Managing PFAS in Sewage Sludge: Exposure Pathways, Impacts, and Treatment Innovations
Abstract
1. Introduction
2. Analytical Methods for PFAS Detection in Sludge
2.1. Methodology for Screening the Relevant Literature
2.2. PFAS Analysis in Sludge Matrices
| Matrix | Key Preparation Steps | Notes | Study |
|---|---|---|---|
| Sewage biosolids | Freeze-drying → Grinding → Spike with standards → Methanol extraction → Ultrasonication → Centrifugation → Cleanup via mixed sorbents (C18, WAX, PSA) | Combined sorbents improved recovery; reducing the PSA/C18 amount increased efficiency (40–100% → 80–180%). | [28] |
| Sediment/sludge | Basic-methanol extraction → Ultrasonic bath → Graphitized carbon cleanup | Developed for freeze-dried and wet matrices; evaluated recovery, MDLs, matrix effects. | [30] |
| Sewage sludge | Oven-drying → Pulverizing → Ultrasonication with persulfate (acid–microwave) → Focused on degradation process, not cleanup | Tested ultrasonic and oxidative methods; found ineffective for PFAS destruction but informative for treatment. | [31] |
| Sewage sludge | Hydrothermal treatment → Sampling → LC-MS/MS analysis | Provided complete PFAS concentration profiles before/after thermal treatment. | [32] |
2.3. Analytical Strategies for Identifying PFAS Compounds
| Method | Target PFAS | Advantages | Limitations | References |
|---|---|---|---|---|
| LC-MS/MS | Ionic PFAS (e.g., PFSA, PFCA) | High sensitivity; widely used; suitable for a broad range of PFAS | Time consuming; costly; limited for volatile/neutral PFAS | [17,38,42,43] |
| GC-MS (with derivatization) | Volatile, semi-volatile, and some ionic PFAS | Better separation; reduced contamination; and detection of derivatized ionic PFAS | Requires derivatization; less reproducible; limited compound range | [40,44,45,46] |
| LC-HRMS (High-Resolution MS) | PFAS and unknown precursors | Non-targeted screening; identification of unknown compounds | Requires complex data analysis; expensive equipment | [47,48] |
| TOF-MS (Time-of-Flight MS) | Precursors and transformation products | High mass accuracy; ideal for structure elucidation | Lower sensitivity for trace quantification | [49] |
| Orbitrap MS | Emerging PFAS and transformation products | Ultra-high resolution; suitable for complex mixtures | High cost; specialized expertise needed | [50] |
| GCxGC-MS (Two-dimensional GC) | Volatile/neutral PFAS | Enhanced separation; functional for complex samples | Technically demanding; not suitable for ionic PFAS | [51] |
2.4. Challenges in Quantifying PFAS and Emerging Techniques
3. Occurrence of PFAS in Sludge Matrices
3.1. Global PFAS Concentrations in Sewage Sludge
| Continent | Country | Period | PFAS Type | Concentration in Sludge (ng/g DW) | References |
|---|---|---|---|---|---|
| North America | Canada | 2009–2010 | 13 PFAAs | 2.1–17,000 | [56] |
| 2015–2016 | 11 PFAAs | 1316 (as Fluor) | [55] | ||
| 2012–2017 | 22 PFAS | 4.93–92.6 | [57] | ||
| USA | 2001 | PFCAs, PFSAs | 402 | [58] | |
| 2005–2013 | 8 PFCAs, 4 PFSAs | 22.5 | [59] | ||
| 2018 | 24 PFAS | 195 | [62] | ||
| 2019 | 24 PFAS | 16–204 | [63] | ||
| 2021 | 92 PFAS | 182–1650 | [60] | ||
| 2022 | 40 PFAS | 114–206 | [61] | ||
| ON | 24 PFAS | 1–3200 | [64] | ||
| Europe | Germany | 2008–2013 | 11 PFAAs | >500,000 | [65] |
| 2010–2016 | PFOA, PFOS | 702 | [66] | ||
| Sweden | 2004–2017 | 79 PFAS | 50–1124 | [67] | |
| France | 1976–2017 | 42 PFAS | 220 | [68] | |
| Spain | 2011 | 8 PFAAs | <0.01–287 | [69] | |
| Greece | 2009–2010 | 18 PFAS | <0.26–237.2 | [70] | |
| Denmark | 2017 | 73 PFAS | 142 | [71] | |
| PFOA, PFNA, PFDA, PFOS | 0.4–34.1 | [72] | |||
| Finland | 2017 | 73 PFAS | 129 | [71] | |
| Sweden | 2017 | 73 PFAS | 102 | [71] | |
| Norway | 2017 | 73 PFAS | 75 | [71] | |
| Italy | 2018 | PFOA, PFOS | 2.5–22.4 | [36] | |
| Switzerland | 2008–2011 | PFAAs | 4–2480 | [73] | |
| Netherlands | 2008–2011 | PFBA, PFOS | 0.8–2440 | [73] | |
| Asia | S. Korea | 2010 | 15 PFAS | 0.8–1400 | [74] |
| China | 2011 | C3–C14 PFAAs | 126–809 | [75] | |
| 2010 | PFHxA, PFOS | 0.35–135 | [76] | ||
| Singapore | 2006–2007 | PFOA, PFOS | 6.5–702.2 | [77] | |
| Thailand | 2009 | 10 PFAAs | 1534.5 | [78] | |
| Hong Kong | 2008 | 19 PFAS | 18.7–7466.2 | [79] | |
| Africa | Nigeria | 2012 | 7 PFCAs, 3 PFSAs | 0.01–0.597 | [80] |
| Kenya | 2013 | 10 PFAAs | 0.098–0.683 | [81] | |
| Oceania | Australia | 2014 | 9 PFAAs | 5.2–150 | [82] |
| 2018 | 44 PFAS | 4.2–910 | [35] |
3.2. Factors Influencing PFAS Concentrations in Sewage Sludge
3.3. PFAS Fate and Pathways via Sludge Management
4. Regulatory Landscape for PFAS in Sludge
4.1. International Regulations and Advisory Levels
| Country/Region | Matrix | Regulated Compounds | Limit/Advisory Level | Reference |
|---|---|---|---|---|
| USA—Michigan | Biosolids | PFOS | ≥50 ng/g: remediation required | [106] |
| USA—Michigan | Biosolids | PFOS | ≥125 ng/g: land application prohibited | [106] |
| USA—New York | Biosolids | PFOA, PFOS | ≥20 ng/g: remediation required | [106] |
| USA—New York | Biosolids | PFOA, PFOS | ≥50 ng/g: land application prohibited | [106] |
| USA—Maine | Biosolids | PFBS, PFOS, PFOA | PFBS: 1900 ng/g; PFOS: 5.2 ng/g; PFOA: 2.5 ng/g | [110] |
| USA—Maine | Biosolids | General | Land application banned | [111] |
| Germany | Biosolids/Soil | PFOS + PFOA | 100 ng/g | [85] |
| UK | Sewage Sludge | PFOS | 46 ng/g | [112] |
| Austria | Sewage Sludge | PFOS + PFOA | 100 ng/g | [112] |
| Canada | Agricultural Soil | PFOS | 10 ng/g | [108] |
| Australia | Soil (post-application) | PFOA, PFOS | 4 ng/g (each) | [109] |
| Australia | Soil (post-application) | C9–C14 PFCAs | 10 ng/g | [109] |
| Netherlands | Soil | PFOS, PFOA | PFOS: 0.9 μg/kg, PFOA: 0.8 μg/kg | [107] |
| Denmark | Soil | PFOS | 390 ng/g | [113] |
| Denmark | Soil | PFOA | 1300 ng/g | [113] |
4.2. PFAS Regulation in Romania: National Context
5. Environmental and Health Risks Related to PFAS in Biosolids
5.1. Risk of PFAS Uptake by Crops and Food Chain Contamination
5.2. Human Exposure Pathways via Soil, Air, and Water
| Country | Surface Water | Groundwater | Drinking Water | Sampling Context | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| PFOA (ng/L) | PFOS (ng/L) | PFOA (ng/L) | PFOS (ng/L) | PFOA (ng/L) | PFOS (ng/L) | |||
| Italy | 15.9 | 38.5 | - | - | 1475 | 117 | Monitoring near Lake Maggiore, influenced by industrial/urban sources | [141,142] |
| Spain | 2.6 | 4.3 | - | - | 29 | 140 | Municipal drinking water survey as part of multi-country study | [143,144] |
| Germany | 3640 | 193 | 160 | 8350 | 519 | 22 | Fire training area contamination in Cologne, private well sampling | [133,145] |
| Sweden | 522 | 2280 | 4470 | 42,200 | 100 | 8000 | Sites near firefighting training areas, biomonitoring study | [131,136,146] |
| UK | 370 | 17 | 230 | 208 | 263 | 130 | National survey of PFOS, PFOA in drinking/source waters | [147] |
| Netherlands | 2060 | 110 | 11.1 | 5 | - | - | National groundwater/drinking water PFAS survey | [148,149] |
| France | 7 | 62 | 16 | 50 | 18 | 11 | National screening of raw/treated tap water | [150,151] |
| Ireland | - | - | 96 | 1.3 | 1.8 | 7.1 | Groundwater and landfill-impacted drinking water | [138,152] |
| Greece | - | - | - | - | 3.6 | - | Urban drinking water sampling | [149] |
| China | 223.8 | 30.2 | 2510 | 403 | 9.7 | 2.7 | Near fluorochemical park and urban water sources | [134,153,154] |
| Japan | 360 | 97 | 1800 | 990 | 12 | 11 | Urban and coastal water monitoring | [135,155] |
| Korea | 730 | 550 | 6.72 | 2.35 | 20.7 | 10.1 | Post-leakage surveys, national water monitoring | [156,157,158] |
| Philippines | 8.4 | 2.9 | - | - | 3 | 0.4 | Urban drinking and source water sampling | [159] |
| Thailand | 10.7 | 1.3 | 34.96 | 25.88 | 16.5 | 6.3 | Groundwater near waste sites, metropolitan tap water | [159,160,161] |
| India | 1.18 | 1.73 | 0.76 | 1.13 | - | - | Urban drinking water sampling | [83] |
| Vietnam | 53.5 | 40.2 | 5.48 | 1.42 | 0.5 | - | National surface water survey | [162] |
| Taiwan | 68.9 | 61.2 | 40.3 | 76.8 | - | - | Drinking water sources and groundwater in urban areas | [163,164] |
| Ghana | 321.1 | 276.6 | - | - | 190 | 168.3 | River basin surface and tap water sampling | [165] |
| Canada | 21 | 15 | 3260 | 1450 | 7.6 | 5.9 | Groundwater treatment and provincial surveys | [137,166] |
| USA | 11,000 | 1090 | 24,000 | 1600 | 4300 | 15 | National survey of source and treated waters | [132,139] |
| Australia | 11 | 34 | 580 | 13,000 | 9.7 | 15.6 | Surface/groundwater monitoring, flood impact study | [167,168,169] |
5.3. Toxicological Effects and Regulatory Implications
5.4. PFAS-Induced Hepatotoxicity
6. PFAS Removal and Treatment Technologies for Sludge
6.1. Thermal and Hydrothermal Treatments
6.2. Adsorption and Advanced Oxidation Processes
6.3. Biotransformation and Innovative Remediation Approaches
| PFAS | Initial Concentration | Microbial Strains | Biodegradation and Defluorination Efficiency | Intermediates | Reference |
|---|---|---|---|---|---|
| PFOA | 500 mg/L | Pseudomonas parafulva | 32% in 72 h; 48% with 1 g/L glucose in 96 h | - | [236] |
| PFOS | - | Pseudomonas plecoglossicida 2.4-D | 75%, with fluoride release | PFHpA | [93] |
| PFOA | 0.1/100 mg/L | Acidimicrobium sp. strain A6 | 63% (0.1 mg/L), 100 d 50% (100 mg/L), 100 d | PFBA; PFPeA; PFHxA; PFHpA | [235] |
| PFOS | 0.1/100 mg/L | Acidimicrobium sp. strain A6 | 60% (0.1 mg/L), 100 d 47% (100 mg/L), 100 d | PFBA; PFBS | [235] |
| PFOA/ PFOS | 5 mg/L each | Mixed culture | 0% (aerobic), 30 d 100% (anaerobe), 30 d | Not reported | [242] |
| PFOA | 500 mg/L | Pseudomonas parafulva strain YAB1 | 48%, 5 d | Not reported | [236] |
| PFOS | 1.8 mg/L | Pseudomonas aeruginosa strain HJ4 | 67%, 2 d | PFBS; PFHxS | [243] |
| PFOS | 1000 mg/L | Pseudomonas plecoglossicida 2.4-D | 100%, 6 d | PFHpA | [244] |
| PFHxS | 0.2 mg/L | Pseudomonas sp. strain PS27 | 32%, 10 d | Not reported | [245] |
| PFHxS | 0.2 mg/L | Pseudomonas sp. strain PDMF10 | 28%, 10 d | Not reported | [245] |
| PFOA | 10 mg/L | Pseudomonas aeruginosa | 29%, 4 d | PFHxA | [237] |
| PFOA | 10 mg/L | Pseudomonas putida | 19%, 4 d | PFPeA; PFPxA; PFHpA | [237] |
| PFOS | 10 mg/L | Pseudomonas aeruginosa | 47%, 4 d | PHHxA; PFHpA | [237] |
| PFOS | 10 mg/L | Pseudomonas putida | 47%, 4 d | PHHxA; PFHpA | [237] |
| My-C4c | 75 µM | Dehalococcoides | 100% removal in 1 day; 100% defluorination in 2 weeks | CoA forms (undetected) | [242] |
| My-C5d | 75 µM | Dehalococcoides | 50% removal; 82% defluorination | MeUC5d_TP209; TP121; TP139 | [241] |
| 6:2 FTUCA | 75 µM | Dehalococcoides | 10% defluorination | PFHxA; PFPeA; 2H-PFHpA; 2H-PFHxA | [241] |
| 3,3,3-trifluoropropionic acid | 50 µM | Activated sludge community | 100% removal; 85% defluorination | - | [240] |
| 2-fluoropropionic acid | 50 µM | Activated sludge community | 100% removal; 21% defluorination | Volatile alkanes | [240] |
| 5,5,5-trifluoropentanoic acid | 50 µM | Activated sludge community | 100% removal; 37% defluorination | Not reported | [240] |
| 4,5,5-trifluoropent-4-enoic acid | 50 µM | Activated sludge community | 30% removal; 71% defluorination | Monofluoromalonyl-CoA | [240] |
| 6:2 diPAP | 4.22 nmol/g soil | Soil microbes | - | 5:2 sFTOH; 6:2 FTOH; PFBA; PFPeA; PFHxA; 5:3 Acid | [246] |
| 8:2 diPAP | 3.37 nmol/g soil | Soil microbes | - | PFOA; PFHxA; 7:3 Acid; PFHpA | [246] |
| PFMeUPA | 75 µM | Dehalococcoides | 100% transformation; 10% defluorination | Not reported | [241] |
| My-C5d | 75 µM | Dehalococcoides | 100% transformation; 78% defluorination | MeUC5d_TP209 | [103] |
6.4. Comparative Evaluation of PFAS-Biosolids Treatment Technologies
7. Research Gaps and Future Perspectives
7.1. Analytical Standardization Needs
7.2. Policy and Monitoring Priorities
7.3. Emerging PFAS and Unknown Precursors
7.4. Need for Long-Term Field Studies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalmar, A.F.; Groffen, T.; Vereecke, H.; Teunkens, A.; Dewinter, G.; Mulier, H.; Struys, M.M.R.F.; Rex, S. Volatile anaesthetics and PFAS forever chemicals: A critical gap in environmental impact assessments. Best Pract. Res. Clin. Anaesthesiol. 2024, 38, 342–348. [Google Scholar] [CrossRef]
- Dias, D.; Bons, J.; Kumar, A.; Kabir, M.H.; Liang, H. Forever Chemicals, Per-and Polyfluoroalkyl Substances (PFAS), in Lubrication. Lubricants 2024, 12, 114. [Google Scholar] [CrossRef]
- Perera, D.C.; Meegoda, J.N. PFAS: The Journey from Wonder Chemicals to Environmental Nightmares and the Search for Solutions. Appl. Sci. 2024, 14, 8611. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Pirvu, F.; Paun, I.; Petre, V.A. Perfluoroalkyl substances in Romanian wastewater treatment plants: Transfer to surface waters, environmental and human risk assessment. Sci. Total Environ. 2023, 892, 164576. [Google Scholar] [CrossRef] [PubMed]
- Cimpean, I.-A.; Paun, I.; Pirvu, F.; Iancu, V.I.; Chiriac, F.L. Unregulated and Regulated PFASs in Bottled and Tap Water: Occurrence, Co-Occurrence Patterns, and Implications for Human Health and Regulatory Frameworks. J. Xenobiot. 2025, 15, 81. [Google Scholar] [CrossRef]
- Monk, J.R.; Hooda, P.S.; Busquets, R.; Sims, D. Occurrence of pharmaceuticals, illicit drugs and PFAS in global surface waters: A meta-analysis-based review. Environ. Pollut. 2025, 378, 126412. [Google Scholar] [CrossRef]
- Rey, D.M.; Briggs, M.A.; Tokranov, A.K.; Lind, H.G.; Scordato, P.T.; Iery, R.D.; Moore, H.E.; Slater, L.D.; LeBlanc, D.R. Groundwater flowpath characteristics drive variability in per- and polyfluoroalkyl substances (PFAS) loading across a stream-wetland system. Sci. Total Environ. 2025, 964, 178533. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, C.; Feng, G.; Zhang, B.-X.; Xu, Z.-Y.; Wang, Y.; Tleubergenova, A.; Zhang, Y.; Meng, X.-Z. Downward migration of per- and polyfluoroalkyl substances (PFAS) in lake sediments: Reconsideration of temporal trend analysis. J. Hazard. Mater. 2025, 492, 138290. [Google Scholar] [CrossRef]
- Beriro, D.J.; Bearcock, J.M.; Vane, C.H.; Marchant, B.; Martin, I.; Haslam, A.; Pickering, H.; Hughes, M.; James, A. A comparative analysis of PFAS in archive and fresh soil samples in England and implications for large-scale surveys. Environ. Pollut. 2025, 379, 126401. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Corrà, F.; Bellio, M.; Guidolin, L.; Tallandini, L.; Irato, P.; Santovito, G. PFAS environmental pollution and antioxidant responses: An overview of the impact on human field. Int. J. Environ. Res. Public Health 2020, 17, 8020. [Google Scholar] [CrossRef]
- Ehrlich, V.; Bil, W.; Vandebriel, R.; Granum, B.; Luijten, M.; Lindeman, B.; Grandjean, P.; Kaiser, A.-M.; Hauzenberger, I.; Hartmann, C.; et al. Consideration of pathways for immunotoxicity of per- and polyfluoroalkyl substances (PFAS). Environ. Health 2023, 22, 19. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Ahrens, L.; Norström, K.; Viktor, T.; Cousins, A.P.; Josefsson, S. Stockholm Arlanda Airport as a source of per- and polyfluoroalkyl substances to water, sediment and fish. Chemosphere 2015, 129, 33–38. [Google Scholar] [CrossRef]
- Reinikainen, J.; Bouhoulle, E.; Sorvari, J. Inconsistencies in the EU regulatory risk assessment of PFAS call for readjustment. Environ. Int. 2024, 186, 108614. [Google Scholar] [CrossRef]
- European Parliament and Council. Directive (EU) 2020/2184 of 16 December 2020 on the quality of water intended for human consumption (recast). Off. J. Eur. Union 2020, 435, 1–62. [Google Scholar]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; Nebbia, C.S.; et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, X.; Liu, H.; Dong, S.; Zhang, Z.; Wang, Z.; Li, J.; Nghiem, L.D.; Khan, S.J.; Wang, Q. Occurrence, fate, and remediation for per- and polyfluoroalkyl substances (PFAS) in sewage sludge: A comprehensive review. J. Hazard. Mater. 2024, 466, 133637. [Google Scholar] [CrossRef]
- Duru, C.I.; Sherchan, S.P. A systematic review and meta analysis on the occurrence of per and polyfluoroalkyl substances (PFAS) in wastewater treatment plants. Water Air Soil Pollut. 2025, 236, 627. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, L.; Ding, Y.; Liu, S.; Li, Y.; Zhou, Z.; Liang, Y. Trends in the analysis and exploration of per- and polyfluoroalkyl substances (PFAS) in environmental matrices: A review. Crit. Rev. Anal. Chem. 2024, 54, 1–24. [Google Scholar] [CrossRef]
- Sørmo, E.; Lade, C.B.M.; Zhang, J.; Asimakopoulos, A.G.; Åsli, G.W.; Hubert, M.; Goranov, A.I.; Arp, H.P.H.; Cornelissen, G. Stabilization of PFAS-contaminated soil with sewage sludge- and wood-based biochar sorbents. Sci. Total Environ. 2024, 922, 170971. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Petre, A.V.; Cimpean, A.I.; Cojocaru, V.C.; Paun, I.; Pirvu, F.; Iancu, V.I. New LC-MS/MS method for the determination of unconventional organic pollutants: Perfluoroalkyl sulfonic acids in wastewater, surface water, and drinking water. Rom. J. Ecol. Environ. Chem. 2024, 6. [Google Scholar] [CrossRef]
- Rehman, A.U.; Crimi, M.; Andreescu, S. Current and emerging analytical techniques for the determination of PFAS in environmental samples. Trends Environ. Anal. Chem. 2023, 37, e00198. [Google Scholar] [CrossRef]
- Armstrong, D.L.; Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Temporal trends of perfluoroalkyl substances in limed biosolids from a large municipal water resource recovery facility. J. Environ. Manag. 2016, 165, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Moodie, D.; Coggan, T.; Berry, K.; Kolobaric, A.; Fernandes, M.; Lee, E.; Reichman, S.; Nugegoda, D.; Clarke, B.O. Legacy and emerging per- and polyfluoroalkyl substances (PFASs) in Australian biosolids. Chemosphere 2021, 270, 129143. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Draft Method 1633—Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS; U.S. Environmental Protection Agency: Washington, DC, USA, 2022. [Google Scholar]
- Kumar, R.; Vuppaladadiyam, A.K.; Antune, E.; Whelan, A.; Fearon, R.; Sheehan, M.; Reeves, L. Emerging contaminants in biosolids: Presence, fate and analytical techniques. Emerg. Contam. 2022, 8, 162–194. [Google Scholar] [CrossRef]
- Fredriksson, F.; Eriksson, U.; Karrman, A.; Yeung, L.W.Y. Per- and polyfluoroalkyl substances (PFAS) in sludge from wastewater treatment plants in Sweden-first findings of novel fluorinated copolymers in Europe including temporal analysis. Sci. Total Environ. 2022, 846, 157406. [Google Scholar] [CrossRef]
- Ozelcaglayan, A.; Parker, W.; Pham, A. The analysis of per- and polyfluoroalkyl substances in wastewater sludges and biosolids: Which adsorbents should be used for the cleanup of extracts? Environ. Sci. Water Res. Technol. 2023, 9, 794. [Google Scholar] [CrossRef]
- Drábová, L.; Dvořáková, D.; Urbancová, K.; Gramblička, T.; Hajšlová, J.; Pulkrabová, J. Critical assessment of clean-up techniques employed in simultaneous analysis of persistent organic pollutants and polycyclic aromatic hydrocarbons in fatty samples. Toxics 2022, 10, 12. [Google Scholar] [CrossRef]
- McNamara, M.; Hill, N.; Cashman, M.; Robuck, A. A method development study to comparatively measure diverse PFAS in wet and freeze-dried sediment. In Proceedings of the SETAC North America 45th Annual Meeting, Fort Worth, TX, USA, 20–24 October 2024; Available online: https://www.setac.org/discover-events/global-meetings/setac-north-america-45th-annual-meeting.html (accessed on 9 May 2025).
- Fournie, T.; Rashwan, T.L.; Switzer, C.; Gerhard, J.I. Smouldering to treat PFAS in sewage sludge. Waste Manag. 2023, 164, 219–227. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Y. Effects of hydrothermal treatments on destruction of per- and polyfluoroalkyl substances in sewage sludge. Environ. Pollut. 2021, 285, 117276. [Google Scholar] [CrossRef] [PubMed]
- Idowu, I.G.; Ekpe, O.D.; Megson, D.; Bruce-Vanderpuije, P.; Sandau, C.D. A systematic review of methods for the analysis of total per- and polyfluoroalkyl substances (PFAS). Sci. Total Environ. 2025, 967, 178644. [Google Scholar] [CrossRef]
- Strynar, M.; McCord, J.; Newton, S.; Washington, J.; Barzen-Hanson, K.; Trier, X.; Liu, Y.; Dimzon, I.K.; Bugsel, B.; Zwiener, C.; et al. Practical application guide for the discovery of novel PFAS in environmental samples using high resolution mass spectrometry. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Thai, P.K.; Kaserzon, S.L.; O′Brien, J.W.; Mueller, J.F. Nationwide occurrence and discharge mass load of per- and polyfluoroalkyl substances in effluent and biosolids: A snapshot from 75 wastewater treatment plants across Australia. J. Hazard. Mater. 2024, 470, 134203. [Google Scholar] [CrossRef]
- Riva, F.; Zuccato, E.; Pacciani, C.; Colombo, A.; Castiglioni, S. A multi-residue analytical method for extraction and analysis of pharmaceuticals and other selected emerging contaminants in sewage sludge. Anal. Methods 2021, 13, 526–535. [Google Scholar] [CrossRef]
- Kundu, S.; Patel, S.; Halder, P.; Patel, T.; Marzbali, M.H.; Pramanik, B.K.; Paz-Ferreiro, J.; de Figueiredo, C.C.; Bergmann, D.; Surapaneni, A.; et al. Removal of PFASs from biosolids using a semi-pilot scale pyrolysis reactor and the application of biosolids derived biochar for the removal of PFASs from contaminated water. Environ. Sci. Water Res. Technol. 2021, 7, 638–649. [Google Scholar] [CrossRef]
- Semerád, J.; Hatasová, N.; Grasserová, A.; Černá, T.; Filipová, A.; Hanč, A.; Innemanová, P.; Pivokonský, M.; Cajthaml, T. Screening for 32 per- and polyfluoroalkyl substances (PFAS) including GenX in sludges from 43 WWTPs located in the Czech Republic—Evaluation of potential accumulation in vegetables after application of biosolids. Chemosphere 2020, 261, 128018. [Google Scholar] [CrossRef]
- Gao, K.; Chen, Y.; Xue, Q.; Fu, J.; Fu, K.; Fu, J.; Zhang, A.; Cai, Z.; Jiang, G. Trends and perspectives in per- and polyfluorinated alkyl substances (PFASs) determination: Faster and broader. TrAC Trends Anal. Chem. 2020, 133, 116114. [Google Scholar] [CrossRef]
- Alzaga, R.; Bayona, J.M.A. Determination of perfluorocarboxylic acids in aqueous matrices by ion-pair solid-phase microextraction–in-port derivatization–gas chromatography–negative ion chemical ionization mass spectrometry. J. Chromatogr. A 2004, 1042, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Harada, K.H.; Zou, X.; Sun, C. Investigating isomers/enantiomers of perfluorooctanoic acid in river water by gas chromatography–mass spectrometry with chiral derivatization. Chemosphere 2019, 238, 124617. [Google Scholar] [CrossRef]
- Ganesan, S.; Chawengkijwanich, C.; Gopalakrishnan, M.; Janjaroen, D. Detection methods for sub-nanogram level of emerging pollutants—per and polyfluoroalkyl substances. Food Chem. Toxicol. 2022, 168, 113377. [Google Scholar] [CrossRef] [PubMed]
- Oza, S.; Bell, K.Y.; Xu, Z.; Wang, Y.; Wells, M.J.M.; Norton Jr., J.W.; Winchell, L.J.; Huang, Q.; Li, H. Surveillance of PFAS in sludge and biosolids at 12 water resource recovery facilities. J. Environ. Qual. 2025, 54, 6–19. [Google Scholar] [CrossRef]
- Atapattu, S.N.; Temerdashev, A. Recent advances in gas chromatography injection port derivatization in analytical method development. TrAC Trends Anal. Chem. 2023, 168, 117334. [Google Scholar] [CrossRef]
- Ye, R.; Di Lorenzo, R.A.; Clouthier, J.T.; Young, C.J.; VandenBoer, T.C. A rapid derivatization for quantitation of perfluorinated carboxylic acids from aqueous matrices by gas chromatography–mass spectrometry. Anal. Chem. 2023, 95, 7399–7407. [Google Scholar] [CrossRef]
- Naile, J.E.; Garrison, A.W.; Avants, J.K.; Washington, J.W. Isomers/enantiomers of perfluorocarboxylic acids: Method development and detection in environmental samples. Chemosphere 2016, 144, 1722–1728. [Google Scholar] [CrossRef]
- Liu, Y.; D’Agostino, L.A.; Qu, G.; Jiang, G.; Martin, J.W. High-resolution mass spectrometry (HRMS) methods for nontarget discovery and characterization of poly- and per-fluoroalkyl substances (PFASs) in environmental and human samples. TrAC Trends Anal. Chem. 2019, 121, 115420. [Google Scholar] [CrossRef]
- Koronaiou, L.-A.; Nannou, C.; Xanthopoulou, N.; Seretoudi, G.; Bikiaris, D.; Lambropoulou, D.A. High-resolution mass spectrometry-based strategies for the target analysis and suspect screening of per- and polyfluoroalkyl substances in aqueous matrices. Microchem. J. 2022, 179, 107457. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Smith, A.M.; Qiu, T.A. Detection, Quantification, and Isomer Differentiation of Per- and Polyfluoroalkyl Substances (PFAS) Using MALDI-TOF with Trapped Ion Mobility. J. Am. Soc. Mass Spectrom. 2024, 35, 317–325. [Google Scholar] [CrossRef]
- Zacs, D.; Bartkevics, V. Trace Determination of Perfluorooctane Sulfonate and Perfluorooctanoic Acid in Environmental Samples (Surface Water, Wastewater, Biota, Sediments, and Sewage Sludge) Using Liquid Chromatography—Orbitrap Mass Spectrometry. J. Chromatogr. A 2016, 1473, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rodriguez, R.; Piperakis, A.; Grandy, J.; McGregor, L.; Boegelsack, N.; Calder, H.; Edwards, M.; Papas, W.; Che, J.; Shields, S. PFAS emissions from functional textiles using micro-chamber and thermal desorption coupled to two-dimensional gas chromatography-time of flight mass spectrometry (TD-GC×GC-TOF MS). J. Chromatogr. A 2024, 1733, 465219. [Google Scholar] [CrossRef]
- Wang, Y.; Darling, S.B.; Chen, J. Selectivity of Per- and Polyfluoroalkyl Substance Sensors and Sorbents in Water. ACS Appl. Mater. Interfaces 2021, 13, 60789–60814. [Google Scholar] [CrossRef]
- Menger, R.F.; Funk, E.; Henry, C.S.; Borch, T. Sensors for detecting per- and polyfluoroalkyl substances (PFAS): A critical review of development challenges, current sensors, and commercialization obstacles. Chem. Eng. J. 2021, 417, 129133. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, S.; Han, D.; Yan, H. Advancements in detection techniques for per- and polyfluoroalkyl substances: A comprehensive review. TrAC Trends Anal. Chem. 2024, 176, 117754. [Google Scholar] [CrossRef]
- Lakshminarasimman, N.; Gewurtz, S.B.; Parker, W.J.; Smyth, S.A. Removal and formation of perfluoroalkyl substances in Canadian sludge treatment systems—A mass balance approach. Sci. Total Environ. 2021, 754, 142431. [Google Scholar] [CrossRef]
- Guerra, P.; Kim, M.; Kinsman, L.; Ng, T.; Alaee, M.; Smyth, S.A. Parameters affecting the formation of perfluoroalkyl acids during wastewater treatment. J. Hazard. Mater. 2014, 272, 148–154. [Google Scholar] [CrossRef]
- Letcher, R.J.; Chu, S.; Smyth, S.-A. Side-chain fluorinated polymer surfactants in biosolids from wastewater treatment plants. J. Hazard. Mater. 2020, 388, 122044. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.K.; Halden, R.U. National inventory of perfluoroalkyl substances in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. J. Hazard. Mater. 2013, 252–253, 413–418. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Hooper, J.; Modiri-Gharehveran, M.; Drennan, D.M.; Beecher, N.; Lee, L. Release of poly- and perfluoroalkyl substances from finished biosolids in soil mesocosms. Water Res. 2022, 217, 118405. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.A.; Mortazavian, S.; Gonzalez, D.J.; Bott, C.; Hooper, J.; Schaefer, C.E. Poly- and perfluoroalkyl substances in municipal wastewater treatment plants in the United States: Seasonal patterns and meta-analysis of long-term trends and average concentrations. ACS Est. Water 2022, 2, 690–700. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Hooper, J.L.; Strom, L.E.; Abusallout, I.; Dickenson, E.R.V.; Thompson, K.A. Occurrence of quantifiable and semi-quantifiable poly- and perfluoroalkyl substances in United States wastewater treatment plants. Water Res. 2023, 233, 119724. [Google Scholar] [CrossRef]
- Michigan Department of Environment, Great Lakes, and Energy (EGLE). Biosolids and PFAS: Quick Facts for Landowners/Farmers. 2022. Available online: https://www.michigan.gov/-/media/Project/Websites/egle/Documents/Programs/WRD/Biosolids/biosolids-pfas-facts-landowners-farmers.pdf?rev=641c693bd1c24188a5a14a83704302cf (accessed on 12 May 2025).
- Tavasoli, E.; Luek, J.L.; Malley, J.P.; Mouser, P.J. Distribution and fate of per- and polyfluoroalkyl substances (PFAS) in wastewater treatment facilities. Environ. Sci. Process. Impacts 2021, 23, 903–913. [Google Scholar] [CrossRef]
- Link, G.W.; Reeves, D.M.; Cassidy, D.P.; Coffin, E.S. Per- and polyfluoroalkyl substances (PFAS) in final treated solids (biosolids) from 190 Michigan wastewater treatment plants. J. Hazard. Mater. 2024, 463, 132734. [Google Scholar] [CrossRef]
- Ulrich, H.; Freier, K.P.; Gierig, M. Getting on with persistent pollutants: Decreasing trends of perfluoroalkyl acids (PFAAs) in sewage sludge. Chemosphere 2016, 161, 527–535. [Google Scholar] [CrossRef]
- Stahl, T.; Gassmann, M.; Falk, S.; Brunn, H. Concentrations and distribution patterns of perfluoroalkyl acids in sewage sludge and in biowaste in Hesse, Germany. J. Agric. Food Chem. 2018, 66, 10147–10153. [Google Scholar] [CrossRef]
- Gobelius, L.; Glimstedt, L.; Olsson, J.; Wiberg, K.; Ahrens, L. Mass flow of per- and polyfluoroalkyl substances (PFAS) in a Swedish municipal wastewater network and wastewater treatment plant. Chemosphere 2023, 336, 139182. [Google Scholar] [CrossRef] [PubMed]
- Munoz, G.; Michaud, A.M.; Liu, M.; Vo Duy, S.; Montenach, D.; Resseguier, C. Target and nontarget screening of PFAS in biosolids, composts, and other organic waste products for land application in France. Environ. Sci. Technol. 2022, 56, 6056–6068. [Google Scholar] [CrossRef]
- Navarro, I.; Sanz, P.; Martínez, M.Á. Analysis of perfluorinated alkyl substances in Spanish sewage sludge by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 1277–1286. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Andersen, H.R.; Thomaidis, N.S.; Stasinakis, A.S. Sorption of perfluorinated compounds onto different types of sewage sludge and assessment of its importance during wastewater treatment. Chemosphere 2014, 111, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Aro, R.; Eriksson, U.; Kärrman, A.; Chen, F.; Wang, T.; Yeung, L.W.Y. Fluorine mass balance analysis of effluent and sludge from Nordic countries. ACS Est. Water 2021, 1, 2087–2096. [Google Scholar] [CrossRef]
- Bossi, R.; Strand, J.; Sortkjær, O.; Larsen, M.M. Perfluoroalkyl compounds in Danish wastewater treatment plants and aquatic environments. Environ. Int. 2008, 34, 443–450. [Google Scholar] [CrossRef]
- Alder, A.C.; Van Der Voet, J. Occurrence and point source characterization of perfluoroalkyl acids in sewage sludge. Chemosphere 2015, 129, 62–73. [Google Scholar] [CrossRef]
- Kim, S.-K.; Im, J.-K.; Kang, Y.-M.; Jung, S.-Y.; Kho, Y.L.; Zoh, K.-D. Wastewater treatment plants (WWTPs)-derived national discharge loads of perfluorinated compounds (PFCs). J. Hazard. Mater. 2012, 201–202, 82–91. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, C.-J.; Zhou, Q.; Chen, L.; Meng, X.-Z. Short- and long-chain perfluorinated acids in sewage sludge from Shanghai, China. Chemosphere 2012, 88, 1300–1305. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Qu, Y.; Chen, J.; Chen, L.; Liu, Y.; Zhou, Q. Quantitative characterization of short- and long-chain perfluorinated acids in solid matrices in Shanghai, China. Sci. Total Environ. 2010, 408, 617–623. [Google Scholar] [CrossRef]
- Yu, J.; Hu, J.; Tanaka, S.; Fujii, S. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in sewage treatment plants. Water Res. 2009, 43, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Kunacheva, C.; Tanaka, S.; Fujii, S.; Boontanon, S.K.; Musirat, C.; Wongwattana, T.; Shivakoti, B.R. Mass flows of perfluorinated compounds (PFCs) in central wastewater treatment plants of industrial zones in Thailand. Chemosphere 2011, 83, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Shih, K. Perfluorochemicals in wastewater treatment plants and sediments in Hong Kong. Environ. Pollut. 2010, 158, 1354–1362. [Google Scholar] [CrossRef]
- Sindiku, O.; Orata, F.; Weber, R.; Osibanjo, O. Per- and polyfluoroalkyl substances in selected sewage sludge in Nigeria. Chemosphere 2013, 92, 329–335. [Google Scholar] [CrossRef]
- Chirikona, F.; Filipovic, M.; Ooko, S.; Orata, F. Perfluoroalkyl acids in selected wastewater treatment plants and their discharge load within the Lake Victoria basin in Kenya. Environ. Monit. Assess. 2015, 187, 238. [Google Scholar] [CrossRef]
- Gallen, C.; Eaglesham, G.; Drage, D.; Nguyen, T.H.; Mueller, J.F. A mass estimate of perfluoroalkyl substance (PFAS) release from Australian wastewater treatment plants. Chemosphere 2018, 208, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.M.; Bharat, G.K.; Tayal, S.; Larssen, T.; Bečanová, J.; Karásková, P.; Whitehead, P.G.; Futter, M.N.; Nizzetto, L. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: Emissions and implications for human exposure. Environ. Pollut. 2016, 208, 704–713. [Google Scholar] [CrossRef]
- Kumari, A.; Singh Maurya, N.; Kumar, A.; Kant Yadav, R.; Kumar, A. Options for the disposal and reuse of wastewater sludge, associated benefit, and environmental risk. In Sustainable Development; Kılıç Taşeli, B., Ed.; IntechOpen: London, UK, 2023; Volume 5. [Google Scholar] [CrossRef]
- Saliu, T.D.; Sauvé, S. A review of per- and polyfluoroalkyl substances in biosolids: Geographical distribution and regulations. Front. Environ. Chem. 2024, 5, 1383185. [Google Scholar] [CrossRef]
- KimLazcano, R.; Perre, C.; Mashtare, M.L.; Lee, L.S. Per- and polyfluoroalkyl substances in commercially available biosolid-based products: The effect of treatment processes. Water Environ. Res. 2019, 91, 1669–1677. [Google Scholar] [CrossRef]
- Thompson, J.T.; Robey, N.M.; Tolaymat, T.M.; Bowden, J.A.; Solo-Gabriele, H.M.; Townsend, T.G. Underestimation of per- and polyfluoroalkyl substances in biosolids: Precursor transformation during conventional treatment. Environ. Sci. Technol. 2023, 57, 3825–3832. [Google Scholar] [CrossRef]
- Zhang, W.; Pang, S.; Lin, Z.; Mishra, S.; Bhatt, P.; Chen, S. Biotransformation of perfluoroalkyl acid precursors from various environmental systems: Advances and perspectives. Environ. Pollut. 2021, 272, 115908. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Lewis, A.J.; Sales, C.M.; Suri, R.; McKenzie, E.R. Linking PFAS partitioning behavior in sewage solids to the solid characteristics, solution chemistry, and treatment processes. Chemosphere 2021, 271, 129530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, H.; Li, F.; Hu, X.; Zhou, Q. Sorption of short- and long-chain perfluoroalkyl surfactants on sewage sludges. J. Hazard. Mater. 2013, 260, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, L.; Qiu, J.; Lv, Z.; Xia, W.; Wan, Y.; Li, Y.; Xu, S.Q. Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat offspring. Reprod. Toxicol. 2012, 33, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Coggan, T.L.; Moodie, D.; Kolobaric, A.; Szabo, D.; Shimeta, J.; Crosbie, N.D.; Lee, E.; Fernandes, M. An investigation into per- and polyfluoroalkyl substances (PFAS) in nineteen Australian wastewater treatment plants (WWTPs). Heliyon 2019, 5, e02316. [Google Scholar] [CrossRef]
- Chen, H.; Peng, H.; Yang, M.; Hu, J.; Zhang, Y. Detection, occurrence, and fate of fluorotelomer alcohols in municipal wastewater treatment. Environ. Sci. Technol. 2017, 51, 8953–8961. [Google Scholar] [CrossRef]
- Avendano, S.M.; Liu, J. Production of PFOS from aerobic soil biotransformation of two perfluoroalkyl sulfonamide derivatives. Chemosphere 2015, 119, 1084–1090. [Google Scholar] [CrossRef]
- Cui, D.; Li, X.; Quinete, N. Occurrence, fate, sources and toxicity of PFAS: What we know so far in Florida and major gaps. TrAC Trends Anal. Chem. 2020, 130, 115976. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, W.; Gong, Y.; Yu, Q.; Li, Q.; Sun, J.; Yuan, Z. Technologies for reducing sludge production in wastewater treatment plants: State of the art. Sci. Total Environ. 2017, 587–588, 510–521. [Google Scholar] [CrossRef]
- Ahrens, L.; Shoeib, M.; Harner, T.; Lee, S.; Guo, R.; Reiner, E. Wastewater treatment plant and landfills as sources of polyfluoroalkyl compounds to the atmosphere. Environ. Sci. Technol. 2011, 45, 8098–8105. [Google Scholar] [CrossRef]
- Hamid, H.; Li, L. Role of wastewater treatment plant (WWTP) in environmental cycling of poly- and perfluoroalkyl (PFAS) compounds. Ecocycles 2016, 2, 43–53. [Google Scholar] [CrossRef]
- Wang, B.; Yao, Y.; Chen, H.; Chang, S.; Tian, Y.; Sun, H. Per- and polyfluoroalkyl substances and the contribution of unknown precursors and short-chain (C2–C3) perfluoroalkyl carboxylic acids at solid waste disposal facilities. Sci. Total Environ. 2020, 705, 135832. [Google Scholar] [CrossRef] [PubMed]
- Gallen, C.; Drage, D.; Eaglesham, G.; Grant, S.; Bowman, M.; Mueller, J.F. Australia-wide assessment of perfluoroalkyl substances (PFASs) in landfill leachates. J. Hazard. Mater. 2017, 331, 132–141. [Google Scholar] [CrossRef]
- Dasu, K.; Xia, X.; Siriwardena, D.; Klupinski, T.P.; Seay, B. Concentration profiles of per- and polyfluoroalkyl substances in major sources to the environment. J. Environ. Manag. 2022, 301, 113879. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Liu, H.; Ting, Z.; Qin, Z.; Mou, J.; Sun, J.; Huang, S.; Chaves, A.V.; Gao, L.; et al. Bioproduction and applications of short-chain fatty acids from secondary sludge anaerobic fermentation: A critical review. Renew. Sustain. Energy Rev. 2023, 183, 113502. [Google Scholar] [CrossRef]
- USEPA. Biden-Harris Administration Finalizes First-Ever National Drinking Water Standard to Protect 100M People from PFAS Pollution. U.S. Environmental Protection Agency 2024. Available online: https://www.epa.gov/newsreleases/biden-harris-administration-finalizes-first-ever-national-drinking-water-standard (accessed on 24 May 2025).
- Sauvé, S.; Barbeau, B.; Bouchard, M.F.; Verner, M.-A.; Liu, J. How should we interpret the new water quality regulations for per- and polyfluoroalkyl substances? ACS EST Water 2023, 3, 2810–2815. [Google Scholar] [CrossRef]
- EU. Amending regulation (EC) No 1881/2006 as regards maximum levels of perfluoroalkyl substances in certain foodstuffs. Official J. Eur. Union 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022R2388 (accessed on 9 May 2025).
- Maine Department of Environmental Protection. An Evaluation of Biosolids Management in Maine and Recommendations for the Future. United States: Final Report. 2024. Available online: https://www.maine.gov/tools/whatsnew/attach.php?id=12198306&an=1 (accessed on 21 June 2025).
- The Dutch National Institute for Public Health and the Environment (RIVM). Nitrogen and PFAS Suddenly Big Societal Issues in The Netherlands. RIVM Newsletter. 2019. Available online: https://www.rivm.nl/en/newsletter/content/2020/issue1/nitrogen-pfas-in-NL (accessed on 21 June 2025).
- CCME. Canadian Soil and Groundwater Quality guidelines for the Protection of Environmental and Human Health. Canadian Council of Ministers of the Environment. 2021. Available online: https://ccme.ca/en/res/pfosfactsheeten.pdf (accessed on 15 April 2025).
- End of Waste Code Biosolids. The Queensland End of Waste Code Biosolids in 2020. Queensland Government. 2020. Available online: https://info.awa.asn.au/ (accessed on 23 April 2025).
- Maine PFAS Report. Managing PFAS in Maine. Maine PFAS Task Force. 2020. Available online: https://www.maine.gov/pfastaskforce/materials/report/PFAS-Task-Force-Report-FINAL-Jan2020.pdf (accessed on 14 May 2025).
- Maine Bill, L.D. State of Maine 130th Legislature Second Regular Session. Maine Legislature 1911. Available online: https://legislature.maine.gov/legis/bills/getPDF.asp?paper=HP1417&item=7&snum=130 (accessed on 14 May 2025).
- Department of the Environment and Energy, Australian Government. Commonwealth Environmental Management Guidance on Perfluorooctane Sulfonic Acid (PFOS) and Perfluorooctanoic Acid (PFOA). Aust. Gov. 2016, 1–33. [Google Scholar]
- Larsen, P.B.; Giovalle, E. Perfluoroalkylated Substances: PFOA, PFOS and PFOSA; Evaluation of health hazards and proposal of a health based quality criterion for drinking water, soil and ground water; Environmental Project No. 1665; The Danish Environmental Protection Agency: Copenhagen, Denmark, 2015. [Google Scholar]
- Hamade, A. Fish consumption benefits and PFAS risks: Epidemiology and public health recommendations. Toxicol. Rep. 2024, 13, 101736. [Google Scholar] [CrossRef]
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS exposure pathways for humans and wildlife: A synthesis of current knowledge and key gaps in understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [CrossRef]
- Mei, W.; Sun, H.; Song, M.; Jiang, L.; Li, Y.; Lu, W.; Ying, G.G.; Luo, C.; Zhang, G. Per- and polyfluoroalkyl substances (PFASs) in the soil–plant system: Sorption, root uptake, and translocation. Environ. Int. 2021, 156, 106642. [Google Scholar] [CrossRef]
- Blaine, A.C.; Rich, C.D.; Sedlacko, E.M.; Hyland, K.C.; Stushnoff, C.; Dickenson, E.R.V.; Higgins, C.P. Perfluoroalkyl acid uptake in lettuce (Lactuca sativa) and strawberry (Fragaria ananassa) irrigated with reclaimed water. Environ. Sci. Technol. 2014, 48, 14361–14368. [Google Scholar] [CrossRef]
- Lundgren, M.R.; Des Marais, D.L. Life history variation as a model for understanding trade-offs in plant–environment interactions. Curr. Biol. 2020, 30, 180–189. [Google Scholar] [CrossRef]
- Jiang, J.; Liang, L.; Ma, Q.; Zhao, T. Kernel nutrient composition and antioxidant ability of Corylus spp. in China. Front. Plant Sci. 2021, 12, 690966. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Wu, Y.; Zhang, H.; Liu, Y.; Hu, X.; Huang, H.; Zhang, S. The roles of protein and lipid in the accumulation and distribution of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in plants grown in biosolids-amended soils. Environ. Pollut. 2016, 216, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Felizeter, S.; Jürling, H.; Kotthoff, M.; De Voogt, P.; McLachlan, M.S. Uptake of perfluorinated alkyl acids by crops: Results from a field study. Environ. Sci. Process. Impacts 2021, 23, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Adu, O.; Ma, X.; Sharma, V.K. Bioavailability, phytotoxicity and plant uptake of per- and polyfluoroalkyl substances (PFAS): A review. J. Hazard. Mater. 2023, 447, 130805–130814. [Google Scholar] [CrossRef]
- Lasters, R.; Groffen, T.; Eens, M.; Bervoets, L. Dynamic spatiotemporal changes of per- and polyfluoroalkyl substances (PFAS) in soil and eggs of private gardens at different distances from a fluorochemical plant. Environ. Pollut. 2024, 346, 123613. [Google Scholar] [CrossRef]
- Zeilmaker, M.J.; Janssen, P.; Versteegh, A.; Van Pul, A.; De Vries, W. Risicoschatting Emissie PFOA voor Omwonenden. Locatie: DuPont/Chemours; RIVM Rapport: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Bonato, T.; Pal, T.; Benna, C.; Di Maria, F. Contamination of the terrestrial food chain by per- and polyfluoroalkyl substances (PFAS) and related human health risks: A systematic review. Sci. Total Environ. 2025, 961, 178337. [Google Scholar] [CrossRef]
- Pérez, F.; Llorca, M.; Köck-Schulmeyer, M.; Škrbić, B.; Oliveira, L.S.; da Boit Martinello, K. Assessment of perfluoroalkyl substances in food items at global scale. Environ. Res. 2014, 135, 181–189. [Google Scholar] [CrossRef]
- Su, H.; Shi, Y.; Lu, Y.; Wang, P.; Zhang, M.; Sweetman, A.; Jones, K.; Johnson, A. Home produced eggs: An important pathway of human exposure to perfluorobutanoic acid (PFBA) and perfluorooctanoic acid (PFOA) around a fluorochemical industrial park in China. Environ. Int. 2017, 101, 1–6. [Google Scholar] [CrossRef]
- Xiao, K.; Li, X.; Xu, N.; Wang, X.; Hao, L.; Bao, H.; Zhang, L.; Shi, Y.; Cai, Y. Carry-over rate of per- and polyfluoroalkyl substances to raw milk and human exposure risks in different regions of China. Sci. Total Environ. 2024, 944, 173902. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Environmental impacts, exposure pathways, and health effects of PFOA and PFOS. Ecotoxicol. Environ. Saf. 2023, 267, 115663. [Google Scholar] [CrossRef]
- Filipovic, M.; Woldegiorgis, A.; Norström, K.; Bibi, M.; Lindberg, M.; Österås, A.H. Historical usage of aqueous film forming foam: A case study of the widespread distribution of perfluoroalkyl acids from a military airport to groundwater, lakes, soils and fish. Chemosphere 2015, 129, 39–45. [Google Scholar] [CrossRef]
- Xiao, F.; Simcik, M.F.; Halbach, T.R.; Gulliver, J.S. Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a US metropolitan area: Migration and implications for human exposure. Water Res. 2015, 72, 64–74. [Google Scholar] [CrossRef]
- Weiß, O.; Wiesmüller, G.A.; Bunte, A.; Göen, T.; Schmidt, C.K.; Wilhelm, M.; Hölzer, J. Perfluorinated compounds in the vicinity of a fire training area—Human biomonitoring among 10 persons drinking water from contaminated private wells in Cologne, Germany. Int. J. Hyg. Environ. Health 2012, 215, 212–215. [Google Scholar] [CrossRef]
- Bao, J.; Yu, W.J.; Liu, Y.; Wang, X.; Jin, Y.H.; Dong, G.H. Perfluoroalkyl substances in groundwater and home-produced vegetables and eggs around a fluorochemical industrial park in China. Ecotoxicol. Environ. Saf. 2019, 171, 199–205. [Google Scholar] [CrossRef]
- Kuroda, K.; Murakami, M.; Oguma, K.; Takada, H.; Takizawa, S. Investigating sources and pathways of perfluoroalkyl acids (PFAAs) in aquifers in Tokyo using multiple tracers. Sci. Total Environ. 2014, 488, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fletcher, T.; Mucs, D.; Scott, K.; Lindh, C.H.; Tallving, P.; Jakobsson, K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 2018, 75, 46–51. [Google Scholar] [CrossRef] [PubMed]
- McGregor, R. In Situ treatment of PFAS-impacted groundwater using colloidal activated carbon. Remediation 2018, 28, 33–41. [Google Scholar] [CrossRef]
- Harrad, S.; Wemken, N.; Drage, D.S.; Abdallah, M.A.E.; Coggins, A.M. Perfluoroalkyl substances in drinking water, indoor air and dust from Ireland: Implications for human exposure. Environ. Sci. Technol. 2019, 53, 13449–13457. [Google Scholar] [CrossRef]
- Crone, B.C.; Speth, T.F.; Wahman, D.G.; Smith, S.J.; Abulikemu, G.; Kleiner, E.J.; Pressman, J.G. Occurrence of per- and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. [Google Scholar] [CrossRef]
- US EPA. Drinking Water Health Advisories for PFAS: Fact Sheet for Communities; US Environmental Protection Agency: Washington, DC, USA, 2022. [Google Scholar]
- Loos, R.; Wollgast, J.; Huber, T.; Hanke, G. Polar herbicides, pharmaceutical products, perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and nonylphenol and its carboxylates and ethoxylates in surface and tap waters around Lake Maggiore in Northern Italy. Anal. Bioanal. Chem. 2007, 387, 1469–1478. [Google Scholar] [CrossRef]
- Pitter, G.; Da Re, F.; Canova, C.; Barbieri, G.; Jeddi, M.Z.; Daprà, F.; Manea, F.; Zolin, R.; Bettega, A.M.; Stopazzolo, G.; et al. Serum levels of perfluoroalkyl substances (PFAS) in adolescents and young adults exposed to contaminated drinking water in the Veneto region, Italy: A cross-sectional study based on a health surveillance program. Environ. Health Perspect. 2020, 128, 1–12. [Google Scholar] [CrossRef]
- Borrull, J.; Colom, A.; Fabregas, J.; Pocurull, E.; Borrull, F. A liquid chromatography tandem mass spectrometry method for determining 18 per- and polyfluoroalkyl substances in source and treated drinking water. J. Chromatogr. A 2020, 1629, 461485. [Google Scholar] [CrossRef]
- Schwanz, T.G.; Llorca, M.; Farré, M.; Barceló, D. Perfluoroalkyl substances assessment in drinking waters from Brazil, France and Spain. Sci. Total Environ. 2016, 539, 143–152. [Google Scholar] [CrossRef]
- Skutlarek, D.; Exner, M.; Färber, H. Perfluorinated surfactants in surface and drinking waters. Environ. Sci. Pollut. Res. 2006, 13, 299–307. [Google Scholar] [CrossRef]
- Gobelius, L.; Hedlund, J.; Dürig, W.; Tröger, R.; Lilja, K.; Wiberg, K.; Ahrens, L. Per- and Polyfluoroalkyl Substances in Swedish Groundwater and Surface Water: Implications for Environmental Quality Standards and Drinking Water Guidelines. Environ. Sci. Technol. 2018, 52, 4340–4349. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Blake, S.; Hall, T.; Kanda, R.; Rumsby, P. Survey of the prevalence of perfluorooctane sulphonate (PFOS), perfluorooctanoic acid (PFOA) and related compounds in drinking water and their sources. Report DEFRA 2008, 7585. [Google Scholar]
- Eschauzier, C.; Raat, K.J.; Stuyfzand, P.J.; De Voogt, P. Perfluorinated alkylated acids in groundwater and drinking water: Identification, origin and mobility. Sci. Total Environ. 2013, 458, 477–485. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Costopoulou, D.; Vassiliadou, I.; Leondiadis, L.; Dassenakis, E.; Traag, W.; Hoogenboom, R.L.A.P.; van Leeuwen, S.P.J. Determination of perfluoroalkylated substances (PFASs) in drinking water from the Netherlands and Greece. Food Addit. Contam. Part A 2015, 32, 2048–2057. [Google Scholar] [CrossRef]
- Boiteux, V.; Dauchy, X.; Rosin, C.; Boiteux, J.F.V. National screening study on 10 perfluorinated compounds in raw and treated tap water in France. Arch. Environ. Contam. Toxicol. 2012, 63, 1–12. [Google Scholar] [CrossRef]
- Adewuyi, A.; Li, Q. Emergency of per- and polyfluoroalkyl substances in drinking water: Status, regulation, and mitigation strategies in developing countries. Eco Environ Health 2024, 3, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Drage, D.S.; Sharkey, M.; Berresheim, H. Perfluoroalkyl substances and brominated flame retardants in landfill-related air, soil, and groundwater from Ireland. Sci. Total Environ. 2020, 705, 135834. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiao, X.C.; Gai, N.; Li, X.J.; Wang, X.C.; Lu, G.H.; Piao, H.T.; Rao, Z.; Yang, Y.L. Perfluorinated compounds in soil, surface water, and groundwater from rural areas in eastern China. Environ. Pollut. 2016, 211, 124–131. [Google Scholar] [CrossRef]
- Qu, Y.; Jiang, X.; Cagnetta, G.; Liu, L.; Bao, Y.; Li, W.; Wang, Q.; Liang, C.; Huang, J.; Yang, H.; et al. Poly- and perfluoroalkyl substances in a drinking water treatment plant in the Yangtze River Delta of China: Temporal trend, removal and human health risk. Sci. Total Environ. 2019, 696, 133949. [Google Scholar] [CrossRef]
- Takemine, S.; Matsumura, C.; Yamamoto, K.; Suzuki, M.; Tsurukawa, M.; Imaishi, H.; Nakano, T.; Kondo, A. Discharge of perfluorinated compounds from rivers and their influence on the coastal seas of Hyogo prefecture, Japan. Environ. Pollut. 2014, 184, 397–404. [Google Scholar] [CrossRef]
- Kim, S.K.; Kho, Y.L.; Shoeib, M.; Kim, K.S.; Kim, K.R.; Park, J.E.; Shin, Y.S. Occurrence of perfluorooctanoate and perfluorooctanesulfonate in the Korean water system: Implication to water intake exposure. Environ. Pollut. 2011, 159, 1167–1173. [Google Scholar] [CrossRef]
- Yong, Z.Y.; Kim, K.Y.; Oh, J.E. The occurrence and distributions of per- and polyfluoroalkyl substances (PFAS) in groundwater after a PFAS leakage incident in 2018. Environ. Pollut. 2021, 268, 115395. [Google Scholar] [CrossRef]
- Heo, J.J.; Lee, J.W.; Kim, S.K.; Oh, J.E. Foodstuff analyses show that seafood and water are major perfluoroalkyl acids (PFAAs) sources to humans in Korea. J. Hazard. Mater. 2014, 279, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Guardian, M.G.E.; Boongaling, E.G.; Bernardo-Boongaling, V.R.R.; Gamonchuang, J.; Boontongto, T.; Burakham, R.; Arnnok, P.; Aga, D.S. Prevalence of per- and polyfluoroalkyl substances (PFASs) in drinking and source water from two Asian countries. Chemosphere 2020, 256, 127115. [Google Scholar] [CrossRef]
- Hongkachok, C.; Boontanon, S.K.; Boontanon, N.; Fujii, S.; Tanaka, S.; Suzuki, Y. Levels of perfluorinated compounds (PFCs) in groundwater around improper municipal and industrial waste disposal sites in Thailand and health risk assessment. Water Sci. Technol. 2017, 2017, 457–466. [Google Scholar] [CrossRef]
- Kunacheva, C.; Fujii, S.; Tanaka, S.; Boontanon, S.K.; Poothong, S.; Wongwatthana, T.; Shivakoti, B.R. Perfluorinated compounds contamination in tap water and bottled water in Bangkok, Thailand. J. Water Supply Res. Technol. AQUA 2010, 59, 345–354. [Google Scholar] [CrossRef]
- Lam, N.H.; Cho, C.R.; Kannan, K.; Cho, H.S. A nationwide survey of perfluorinated alkyl substances in waters, sediment and biota collected from aquatic environment in Vietnam: Distributions and bioconcentration profiles. Sci. Total Environ. 2017, 605–606, 415–423. [Google Scholar] [CrossRef]
- Jiang, J.J.; Okvitasari, A.R.; Huang, F.Y.; Tsai, C.S. Characteristics, pollution patterns and risks of perfluoroalkyl substances in drinking water sources of Taiwan. Chemosphere 2021, 264, 128579. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lai, W.W.P.; Tung, H.-H.; Lin, A.Y.C. Occurrence of pharmaceuticals, hormones, and perfluorinated compounds in groundwater in Taiwan. Environ. Monit. Assess. 2015, 187, 256. [Google Scholar] [CrossRef] [PubMed]
- Essumang, D.K.; Eshun, A.; Hogarh, J.N.; Bentum, J.K.; Adjei, J.K.; Negishi, J.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Masunaga, S. Perfluoroalkyl acids (PFAAs) in the Pra and Kakum River basins and associated tap water in Ghana. Sci. Total Environ. 2017, 579, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Kleywegt, S.; Raby, M.; McGill, S.; Helm, P. The impact of risk management measures on the concentrations of per- and polyfluoroalkyl substances in source and treated drinking waters in Ontario, Canada. Sci. Total Environ. 2020, 748, 141195. [Google Scholar] [CrossRef]
- Gallen, C.; Baduel, C.; Lai, F.Y.; Thompson, K.; Thompson, J.; Warne, M.; Mueller, J.F. Spatio-temporal assessment of perfluorinated compounds in the Brisbane River system, Australia: Impact of a major flood event. Mar. Pollut. Bull. 2014, 85, 597–605. [Google Scholar] [CrossRef]
- Bräunig, J.; Baduel, C.; Heffernan, A.; Rotander, A.; Donaldson, E.; Mueller, J.F. Fate and redistribution of perfluoroalkyl acids through AFFF-impacted groundwater. Sci. Total Environ. 2017, 596, 360–368. [Google Scholar] [CrossRef]
- Thompson, J.; Eaglesham, G.; Mueller, J. Concentrations of PFOS, PFOA and other perfluorinated alkyl acids in Australian drinking water. Chemosphere 2011, 83, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, O.S.; Fountoulakis, M.S.; Gatidou, G. Perfluoroalkyl and polyfluoroalkyl substances in sewage sludge: Challenges of biological and thermal treatment processes and potential threats to the environment from land disposal. Environ. Sci. Eur. 2024, 36, 207. [Google Scholar] [CrossRef]
- Post, G.B.; Gleason, J.A.; Cooper, K.R. Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: Contaminants of emerging concern. PLoS Biol. 2017, 15, e2002855. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Q.; Lei, L.; Zhou, W.; Huang, L.; Zhang, J.; Chen, D. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances affects leukocyte telomere length in female newborns. Environ. Pollut. 2018, 235, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Q.; Shi, J.; Shi, L.; Li, B.; Xu, A.; Zhao, G.; Wu, L. Perfluorooctane sulfonate exposure causes gonadal developmental toxicity in Caenorhabditis elegans through ROS-induced DNA damage. Chemosphere 2016, 155, 115–126. [Google Scholar] [CrossRef]
- Xu, Y.; Jurkovic-Mlakar, S.; Lindh, C.H.; Scott, K.; Fletcher, T.; Jakobsson, K.; Engström, K. Associations between serum concentrations of perfluoroalkyl substances and DNA methylation in women exposed through drinking water: A pilot study in Ronneby, Sweden. Environ. Int. 2020, 145, 106148. [Google Scholar] [CrossRef]
- Xu, H.; Mao, Y.; Hu, Y.; Xu, B. Association between exposure to polyfluoroalkyl chemicals and increased fractional exhaled nitric oxide in adults. Environ. Res. 2021, 198, 110450. [Google Scholar] [CrossRef]
- Sobolewski, M.; Conrad, K.; Allen, J.L.; Weston, H.; Martin, K.; Lawrence, B.P.; Cory-Slechta, D.A. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. NeuroToxicology 2014, 45, 121–130. [Google Scholar] [CrossRef]
- Oh, J.; Bennett, D.H.; Calafat, A.M.; Tancredi, D.; Roa, D.L.; Schmidt, R.J.; Hertz-Picciotto, I.; Shin, H.M. Prenatal exposure to per- and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environ. Int. 2021, 147, 106328. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ng, C. Absorption, distribution, and toxicity of per- and polyfluoroalkyl substances (PFAS) in the brain: A review. Environ. Sci. Process. Impacts 2021, 23, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Chang, W.; Zhang, Y.; Zhang, Y.; Liu, W. Neurotoxic effects of perfluoroalkyl acids: Neurobehavioral deficit and its molecular mechanism. Toxicol. Lett. 2019, 305, 65–72. [Google Scholar] [CrossRef]
- Feig, D.I.; Kang, D.-H.; Johnson, R.J. Uric Acid and Cardiovascular Risk. N. Engl. J. Med. 2008, 359, 85. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Dhana, K.; Furtado, J.D.; Rood, J.; Zong, G.; Liang, L.; Qi, L.; Bray, G.A.; DeJonge, L.; Coull, B.; et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med. 2018, 15, e1002502. [Google Scholar] [CrossRef]
- Tian, Y.P.; Zeng, X.W.; Bloom, M.S.; Lin, S.; Wang, S.Q.; Yim, S.H.L.; Yang, M.; Chu, C.; Gurram, N.; Hu, L.W.; et al. Isomers of perfluoroalkyl substances and overweight status among Chinese by sex status: Isomers of C8 Health Project in China. Environ. Int. 2019, 124, 130–138. [Google Scholar] [CrossRef]
- Jain, R.B.; Ducatman, A. Perfluoroalkyl acids serum concentrations and their relationship to biomarkers of renal failure: Serum and urine albumin, creatinine, and albumin creatinine ratios across the spectrum of glomerular function among US adults. Environ. Res. 2019, 174, 143–151. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, H.; Wen, Z.; Wang, Y.; Li, X.; Huang, T.; Mo, J.; Wu, Y.; Zhong, Y.; Ge, R.S. Perfluoroalkyl substances cause Leydig cell dysfunction as endocrine disruptors. Chemosphere 2020, 253, 126764. [Google Scholar] [CrossRef]
- Wan, H.T.; Lai, K.P.; Wong, C.K.C. Comparative analysis of PFOS and PFOA toxicity on sertoli cells. Environ. Sci. Technol. 2020, 54, 3465–3475. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Tong, C.; Fang, F.; Zhao, S.; Tian, Y.; Tao, Y.; Zhang, J. Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ. Health Perspect. 2017, 125, 067012-1–067012-6. [Google Scholar] [CrossRef]
- Vélez, M.P.; Arbuckle, T.E.; Fraser, W.D. Maternal exposure to perfluorinated chemicals and reduced fecundity: The MIREC study. Hum. Reprod. 2015, 30, 701–709. [Google Scholar] [CrossRef]
- Liew, Z.; Luo, J.; Nohr, E.A.; Bech, B.H.; Bossi, R.; Arah, O.A.; Olsen, J. Maternal plasma perfluoroalkyl substances and miscarriage: A nested case–control study in the Danish national birth cohort. Environ. Health Perspect. 2020, 128, 047007-1–047007-10. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.K.; Eckel, S.P.; Habre, R.; Yang, T.; Faham, D.; Amin, M.; Grubbs, B.H.; Farzan, S.F.; Kannan, K.; Robinson, M.; et al. Detected prenatal perfluorooctanoic acid (PFOA) exposure is associated with decreased fetal head biometric parameters in participants experiencing higher perceived stress during pregnancy in the MADRES cohort. Environ. Adv. 2022, 9, 100286. [Google Scholar] [CrossRef] [PubMed]
- Blake, B.E.; Fenton, S.E. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 2020, 443, 152565. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, G.; Rood, J.; Liang, L.; Bray, G.A.; de Jonge, L.; Coull, B.; Furtado, J.D.; Qi, L.; Grandjean, P.; et al. Perfluoroalkyl substances and changes in bone mineral density: A prospective analysis in the POUNDS-LOST study. Environ. Res. 2019, 179, 108775. [Google Scholar] [CrossRef]
- Wiener, R.C.; Waters, C. Perfluoroalkyls/polyfluoroalkyl substances and dental caries experience in children, ages 3–11 years, National Health and Nutrition Examination Survey, 2013–2014. J. Public Health Dent. 2019, 79, 307–319. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, R.; Hua, L.; Guo, Y.; Huang, L.; Zhao, Y.; Wang, X.; Zhang, J. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and childhood atopic dermatitis: A prospective birth cohort study. Environ. Health 2018, 17, 1–12. [Google Scholar] [CrossRef]
- Ait Bamai, Y.; Goudarzi, H.; Araki, A.; Okada, E.; Kashino, I.; Miyashita, C.; Kishi, R. Effect of prenatal exposure to per- and polyfluoroalkyl substances on childhood allergies and common infectious diseases in children up to age 7 years: The Hokkaido study on environment and children’s health. Environ. Int. 2020, 143, 105979. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Ye, J.; Ye, W. Relationship of perfluoroalkyl chemicals with chronic obstructive pulmonary disease: A cross-sectional study. Toxicol. Ind. Health 2025, 41, 176–185. [Google Scholar] [CrossRef]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef]
- Wielsøe, M.; Kern, P.; Bonefeld-Jørgensen, E.C. Serum levels of environmental pollutants is a risk factor for breast cancer in Inuit: A case control study. Environ. Health 2017, 16, 56. [Google Scholar] [CrossRef]
- Chang, E.T.; Adami, H.O.; Boffetta, P.; Cole, P.; Starr, T.B.; Mandel, J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and cancer risk in humans. Crit. Rev. Toxicol. 2014, 44, 1–81. [Google Scholar] [CrossRef] [PubMed]
- Pierozan, P.; Cattani, D.; Karlsson, O. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) induce epigenetic alterations and promote human breast cell carcinogenesis in vitro. Arch. Toxicol. 2020, 94, 3893–3906. [Google Scholar] [CrossRef]
- Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Toxicological interactions of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) with selected pollutants. J. Hazard. Mater. 2012, 201–202, 209–218. [Google Scholar] [CrossRef]
- Wolf, C.J.; Rider, C.V.; Lau, C.; Abbott, B.D. Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-α activation. Toxicology 2014, 316, 43–54. [Google Scholar] [CrossRef]
- Zhou, R.; Cheng, W.; Feng, Y.; Wei, H.; Liang, F.; Wang, Y. Interactions between three typical endocrine-disrupting chemicals (EDCs) in binary mixtures exposure on myocardial differentiation of mouse embryonic stem cell. Chemosphere 2017, 178, 378–383. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Z.; Gao, P.; Yin, D. Multigenerational effects of perfluorooctanoic acid on lipid metabolism of Caenorhabditis elegans and its potential mechanism. Sci. Total Environ. 2020, 703, 134762. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, C.E.; Annunziato, K.M.; Cooper, K.R. Behavioral, morphometric, and gene expression effects in adult zebrafish (Danio rerio) embryonically exposed to PFOA, PFOS, and PFNA. Aquat. Toxicol. 2016, 180, 123–130. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Luo, B.; Yan, S.; Guo, X.; Dai, J. Proteomic analysis of mouse testis reveals perfluorooctanoic acid-induced reproductive dysfunction via direct disturbance of testicular steroidogenic machinery. J. Proteome Res. 2014, 13, 3370–3385. [Google Scholar] [CrossRef] [PubMed]
- Reardon, A.J.F.; Karathra, J.; Ribbenstedt, A.; Benskin, J.P.; Macdonald, A.M.; Kinniburgh, D.W.; Hamilton, T.J.; Fouad, K.; Martin, J.W. Neurodevelopmental and metabolomic responses from prenatal coexposure to perfluorooctanesulfonate (PFOS) and methylmercury (MeHg) in Sprague-Dawley rats. Chem. Res. Toxicol. 2019, 32, 1656–1669. [Google Scholar] [CrossRef]
- Bharal, B.; Ruchitha, C.; Kumar, P.; Pandey, R.; Rachamalla, M.; Niyogi, S.; Naidu, R.; Kaundal, R.K. Neurotoxicity of per- and polyfluoroalkyl substances: Evidence and future directions. Sci. Total Environ. 2024, 955, 176941. [Google Scholar] [CrossRef]
- Brown-Leung, J.M.; Cannon, J.R. Neurotransmission Targets of Per- and Polyfluoroalkyl Substance Neurotoxicity: Mechanisms and Potential Implications for Adverse Neurological Outcomes. Chem. Res. Toxicol. 2022, 35, 1312–1333. [Google Scholar] [CrossRef]
- Starnes, H.M.; Rock, K.D.; Jackson, T.W.; Belcher, S.M. A Critical Review and Meta-Analysis of Impacts of Per- and Polyfluorinated Substances on the Brain and Behavior. Front. Toxicol. 2022, 4, 881584. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, B.N.; Lee, Y.A.; Shin, C.H.; Hong, Y.C.; Dossing, L.D.; Hildebrandt, G.; Lim, Y.H. Association between early-childhood exposure to perfluoroalkyl substances and ADHD symptoms: A prospective cohort study. Sci. Total Environ. 2023, 879, 163081. [Google Scholar] [CrossRef]
- Delcourt, N.; Pouget, A.-M.; Grivaud, A.; Nogueira, L.; Larvor, F.; Marchand, P.; Schmidt, E.; Le Bizec, B. First Observations of a Potential Association between Accumulation of Per- and Polyfluoroalkyl Substances in the Central Nervous System and Markers of Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sammi, S.R.; Foguth, R.M.; Nieves, C.S.; De Perre, C.; Wipf, P.; McMurray, C.T.; Lee, L.S.; Cannon, J.R. Perfluorooctane Sulfonate (PFOS) Produces Dopaminergic Neuropathology in Caenorhabditis elegans. Toxicol. Sci. 2019, 172, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Robarts, D.R.; Paine-Cabrera, M.; Kotulkar, K.K.; Venneman, S.; Gunewardena, L.; Foquet, G.; Bial, U.; Apte, U. Identifying novel mechanisms of per- and polyfluoroalkyl substance-induced hepatotoxicity using FRG humanized mice. Arch. Toxicol. 2024, 98, 3063–3075. [Google Scholar] [CrossRef]
- Mauge-Lewis, K.A.; Ramaiahgari, S.C.; Auerbach, S.S.; Roberts, G.K.; Waidyanatha, S.; Fenton, S.E.; Phadke, D.P.; Balik-Meisner, M.R.; Tandon, A.; Mav, D.; et al. Unraveling Human Hepatocellular Responses to PFAS and Aqueous Film-Forming Foams (AFFFs) for Molecular Hazard Prioritization and In Vivo Translation. Environ. Sci. Technol. 2025, 59, 2423–2435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, L.; Ducatman, A.; Deng, C.; von Stackelberg, K.E.; Danford, C.J.; Zhang, X. Association of per- and polyfluoroalkyl substance exposure with fatty liver disease risk in US adults. JHEP Rep. 2023, 5, 100694. [Google Scholar] [CrossRef]
- Rashwan, T.L.; Gerhard, J.I.; Grant, G.P. Application of self-sustaining smouldering combustion for the destruction of wastewater biosolids. Waste Manag. 2016, 50, 201–212. [Google Scholar] [CrossRef]
- Duchesne, A.L.; Brown, J.K.; Patch, D.J.; Major, D.; Weber, K.P.; Gerhard, J.I. Remediation of PFAS-contaminated soil and granular activated carbon by smoldering combustion. Environ. Sci. Technol. 2020, 54, 12631–12640. [Google Scholar] [CrossRef] [PubMed]
- David, M. Demonstration of Smoldering Combustion Treatment of PFAS-Impacted Investigation-Derived Waste. SERDP Project ER18–1593. 2019, 1–40. Available online: https://serdp-estcp.mil/projects/details/f72ddebf-217b-466f-8e68-afe857bbe983 (accessed on 18 May 2025).
- Ankley, G.T.; Cureton, P.; Hoke, R.A.; Houde, M.; Kumar, A.; Kurias, J.; Lanno, R.; McCarthy, C.; Newsted, J.; Sample, B.E.; et al. Assessing the ecological risks of per- and polyfluoroalkyl substances: Current state-of-the science and a proposed path forward. Environ. Toxicol. Chem. 2021, 40, 564–605. [Google Scholar] [CrossRef]
- Kumar, R.; Dada, T.K.; Whelan, A.; Cannon, P.; Sheehan, M.; Reeves, L.; Antunes, E. Microbial and thermal treatment techniques for degradation of PFAS in biosolids: A focus on degradation mechanisms and pathways. J. Hazard. Mater. 2023, 452, 131212. [Google Scholar] [CrossRef]
- Taylor, P.H.; Yamada, T.; Striebich, R.C.; Graham, J.L.; Giraud, R.J. Corrigendum to “Investigation of waste incineration of fluorotelomer-based polymers as a potential source of PFOA in the environment”. Chemosphere 2022, 298, 134601. [Google Scholar] [CrossRef]
- Garg, A.; Shetti, N.P.; Basu, S.; Nadagouda, M.N.; Aminabhavi, T.M. Treatment technologies for removal of per- and polyfluoroalkyl substances (PFAS) in biosolids. Chem. Eng. J. 2023, 453, 139964. [Google Scholar] [CrossRef]
- Thoma, E.D.; Wright, R.S.; George, I.; Krause, M.; Presezzi, D.; Villa, V.; Preston, W.; Deshmukh, P.; Kauppi, P.; Zemek, P.G. Pyrolysis processing of PFAS-impacted biosolids, a pilot study. J. Air Waste Manag. Assoc. 2022, 72, 309–318. [Google Scholar] [CrossRef]
- Bamdad, H.; Papari, S.; Moreside, E.; Berruti, F. High-temperature pyrolysis for elimination of per- and polyfluoroalkyl substances (PFAS) from biosolids. Processes 2022, 10, 2187. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.; Bergmann, D.; Bulatovic, T.; Surapaneni, A.; Gray, S. Review of influence of critical operation conditions on by-product/intermediate formation during thermal destruction of PFAS in solid/biosolids. Sci. Total Environ. 2022, 854, 158796. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, T.; Liang, Y. Stabilization of per- and polyfluoroalkyl substances (PFAS) in sewage sludge using different sorbents. J. Hazard. Mater. Adv. 2022, 6, 100089. [Google Scholar] [CrossRef]
- Lazcano, R.K.; Choi, Y.J.; Mashtare, M.L.; Lee, L.S. Characterizing and comparing per- and polyfluoroalkyl substances in commercially available biosolid and organic non-biosolid-based products. Environ. Sci. Technol. 2020, 54, 8640–8648. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, H.; Ma, X.; Tsige, M. PFAS removal via adsorption: A synergistic review on advances of experimental and computational approaches. Chemosphere 2025, 377, 144323. [Google Scholar] [CrossRef]
- Ilieva, Z.; Suehring, R.; Bastos, N.; Ezzahraoui, F.-Z.; Hamza, R. Adsorption dynamics of four per- and polyfluoroalkyl substances (PFAS) onto activated sludge (AS) and aerobic granular sludge (AGS). J. Environ. Chem. Eng. 2025, 13, 116377. [Google Scholar] [CrossRef]
- Vu, C.T.; Wu, T. Recent progress in adsorptive removal of per- and poly-fluoroalkyl substances (PFAS) from water/wastewater. Crit. Rev. Environ. Sci. Technol. 2022, 52, 90–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, K.; Wu, Q.; Ma, T.; Liang, J.; Wang, R. A porphyrinic metal-organic framework with cooperative adsorption domains for PFAS removal from water. ChemSusChem 2024, 17, e202400069. [Google Scholar] [CrossRef]
- Franco, L.A.; Stuart, T.D.; Hossain, M.S.; Ramarao, B.V.; VanLeuven, C.C.; Wriedt, M.; Satchwell, M.; Kumar, D. Apple pomace-derived cationic cellulose nanocrystals for PFAS removal from contaminated water. Processes 2024, 12(2), 297. [Google Scholar] [CrossRef]
- Wang, R.; Lin, Z.W.; Klemes, M.J.; Ateia, M.; Trang, B.; Wang, J.; Ching, C.; Helbling, D.E.; Dichtel, W.R. A tunable porous β-cyclodextrin polymer platform to understand and improve anionic PFAS removal. ACS Cent. Sci. 2022, 8, 663–669. [Google Scholar] [CrossRef]
- Ilango, A.K.; Arathala, P.; Musah, R.A.; Liang, Y. Experimental and density functional theory investigation of surface-modified biopolymer for improved adsorption of mixtures of per- and polyfluoroalkyl substances in water. Water Res. 2024, 255, 121458. [Google Scholar] [CrossRef]
- Huang, S.; Jaffe, P.R. Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. Strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef]
- Yi, L.B.; Chai, L.Y.; Xie, Y.; Peng, Q.J.; Peng, Q.Z. Isolation, identification, and degradation performance of a PFOA-degrading strain. Genet. Mol. Res. 2016, 15, 15028043. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Stoica, C.; Iftode, C.; Pirvu, F.; Petre, V.A.; Paun, I.; Pascu, L.F.; Vasile, G.G.; Nita-Lazar, M. Bacterial Biodegradation of Perfluorooctanoic Acid (PFOA) and Perfluorosulfonic Acid (PFOS) Using Pure Pseudomonas Strains. Sustainability 2023, 15, 14000. [Google Scholar] [CrossRef]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Weifeng, J.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Gong, T.; Dong, Q.; Wang, W. Uncovering hydrothermal treatment of per- and polyfluoroalkyl substances. Eco Environ. Health 2023, 2, 21–23. [Google Scholar] [CrossRef]
- Che, S.; Jin, B.; Liu, Z.; Yu, Y.; Liu, J.; Men, Y. Structure-specific aerobic defluorination of short-chain fluorinated carboxylic acids by activated sludge communities. Environ. Sci. Technol. Lett. 2021, 8, 668–674. [Google Scholar] [CrossRef]
- Yu, Y.; Che, S.; Ren, C.; Jin, B.; Tian, Z.; Liu, J.; Men, Y. Microbial defluorination of unsaturated per- and polyfluorinated carboxylic acids under anaerobic and aerobic conditions: A structure specificity study. Environ. Sci. Technol. 2022, 56, 4894–4904. [Google Scholar] [CrossRef]
- Meesters, R.J.; Schröder, H.F. Perfluorooctane Sulfonate—A Quite Mobile Anionic Anthropogenic Surfactant, Ubiquitously Found in the Environment. Water Sci. Technol. 2004, 50, 235–242. [Google Scholar] [CrossRef]
- Kwon, B.G.; Lim, H.J.; Na, S.H.; Choi, B.I.; Shin, D.S.; Chung, S.Y. Biodegradation of Perfluorooctanesulfonate (PFOS) as an Emerging Contaminant. Chemosphere 2014, 109, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Chetverikov, S.P.; Sharipov, D.A.; Korshunova, T.Y.; Loginov, O.N. Degradation of Perfluorooctanyl Sulfonate by Strain Pseudomonas plecoglossicida 2.4-D. Appl. Biochem. Microbiol. 2017, 53, 533–538. [Google Scholar] [CrossRef]
- Presentato, A.; Lampis, S.; Vantini, A.; Manea, F.; Daprà, F.; Zuccoli, S.; Vallini, G. On the Ability of Perfluorohexane Sulfonate (PFHxS) Bioaccumulation by Two Pseudomonas sp. Strains Isolated from PFAS-Contaminated Environmental Matrices. Microorganisms 2020, 8, 92. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J. Aerobic Biotransformation of Polyfluoroalkyl Phosphate Esters (PAPs) in Soil. Environ. Pollut. 2016, 212, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Baqar, M.; Chen, H.; Yao, Y.; Sun, H. Latest Trends in the Environmental Analysis of PFAS Including Nontarget Analysis and EOF-, AOF-, and TOP-Based Methodologies. Anal. Bioanal. Chem. 2025, 417, 555–571. [Google Scholar] [CrossRef]
- Zweigle, J.; Simon, F.; Meermann, B.; Zwiener, C. Can Qualitative Nontarget Data Be Indicative of PFAS Contamination? First Evidence by Correlation with EOF in Environmental Samples. Environ. Sci. Technol. Lett. 2024, 11, 996–1001. [Google Scholar] [CrossRef]
- Shoemaker, J.; Tettenhorst, D. Method 537.1: Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS); U.S. Environmental Protection Agency: Washington, DC, USA, 2020. [Google Scholar]
- ISO 21675:2019; Water Quality—Determination of Selected Polyfluoroalkyl Substances (PFAS) in Water—Method Using Solid Phase Extraction and LC-MS/MS. International Organization for Standardization: Geneva, Switzerland, 2019.
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for grouping per- and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Lendewig, M.; Marquez, R.; Franco, J.; Vera, R.E.; Vivas, K.A.; Forfora, N.; Venditti, R.A.; Gonzalez, R. PFAS regulations and economic impact: A review of U.S. pulp & paper and textiles industries. Chemosphere 2025, 377, 144301. [Google Scholar] [CrossRef]
- Suffill, E.; White, M.P.; Hale, S.; Pahl, S. Regulating “forever chemicals”: Social data are necessary for the successful implementation of the essential use concept. Environ. Sci. Eur. 2024, 36, 111. [Google Scholar] [CrossRef]
- UNEP. Global Monitoring Plan: Protocol 4 for POPs Analysis—PFAS; United Nations Environment Programme: Geneva, Switzerland, 2015. [Google Scholar]
- OECD. Working Towards A Global Emission Inventory of PFASs: Focus on PFCAs—Status Quo and the Way Forward; OECD Publishing: Paris, Francce, 2015. [Google Scholar] [CrossRef]
- USEPA. PFAS Strategic Roadmap: EPA’s Commitments to Action 2021–2024; U.S. Environmental Protection Agency: Washington, DC, USA, 2023. [Google Scholar]
- Feng, S.; Lu, X.; Ouyang, K.; Su, G.; Li, Q.; Shi, B.; Meng, J. Environmental occurrence, bioaccumulation and human risks of emerging fluoroalkylether substances: Insight into security of alternatives. Sci. Total Environ. 2024, 922, 171151. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Hu, J.; Yang, X.; Song, X.; Liang, K.; Huang, M.; Zhao, S.; Liu, H. Elucidating environmental fate and toxicological mechanisms of ultrashort- and short-chain PFAS: Integrating machine learning, molecular modeling, and experimental validation. J. Environ. Manag. 2025, 390, 126277. [Google Scholar] [CrossRef]
- Alvarez-Ruiz, R.; Lee, L.S.; Choi, Y. Fate of per- and polyfluoroalkyl substances at a 40-year dedicated municipal biosolids land disposal site. Sci. Total Environ. 2024, 954, 176540. [Google Scholar] [CrossRef] [PubMed]
- Sanzana, S.; Fenti, A.; Iovino, P.; Panico, A. A review of PFAS remediation: Separation and degradation technologies for water and wastewater treatment. J. Water Process Eng. 2025, 74, 107793. [Google Scholar] [CrossRef]
- Darlington, R.; Barth, E.; McKernan, J. The Challenges of PFAS Remediation. Mil. Eng. 2018, 110, 58–60. [Google Scholar]
- Örnhem Gal, A.O.; Åström, M.E.; Filipsson, M.E.M. A Review of Selected Alternative Remediation Methods for PFAS Contamination. Remediat. J. 2025, 35, e70007. [Google Scholar] [CrossRef]
- Prathima, B.; Sivakumar Babu, G.L. Integrated Framework for Sustainable Remediation of Soil Contamination in India: From Investigation to Implementation. J. Indian Inst. Sci. 2025, 104, 919–945. [Google Scholar] [CrossRef]
- Yu, R.S.; Yu, H.C.; Yang, Y.F.; Singh, S. A Global Overview of Per- and Polyfluoroalkyl Substance Regulatory Strategies and Their Environmental Impact. Toxics 2025, 13, 251. [Google Scholar] [CrossRef] [PubMed]
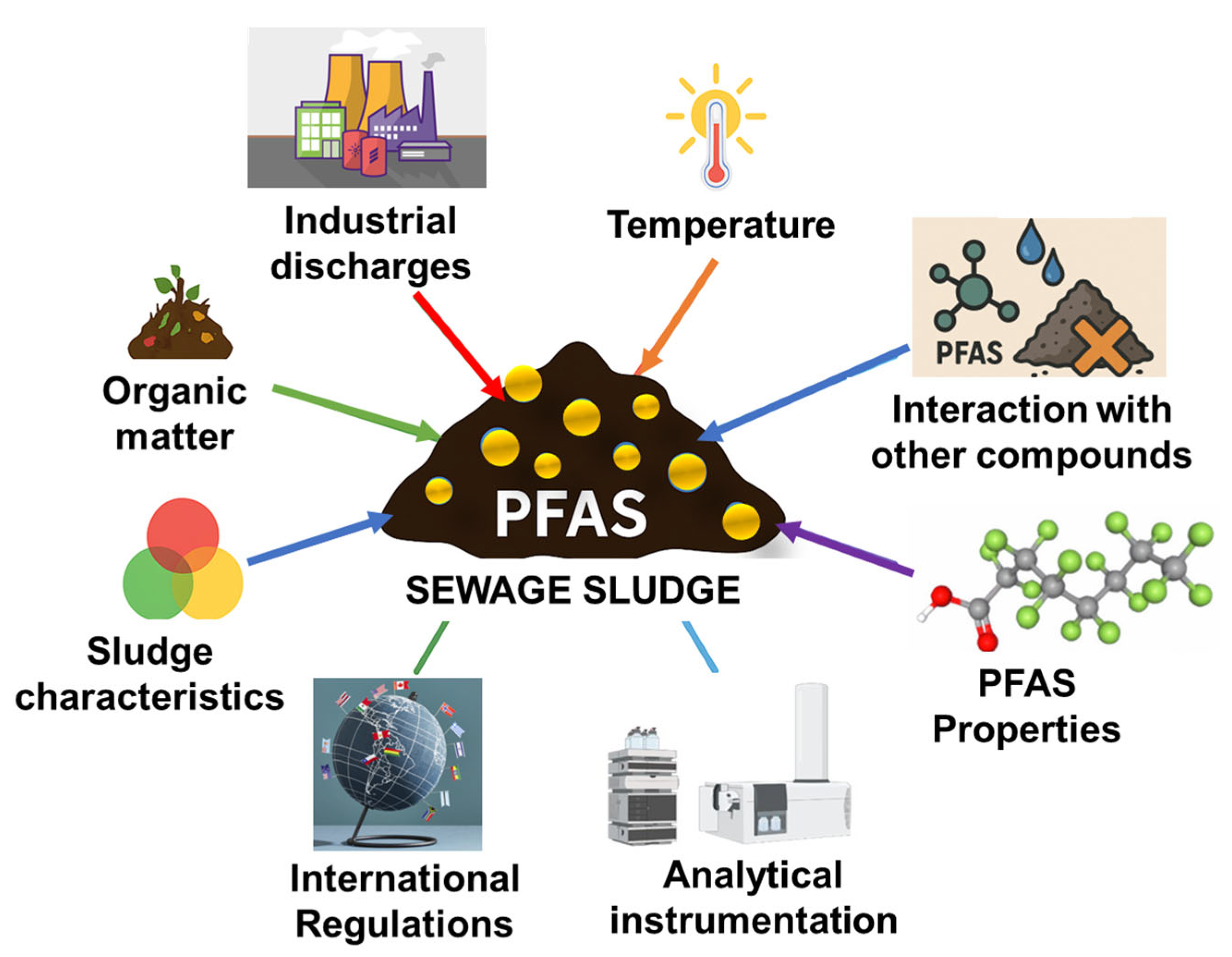
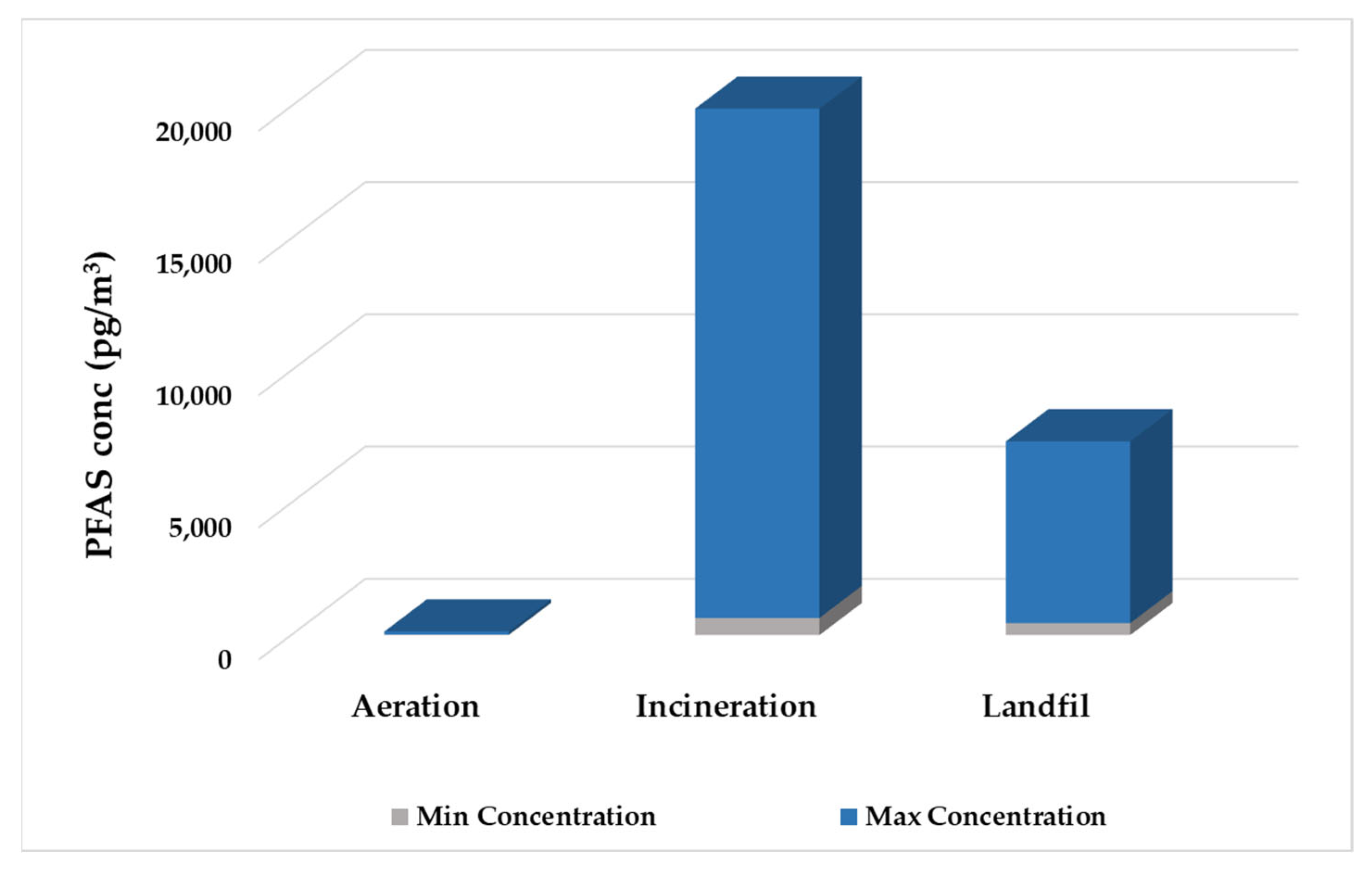

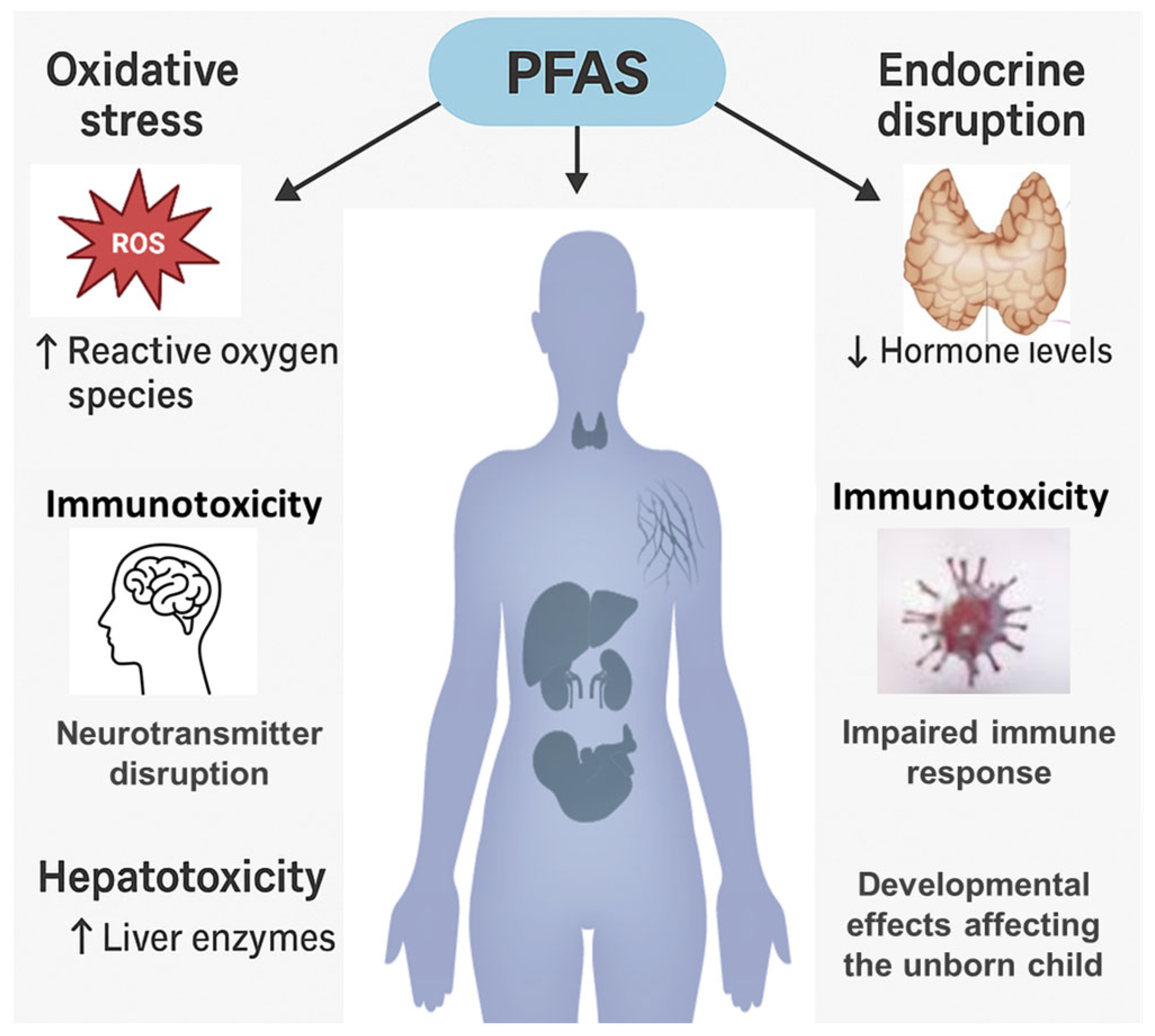

| Scheme | PFAS | Exposure (Concentration and Time) | Effects | References |
|---|---|---|---|---|
| Caenorhabditis elegans | PFOA | 1.0 ng/L, 24 h | ↑ Obesogenic effects; ↑ GPAT, FAS, ACC, ACS; ↓ FAD, FATP, CPT; ↑ Triglyceride synthesis; ↑ MAPK, PPAR signaling; ↓ TGF-β signaling; ↑ Adipogenesis, lipogenesis | [203] |
| Caenorhabditis elegans | PFOS | 0.25–25.0 μM, 72 h | ↓ Gonadal development; ↓ Cell proliferation; ↑ Cell apoptosis; ↑ DNA damage; ↑ ROS production | [173] |
| Zebrafish (Danio rerio) | PFOA | 2 μM, 5 d | ↑ Bdnf gene; ↓ Slco1d1, slco2b1, tgfb1a; Behavioral effects | [204] |
| BALB/c mice | PFOA | 0.31–20 mg/kg, 28 d | ↓ Testicular weight; ↓ Sperm quality; ↑ Germ cell deficiency; ↓ Testosterone and progesterone | [205] |
| Sprague-Dawley rats | PFOS | 1 mg/kg, 21 d | ↑ Behavioral changes | [206] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascu, L.F.; Petre, V.A.; Cimpean, I.A.; Paun, I.; Pirvu, F.; Chiriac, F.L. Managing PFAS in Sewage Sludge: Exposure Pathways, Impacts, and Treatment Innovations. J. Xenobiot. 2025, 15, 135. https://doi.org/10.3390/jox15040135
Pascu LF, Petre VA, Cimpean IA, Paun I, Pirvu F, Chiriac FL. Managing PFAS in Sewage Sludge: Exposure Pathways, Impacts, and Treatment Innovations. Journal of Xenobiotics. 2025; 15(4):135. https://doi.org/10.3390/jox15040135
Chicago/Turabian StylePascu, Luoana Florentina, Valentina Andreea Petre, Ioana Antonia Cimpean, Iuliana Paun, Florinela Pirvu, and Florentina Laura Chiriac. 2025. "Managing PFAS in Sewage Sludge: Exposure Pathways, Impacts, and Treatment Innovations" Journal of Xenobiotics 15, no. 4: 135. https://doi.org/10.3390/jox15040135
APA StylePascu, L. F., Petre, V. A., Cimpean, I. A., Paun, I., Pirvu, F., & Chiriac, F. L. (2025). Managing PFAS in Sewage Sludge: Exposure Pathways, Impacts, and Treatment Innovations. Journal of Xenobiotics, 15(4), 135. https://doi.org/10.3390/jox15040135








