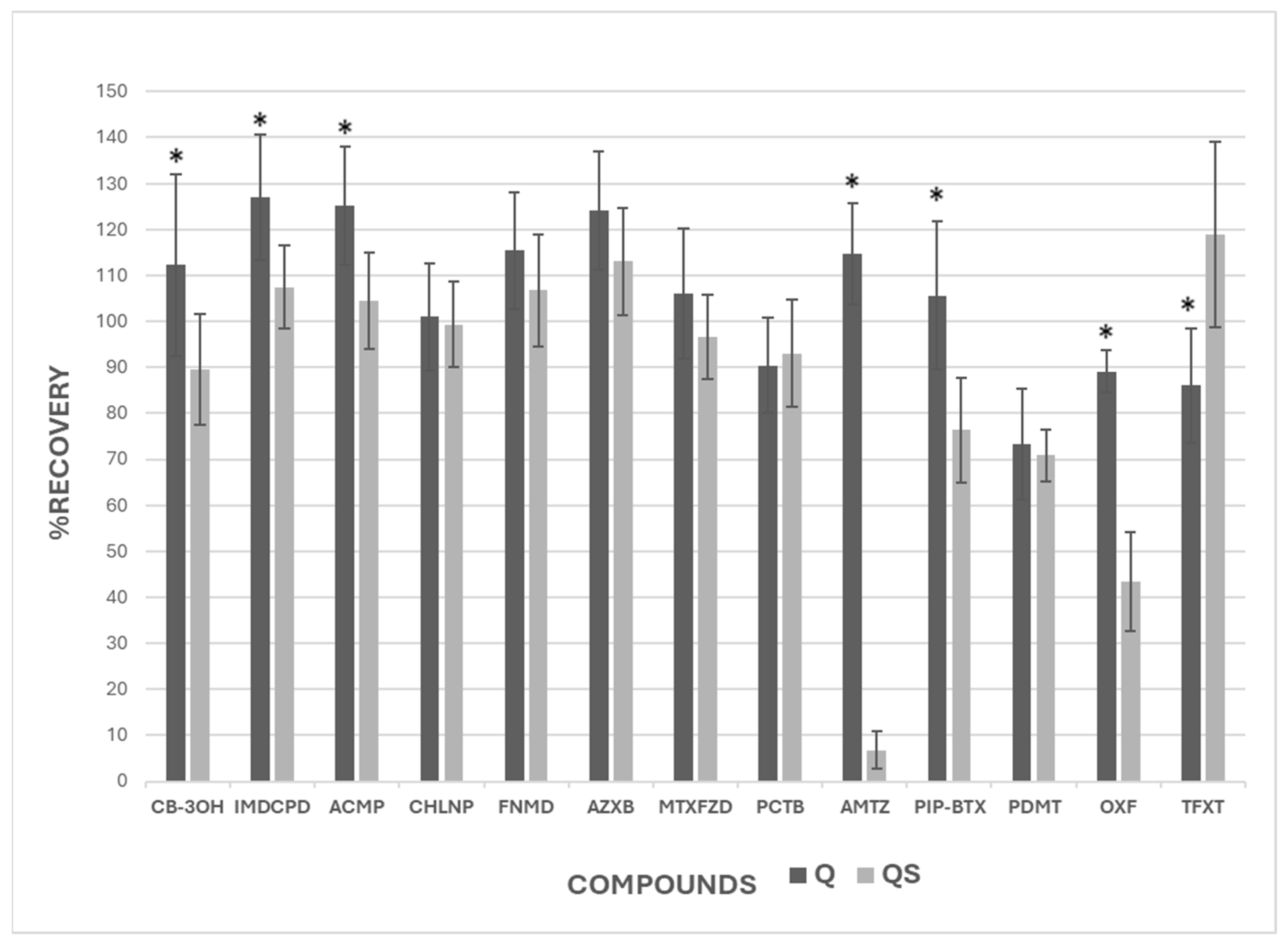
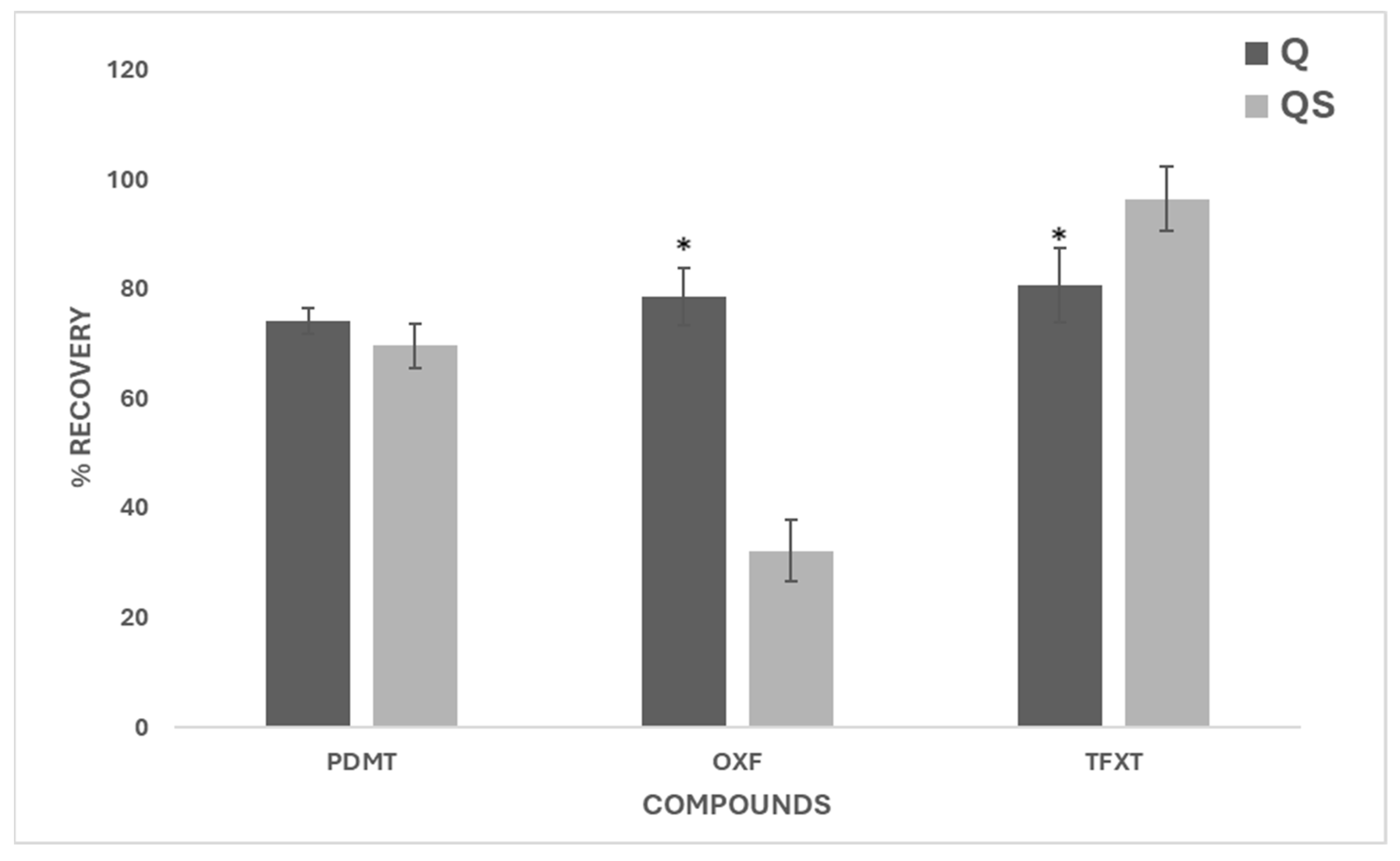
The application of both extraction methods showed that, in most cases, Q and QS recovered a significant proportion of the target compounds, although the efficiency varied depending on the pesticide. Notably, the QS method showed low recoveries for AMTZ, and OXF, showing that it is not a suitable method for their extraction. These results suggest that further research is needed to clarify under which conditions and for which compounds the QS method performs reliably, in order to validate its broader application across different soil types.
Following the evaluation of the persistence of PDMT, OXF, and TFTX in the selected soil and the effect of the application of two organic amendments (biowaste compost and cow manure), it was found that the herbicide OXF was the most persistent, while the fungicide TFXT showed very little persistence; the herbicide PDMT showed an intermediate behavior. PMDT and OXF were persistent in the non-amended soil. The soil persistence of the three pesticides decreased after the application of compost or cow manure. The half-life times obtained in the S+P+C and S+P+E treatments were: 33 d and 50 d for the herbicide PDMT, and 53 d and 58 d for OXF. For the ungicide TFXT, the half-life times were 5.2 d, 5.2 d, and 4.5 d for S+P, S+P+C, and S+P+E, respectively. The application of the two organic amendments, under laboratory conditions, significantly decreased the half-life time of PMDT, OXF, and TFXT. PDMT and OXF significantly reduced their half-life time after application of both compost and cow manure; the TXFT did so after the manure application.
Future studies might perform validation in different soils and evaluate the recovery of other pesticides. In addition, more studies are needed in order to determine whether the application of organic amendments facilitates the degradation of these pesticides and under what conditions they are more effective. It is also necessary to study the impact of these compounds on soil biodiversity and soil properties in order to promote more appropriate legislation and standards for the use of these substances, as well as stricter controls to avoid their excessive use and unnecessary risks.