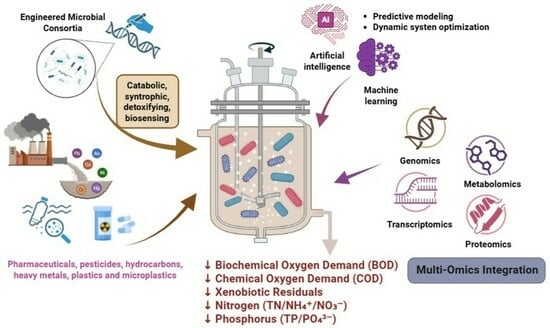
Harnessing Engineered Microbial Consortia for Xenobiotic Bioremediation: Integrating Multi-Omics and AI for Next-Generation Wastewater Treatment
Abstract
1. Introduction
2. Microbial Ecology of WWTS
3. Technologies Enhancing Microbial Bioremediation
3.1. Co-Digestion Strategies
3.2. Biofilm-Based Systems
Biofouling Mitigation in Biofilm-Based Reactors
- Quorum Quenching (QQ): QQ can significantly mitigate biofouling. A full-scale MBR inoculated with Acinetobacter guillouiae ST01 reduced biofilm polysaccharides by 30% and proteins by 47% relative to controls, while halving the fouling rate [86]. In another study, QQ beads introduced into an MBR extended operation time by 2–3×, maintained ~50% QQ activity over 50 days, and reduced polysaccharides and protein by over 40% [87].
- Functionalized Membranes: PVDF ultrafiltration membranes grafted with quorum-sensing inhibitors (poly(vanillin) brushes) showed a 50% increase in stable flux compared to control membranes during dynamic filtration [88].
- Anti-fouling Surface Coatings: Superhydrophobic TiO2/CuO nanocomposite coatings achieved >6 log10 reduction (~99.9%) in E. coli and S. aureus adhesion within 24 h, meeting ISO 22196 antibiofilm criteria [89].
3.3. Use of Conductive Materials for DIET Stimulation
3.4. Bioaugmentation and Engineered Consortia
4. Molecular Approaches in Microbial Community Profiling
4.1. Environmental DNA and Marker Gene Profiling
4.2. Metagenomics for Taxonomic and Functional Profiling
4.3. Functional Inference and Bioinformatics Tools
4.4. Artificial Intelligence and Predictive Analytics
| AI/ML Tool | Target Microbial Taxa | Predictive Output | Application in WWTS | References |
|---|---|---|---|---|
| Artificial Neural Networks (ANNs) | Pseudomonas, Acinetobacter, Sphingomonas | Abundance of xenobiotic degraders, removal efficiency | Prediction of phenolic and pharmaceutical xenobiotic degradation performance | [25,140] |
| Convolutional Neural Networks (CNNs) | Pseudomonas, Escherichia coli, Enterococcus faecalis | ARG occurrence and abundance | Near-real-time ARG monitoring for biosafety and co-selection control | [26,141] |
| Random Forest (RF) | Pseudomonas, Zoogloea, Burkholderiaceae | ARG and heavy metal resistance gene (MRG) trends | Prediction of ARG/MRG proliferation associated with xenobiotic and metal pollution | [142,143] |
| Support Vector Machine (SVM) | Thauera, Sphingomonas, Acinetobacter | Xenobiotic degradation efficiency | Predicting system response to recalcitrant organics (e.g., PAHs, pharmaceuticals) | [144,145] |
| Ensemble Learning Models | Proteobacteria, Firmicutes, Actinobacteria | System resilience under xenobiotic stress | Real-time prediction of treatment performance during xenobiotic loading events | [146,147] |
5. Challenges and Limitations
5.1. Immediate Operational Hurdles
5.1.1. Microbial Community Instability and Functional Redundancy
5.1.2. Biofilm Management and Reactor Fouling
- Operational optimization: Maintaining the filament index (FI) between 1.5 and 3, DO >2.5 mg L−1, and food-to-microorganism (F/M) ratios <0.65 g COD g−1 MLSS d−1 minimized filament growth and stabilized membrane performance. Under these conditions, the increase in TMP remained <2 kPa, whereas deviations can cause an increase in TMP of >14 kPa [151].
- Chemical control: Low concentrations of oxidants such as hydrogen peroxide, chlorine, or foam suppressants inhibit filamentous organisms. However, such interventions must be cautiously applied to avoid disrupting the beneficial microbial communities [154].
- Biological control: Quorum-quenching (QQ) bacteria, such as Pseudomonas putida and B. subtilis, immobilized on microporous carriers disrupt quorum sensing and reduce EPS synthesis and biofilm formation [155].
- Reactor design: Spatial segregation of nitrifier–denitrifiers minimizes fouling while sustaining nutrient removal.
5.1.3. Scale-Up Constraints and Regional Limitations
5.2. Fundamental Scientific and Systemic Barriers
5.2.1. Recalcitrant and Emerging Contaminants
- AOPs, such as ozonation, UV/H2O2 treatment, and Fenton reactions, chemically oxidize recalcitrant organics.
- BESs, such as CWs and microbial fuel cell hybrids, integrate microbial and electrochemical degradation for EC breakdown [160].
5.2.2. Antibiotic Resistance Genes and Horizontal Gene Transfer
5.2.3. Regulatory Gaps, Policy Challenges, and Biosafety Considerations
6. Future Perspectives: Towards Precision Microbial WWTS
6.1. Engineering Resilient Microbial Consortia
6.2. Scaling up Bioelectrochemical and Energy-Positive Systems
6.3. Integration of Artificial Intelligence and Smart Diagnostics
6.4. Microbial Standardization and Regionalization
6.5. Policy Innovation and Biosecurity Models
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renganathan, P.; Gaysina, L.A.; Holguín-Peña, R.J.; Sainz-Hernández, J.C.; Ortega-García, J.; Rueda-Puente, E.O. Phycoremediated microalgae and cyanobacteria biomass as biofertilizer for sustainable agriculture: A holistic biorefinery approach to promote circular bioeconomy. Biomass 2024, 4, 1047–1077. [Google Scholar] [CrossRef]
- Zheng, Z.; Liao, C.; Chen, Y.; Ming, T.; Jiao, L.; Kong, F.; Su, X.; Xu, J. Revealing the functional potential of microbial community of activated sludge for treating tuna processing wastewater through metagenomic analysis. Front. Microbiol. 2024, 15, 1430199. [Google Scholar] [CrossRef]
- Fan, X.; Ji, M.; Mu, D.; Zeng, X.; Tian, Z.; Sun, K.; Gao, R.; Liu, Y.; He, X.; Wu, L.; et al. Global diversity and biogeography of DNA viral communities in activated sludge systems. Microbiome 2023, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, X.; Zhu, L. Structure and function of the microbial consortia of activated sludge in typical municipal wastewater treatment plants in winter. Sci. Rep. 2017, 7, 17930. [Google Scholar] [CrossRef] [PubMed]
- Abate, R.; Oon, S.; Oon, L.; Bi, Y. Microalgae-bacteria nexus for environmental remediation and renewable energy resources: Advances, mechanisms and biotechnological applications. Heliyon 2024, 10, e31170. [Google Scholar] [CrossRef] [PubMed]
- Ningombam, L.; Mana, T.; Pradhan, S.; Apum, G.; Singh, Y.D. Fungal bioremediation in environmental pollution and recent strategies. Discov. Environ. 2025, 3, 100. [Google Scholar] [CrossRef]
- Aguilar-Romero, I.; Madrid, F.; Villaverde, J.; Morillo, E. Ibuprofen-enhanced biodegradation in solution and sewage sludge by a mineralizing microbial consortium: Shift in associated bacterial communities. J. Hazard. Mater. 2024, 464, 132970. [Google Scholar] [CrossRef]
- Mireisz, T.; Horváth, F.B.; Kashaija, N.T.; Farkas, R.; Boldizsár, I.; Tóth, E. Drug-degrading bacteria isolated from the effluent water of a sewage plant. Biol. Futur. 2024, 75, 351–359. [Google Scholar] [CrossRef]
- Narwal, S.K.; Gupta, R. Biodegradation of xenobiotic compounds: An overview. In Handbook of Research on Inventive Bioremediation Techniques; IGI Global: Hershey, PA, USA, 2017; pp. 186–212. [Google Scholar]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, W.; Bhatt, P.; Chen, S. Recent advanced technologies for the characterization of xenobiotic-degrading microorganisms and microbial communities. Front. Bioeng. Biotechnol. 2021, 9, 632059. [Google Scholar] [CrossRef]
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.; Khanna, S.; Rawat, G.; Panda, A.; Bisht, S.; Upadhyay, J.; Ansari, M. Degradation of xenobiotic pollutants: An environmentally sustainable approach. Metabolites 2022, 12, 598. [Google Scholar] [CrossRef]
- Yadav, R.; Rajput, V.; Dharne, M. Functional metagenomic landscape of polluted river reveals potential genes involved in degradation of xenobiotic pollutants. Environ. Res. 2020, 192, 110332. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.P. Microbiological removal of phenol by an application of Pseudomonas spp. ETL: An innovative biotechnological approach providing answers to the problems of FETP. J. Appl. Environ. Microbiol. 2014, 1, 1–7. [Google Scholar]
- Rubén, A.; Alberto, L.; María, A.; César, J. Emerging pollutants in wastewater: Advanced oxidation processes as an alternative treatment and perspectives. Processes 2022, 10, 1041. [Google Scholar] [CrossRef]
- Tiwari, S.; Tripathi, A.; Gaur, R. Bioremediation of plant refuges and xenobiotics. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer: Singapore, 2016; pp. 85–142. [Google Scholar]
- Bharadwaj, A. Bioremediation of xenobiotics: An eco-friendly cleanup approach. In Green Chemistry in Environmental Sustainability and Chemical Education: Proceedings of ICGC 2016, New Delhi; Springer: Singapore, 2018; pp. 1–13. [Google Scholar]
- Bôto, M.; Almeida, C.M.; Mucha, A.P. Potential of constructed wetlands for removal of antibiotics from saline aquaculture effluents. Water 2016, 8, 465. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, M.; Zhu, Y.; Wang, D.; Li, Z.; Zeng, G.; Zhang, C.; Huang, J.; Xu, P. Collision of emerging and traditional methods for antibiotics removal: Taking constructed wetlands and nanotechnology as an example. NanoImpact 2019, 15, 100175. [Google Scholar] [CrossRef]
- Ma, J.; Cui, Y.; Li, A.; Zou, X.; Ma, C.; Chen, Z. Antibiotics and antibiotic resistance genes from wastewater treated in constructed wetlands. Ecol. Eng. 2022, 177, 106548. [Google Scholar] [CrossRef]
- Stoeck, T.; Pan, H.; Dully, V.; Forster, D.; Jung, T. Towards an eDNA metabarcode-based performance indicator for full-scale municipal wastewater treatment plants. Water Res. 2018, 144, 322–331. [Google Scholar] [CrossRef]
- Numberger, D.; Ganzert, L.; Zoccarato, L.; Mühldorfer, K.; Sauer, S.; Grossart, P.; Greenwood, A.D. Characterization of bacterial communities in wastewater with enhanced taxonomic resolution by full-length 16S rRNA sequencing. Sci. Rep. 2019, 9, 9673. [Google Scholar] [CrossRef]
- Shen, M.; Hu, X.; Li, M.; Lyu, C.; Hu, Y.; Bu, X.; Chen, T.; Cai, H.; Li, C.; Liu, J.; et al. Distribution of antibiotic resistance genes and their association with microbes in wastewater treatment plants: A metagenomics analysis. Water 2022, 15, 1587. [Google Scholar] [CrossRef]
- Inoue, Y.; Miyata, K.; Yamane, M.; Honda, H. Environmental nucleic acid pollution: Characterization of wastewater generating false positives in molecular ecological surveys. ACS ES&T Water 2023, 3, 756–764. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, H.; Zhang, L.; Yin, W.; Li, L.; Chen, T.; Li, Z.; Wang, A.; Ding, C. Intermittent electrostimulation-modified direct interspecies electron transfer for enhanced methanogenesis in anaerobic digestion of sulfate-rich wastewater. Bioresour. Technol. 2024, 406, 130992. [Google Scholar] [CrossRef]
- Liu, X.; Nie, Y.; Wu, X.L. Predicting microbial community compositions in wastewater treatment plants using artificial neural networks. Microbiome 2023, 11, 93. [Google Scholar] [CrossRef]
- Jang, J.; Abbas, A.; Kim, M.; Shin, J.; Kim, Y.M.; Cho, K.H. Prediction of antibiotic-resistance genes occurrence at a recreational beach with deep learning models. Water Res. 2021, 196, 117001. [Google Scholar] [CrossRef]
- Cardozo, G.S.; Ruas, G.; Fiore, F.A.; Silva, G.H. Impacts of tropical climate on outdoor treatment of anaerobically digested sanitary wastewater using native microalgae. Heliyon 2025, 11, e41848. [Google Scholar] [CrossRef]
- Valentin, M.T.; Luo, G.; Zhang, S.; Białowiec, A. Direct interspecies electron transfer mechanisms of a biochar-amended anaerobic digestion: A review. Biotechnol. Biofuels Bioprod. 2023, 16, 146. [Google Scholar] [CrossRef]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnol. 2019, 52, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Phulpoto, I.; Hu, B.; Wang, Y.; Ndayisenga, F.; Li, J.; Yu, Z. Effect of natural microbiome and culturable biosurfactants-producing bacterial consortia of freshwater lake on petroleum-hydrocarbon degradation. Sci. Total Environ. 2020, 751, 141639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, Y.; Wang, C.; Liu, X.; Li, S. Biodegradation of n-alkanes in crude oil by three identified bacterial strains. Fuel 2020, 275, 117953. [Google Scholar] [CrossRef]
- Xie, F.; Thiri, M.; Wang, H. Simultaneous heterotrophic nitrification and aerobic denitrification by a novel isolated Pseudomonas mendocina X49. Bioresour. Technol. 2020, 319, 124195. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Piercy, E.; Sun, X.; Ellis, P.R.; Taylor, M.; Guo, M. Temporal dynamics of microbial communities in anaerobic digestion: Influence of temperature and feedstock composition on reactor performance and stability. Water Res. 2025, 284, 123974. [Google Scholar] [CrossRef]
- Robles-Morales, D.L.; Reyes Cervantes, A.; Díaz-Godínez, R.; Tovar-Jiménez, X.; Medina-Moreno, S.A.; Jiménez-González, A. Design and performance evaluation of a fungi-bacteria consortium to biodegrade organic matter at high concentration on synthetic slaughterhouse wastewater. Water Air Soil Pollut. 2021, 232, 223. [Google Scholar] [CrossRef]
- Civzele, A.; Stipniece-Jekimova, A.A.; Mezule, L. Biodegradation of screenings from sewage treatment by white rot fungi. Fungal Biol. Biotechnol. 2025, 12, 7. [Google Scholar] [CrossRef]
- Rathour, R.K.; Sharma, D.; Ullah, S.; Mahmoud, E.H.; Sharma, N.; Kumar, P.; Bhatt, A.K.; Ahmad, I.; Bhatia, R.K. Bacterial–microalgal consortia for bioremediation of textile industry wastewater and resource recovery for circular economy. Biotechnol. Environ. 2024, 1, 6. [Google Scholar] [CrossRef]
- Smetana, G.; Grosser, A. The application of an upflow anaerobic sludge blanket reactor in the treatment of brewery and dairy wastewater: A critical review. Energies 2024, 17, 1504. [Google Scholar] [CrossRef]
- Da Motta, I.G.; Santana, L.A.; Pereira, H.P.; De Paula, V.R.; Martins, M.F.; Otenio, M.H. Population dynamics of methanogenic archaea in co-digestion systems operating different industrial residues for biogas production. Sustainability 2022, 14, 11536. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.; Lee, J.; Shin, J.; Shin, S.G.; Lee, J. Effect of magnetite supplementation on mesophilic anaerobic digestion of phenol and benzoate: Methane production rate and microbial communities. Bioresour. Technol. 2022, 350, 126943. [Google Scholar] [CrossRef]
- Cai, M.; Wilkins, D.; Chen, J.; Ng, S.; Lu, H.; Jia, Y.; Lee, P. Metagenomic reconstruction of key anaerobic digestion pathways in municipal sludge and industrial wastewater biogas-producing systems. Front. Microbiol. 2016, 7, 1625. [Google Scholar] [CrossRef]
- Elliott, J.; Krohn, C.; Ball, A. Source-separated industrial wastewater is a candidate for biogas production through anaerobic digestion. Fermentation 2024, 10, 165. [Google Scholar] [CrossRef]
- Trinh, H.P.; Lee, S.H.; Nguyen, T.V.; Park, H.D. Contribution of the microbial community to operational stability in an anammox reactor: Neutral theory and functional redundancy perspectives. Bioresour. Technol. 2025, 419, 132029. [Google Scholar] [CrossRef]
- Xiao, R.; Kang, D.; Zhao, H.; Fan, M.; Peng, Y.; Niu, J. Metagenomic insights into the microbial community of activated sludge in oxytetracycline wastewater treatment. Water 2024, 16, 3680. [Google Scholar] [CrossRef]
- Neria-González, M.I.; Aguilar-López, R. Heavy metal removal processes by sulfate-reducing bacteria. In Environmental Pollution and Remediation; Prasad, R., Ed.; Springer: Singapore, 2021; pp. 305–328. [Google Scholar]
- Sidhu, C.; Vikram, S.; Pinnaka, A.K. Unraveling the microbial interactions and metabolic potentials in pre- and post-treated sludge from a wastewater treatment plant using metagenomic studies. Front. Microbiol. 2017, 8, 1382. [Google Scholar] [CrossRef] [PubMed]
- Malwe, A.S.; Longwani, U.; Sharma, V.K. XenoBug: Machine learning-based tool to predict pollutant-degrading enzymes from environmental metagenomes. NAR Genom. Bioinform. 2025, 7, lqaf037. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Krah, D.; Dötsch, A.; Ghattas, A.; Wick, A.; Ternes, T. Insights into the variability of microbial community composition and micropollutant degradation in diverse biological wastewater treatment systems. Water Res. 2018, 143, 313–324. [Google Scholar] [CrossRef]
- Kelly, J.; London, M.; McCormick, A.; Rojas, M.; Scott, J.; Hoellein, T. Wastewater treatment alters microbial colonization of microplastics. PLoS ONE 2021, 16, e0244443. [Google Scholar] [CrossRef]
- Kim, E.; Yulisa, A.; Kim, S.; Hwang, S. Monitoring microbial community structure and variations in a full-scale petroleum refinery wastewater treatment plant. Bioresour. Technol. 2020, 306, 123178. [Google Scholar] [CrossRef]
- Bouabidi, Z.; El-Naas, M.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Aghalari, Z.; Dahms, H.; Sillanpää, M.; Sosa-Hernández, J.; Parra-Saldívar, R. Effectiveness of wastewater treatment systems in removing microbial agents: A systematic review. Glob. Health 2020, 16, 13. [Google Scholar] [CrossRef]
- Wang, D.; Thunéll, S.; Lindberg, U.; Jiang, L.; Trygg, J.; Tysklind, M.; Souihi, N. A machine learning framework to improve effluent quality control in wastewater treatment plants. Sci. Total Environ. 2021, 784, 147138. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, C.; Zhang, L.; Ai, W.; Ulbricht, M.; Wei, Y. Anaerobic membrane bioreactor for hygiene wastewater treatment in controlled ecological life support systems: Degradation of surfactants and microbial community succession. Bioresour. Technol. 2023, 374, 128699. [Google Scholar] [CrossRef]
- Heisi, H.; Nkuna, R.; Matambo, T. Rhizosphere microbial community structure and PICRUSt2 predicted metagenomes function in heavy metal contaminated sites: A case study of the Blesbokspruit wetland. Sci. Total Environ. 2024, 959, 178147. [Google Scholar] [CrossRef] [PubMed]
- Vera, A.; Wilson, F.; Cupples, A. Predicted functional genes for the biodegradation of xenobiotics in groundwater and sediment at two contaminated naval sites. Appl. Microbiol. Biotechnol. 2022, 106, 835. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Abu-Reesh, I.; He, Z. Domestic wastewater treatment towards reuse by “self-supplied” microbial electrochemical system assisted UV/H2O2 process. Water Res. 2024, 267, 119277. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, Y.; Ye, L.; Qian, Y.; Shi, Y.; Xu, K.; Ren, H.; Geng, J. Microbial roles in dissolved organic matter transformation in full-scale wastewater treatment processes revealed by reactomics and comparative genomics. Environ. Sci. Technol. 2021, 55, 15249–15259. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Zielińska, M. Bacterial communities in full-scale wastewater treatment systems. World J. Microbiol. Biotechnol. 2016, 32, 66. [Google Scholar] [CrossRef]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; McCabe, M.; Cormican, P.; Zhan, X.; Gardiner, G.E. Exploring the roles of and interactions among microbes in dry co-digestion of food waste and pig manure using high-throughput 16S rRNA gene amplicon sequencing. Biotechnol. Biofuels 2019, 12, 5. [Google Scholar] [CrossRef]
- Saini, S.; Tewari, S.; Dwivedi, J.; Sharma, V. Biofilm-mediated wastewater treatment: A comprehensive review. Mater. Adv. 2023, 4, 1415–1443. [Google Scholar] [CrossRef]
- Shan, X.; Guo, H.; Ma, F.; Shan, Z. Enhanced treatment of synthetic wastewater by bioaugmentation with a constructed consortium. Chemosphere 2023, 338, 139520. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, T.; Wang, W.; Lv, Y.; Deng, H.; Lu, W.; Cheng, X. Enhancement from anaerobic digestion of food waste by conductive materials: Performance and mechanism. ACS Omega 2022, 7, 40782–40788. [Google Scholar] [CrossRef]
- Mudzanani, K.; Van Heerden, E.; Mbhele, R.; Daramola, M.O. Enhancement of biogas production via co-digestion of wastewater treatment sewage sludge and brewery spent grain: Physicochemical characterization and microbial community. Sustainability 2020, 13, 8225. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Ifran, M.; Li, A. Anaerobic co-digestion of sewage sludge, food waste and yard waste: Synergistic enhancement on process stability and biogas production. Sci. Total Environ. 2020, 704, 135429. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, E.; Hwang, S. Methanogenic diversity changes in full-scale anaerobic digesters by co-digestion of food waste and sewage sludge. J. Mater. Cycles Waste Manag. 2022, 24, 2669–2676. [Google Scholar] [CrossRef]
- Bae, J.S.; Yoon, Y.M.; Shin, S.K.; Lee, D.J.; Seo, D.C. Biogas potential and methanogenic community shift in in-situ anaerobic sewage sludge digestion with food waste leachate additions. Appl. Biol. Chem. 2020, 63, 84. [Google Scholar] [CrossRef]
- Shin, J.; Kim, Y.B.; Jeon, J.H.; Choi, S.; Park, I.K.; Kim, Y.M. Biomethanation of sewage sludge with food waste leachate via co-digestion. J. Microbiol. Biotechnol. 2017, 27, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ni, J.; Cheng, H.; Zhu, A.; Guo, G.; Qin, Y.; Li, Y.Y. Methanogenic performance and microbial community during thermophilic digestion of food waste and sewage sludge in a high-solid anaerobic membrane bioreactor. Bioresour. Technol. 2021, 342, 125938. [Google Scholar] [CrossRef]
- Lee, W.; Kim, Y.; Kim, H.; Kim, M. Comparison of anaerobic co-digestion of food waste and livestock manure at various mixing ratios under mesophilic and thermophilic temperatures. Sustainability 2023, 16, 7653. [Google Scholar] [CrossRef]
- Cheong, W.L.; Chan, Y.J.; Tiong, T.J.; Chong, W.C.; Kiatkittipong, W.; Kiatkittipong, K.; Mohamad, M.; Daud, H.; Suryawan, I.W.; Sari, M.M.; et al. Anaerobic co-digestion of food waste with sewage sludge: Simulation and optimization for maximum biogas production. Water 2021, 14, 1075. [Google Scholar] [CrossRef]
- Cengiz, A.I.; Guven, H.; Ozgun, H.; Ersahin, M.E. Enhanced energy recovery in municipal wastewater treatment plants through co-digestion by anaerobic membrane bioreactors: Current status and future perspectives. Rev. Environ. Sci. Biotechnol. 2024, 23, 385–410. [Google Scholar] [CrossRef]
- Yekta, S.S.; Liu, T.; Anacleto, T.M.; Bjerg, M.A.; Šafarič, L.; Goux, X.; Karlsson, A.; Björn, A.; Schnürer, A. Effluent solids recirculation to municipal sludge digesters enhances long-chain fatty acids degradation capacity. Biotechnol. Biofuels 2021, 14, 56. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Osman, A.I.; Ai, P.; Zhou, Z.; Meng, F.; Rooney, D.W. Bioenergy production from chicken manure: A review. Environ. Chem. Lett. 2023, 21, 2707–2727. [Google Scholar] [CrossRef]
- Maurya, A.; Kumar, R.; Raj, A. Biofilm-based technology for industrial wastewater treatment: Current technology, applications and future perspectives. World J. Microbiol. Biotechnol. 2023, 39, 31. [Google Scholar] [CrossRef]
- Philipp, L.; Bühler, K.; Ulber, R.; Gescher, J. Beneficial applications of biofilms. Nat. Rev. Microbiol. 2023, 22, 276–290. [Google Scholar] [CrossRef]
- Xiong, H.; Zhou, X.; Cao, Z.; Xu, A.; Dong, W.; Jiang, M. Microbial biofilms as a platform for diverse biocatalytic applications. Bioresour. Technol. 2024, 386, 129396. [Google Scholar] [CrossRef]
- Pippo, F.; Gregorio, L.; Congestri, R.; Tandoi, V.; Rossetti, S. Biofilm growth and control in cooling water industrial systems. FEMS Microbiol. Ecol. 2018, 94, fiy044. [Google Scholar] [CrossRef]
- Deena, S.; Kumar, G.; Vickram, A.; Singhaniam, R.; Dong, C.; Rohini, K.; Anbarasu, K.; Thanigaivel, S.; Ponnusamy, V. Efficiency of various biofilm carriers and microbial interactions with substrate in moving bed-biofilm reactor for wastewater treatment: A review. Bioresour. Technol. 2022, 359, 127421. [Google Scholar]
- Jang, D.; Won, J.; Jo, Y.; Kim, Y.; Jang, A. The effect of biocarriers on the nitrification and microbial community in moving bed biofilm reactor for anaerobic digestion effluent treatment. Environ. Res. 2023, 216, 114707. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhou, J.; Sheng, D.; Wu, D.; Zhou, H.; Yang, Z.; Yin, J.; Xia, C.; Kan, Y.; He, J. Integration of a pure moving bed biofilm reactor process into a large micro-polluted water treatment plant. Water Sci. Technol. 2022, 86, 3051–3066. [Google Scholar] [CrossRef] [PubMed]
- Lusinier, N.; Seyssiecq, I.; Sambusiti, C.; Jacob, M.; Lesage, N.; Roche, N. Application of moving bed biofilm reactor and fixed bed hybrid biological reactor for oilfield produced water treatment: Influence of total dissolved solids concentration. Energies 2020, 14, 7297. [Google Scholar] [CrossRef]
- Eshamuddin, M.; Zuccaro, G.; Nourrit, G.; Albasi, C. The influence of process operating conditions on the microbial community structure in the moving bed biofilm reactor at phylum and class level: A review. J. Environ. Chem. Eng. 2024, 12, 113266. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Chen, J.; Yan, Y.; Kobayashi, T.; Hu, Y.; Zhang, X. Food waste anaerobic digestion under high organic loading rate: Inhibiting factors, mechanisms, and mitigation strategies. Processes 2025, 13, 2090. [Google Scholar] [CrossRef]
- Hu, Y.; Kobayashi, T.; Zhen, G.; Shi, C.; Xu, K. Effects of lipid concentration on thermophilic anaerobic co-digestion of food waste and grease waste in a siphon-driven self-agitated anaerobic reactor. Biotechnol. Rep. 2018, 19, e00269. [Google Scholar] [CrossRef]
- Tabraiz, S.; Shamurad, B.; Petropoulos, E.; Quintela-Baluja, M.; Charlton, A.; Dolfing, J.; Sallis, P.J. Mitigation of membrane biofouling in membrane bioreactor treating sewage by novel quorum quenching strain of Acinetobacter originating from a full-scale membrane bioreactor. Bioresour. Technol. 2021, 334, 125242. [Google Scholar] [CrossRef]
- Wang, R.; An, Z.; Fan, L.; Zhou, Y.; Su, X.; Zhu, J.; Zhang, Q.; Chen, C.; Lin, H.; Sun, F. Effect of quorum quenching on biofouling control and microbial community in membrane bioreactors by Brucella sp. ZJ1. J. Environ. Manag. 2023, 339, 117961. [Google Scholar] [CrossRef]
- Ren, S.; Huang, G.; Wang, Y. Quorum quenching-mediated biofilm mitigation on functionalized ultrafiltration membranes via atom transfer radical polymerization. ACS ES&T Eng. 2022, 2, 2275–2286. [Google Scholar] [CrossRef]
- Pal, S.; Villani, S.; Mansi, A.; Marcelloni, A.M.; Chiominto, A.; Amori, I.; Proietto, A.R.; Calcagnile, M.; Alifano, P.; Bagheri, S.; et al. Antimicrobial and superhydrophobic CuONPs/TiO2 hybrid coating on polypropylene substrates against biofilm formation. ACS Omega 2024, 9, 45376. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kang, H.; Park, K.; Park, H. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, S.; Liang, D.; Li, N. Conductive materials in anaerobic digestion: From mechanism to application. Bioresour. Technol. 2019, 289, 121619. [Google Scholar] [CrossRef]
- Gahlot, P.; Ahmed, B.; Tiwari, S.; Aryal, N.; Khursheed, A.; Kazmi, A.; Tyagi, V. Conductive material engineered direct interspecies electron transfer (DIET) in anaerobic digestion: Mechanism and application. Environ. Technol. Innov. 2020, 20, 101056. [Google Scholar] [CrossRef]
- Wang, Z.K.; Liu, Q.H.; Yang, Z.M. Nano magnetite-loaded biochar boosted methanogenesis through shifting microbial community composition and modulating electron transfer. Sci. Total Environ. 2023, 861, 160597. [Google Scholar] [CrossRef]
- Hasani, Z.; Nayak, J.; Balushi, N.; Al-Mamun, A.; Samal, K. Prospect of conductive materials in the anaerobic digester matrix for methane production: Electron transfer and microbial communication. Water 2025, 17, 1321. [Google Scholar] [CrossRef]
- Nabi, M.; Liang, H.; Cheng, L.; Yang, W.; Gao, D. A comprehensive review on the use of conductive materials to improve anaerobic digestion: Focusing on landfill leachate treatment. J. Environ. Manag. 2022, 309, 114683. [Google Scholar] [CrossRef]
- Martins, G.; Salvador, A.; Pereira, L.; Alves, M. Methane production and conductive materials: A critical review. Environ. Sci. Technol. 2018, 52, 10241–10253. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, G. Advances in direct interspecies electron transfer and conductive materials: Electron flux, organic degradation and microbial interaction. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef]
- Zhong, Y.; He, J.; Zhang, P.; Zou, X.; Pan, X.; Zhang, J. Novel nitrogen-doped biochar supported magnetite promotes anaerobic digestion: Material characterization and metagenomic analysis. Bioresour. Technol. 2023, 369, 128492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Mazarji, M.; Li, M.; Li, A.; Wang, Y.; Yang, Y.; Lee, J.T.; Rene, E.R.; Yuan, X.; Pan, J. Exploring magnetic nanomaterials with a focus on magnetic biochar in anaerobic digestion: From synthesis to application. Biochar 2024, 6, 63. [Google Scholar] [CrossRef]
- Tang, J.; Zhuang, L.; Ma, J.; Tang, Z.; Yu, Z.; Zhou, S. Secondary mineralization of ferrihydrite affects microbial methanogenesis in Geobacter-Methanosarcina cocultures. Appl. Environ. Microbiol. 2016, 82, 5869–5877. [Google Scholar] [CrossRef] [PubMed]
- Ustra, A.T.; Mendonça, C.; Leite, A.D.; Macouin, M.; Doherty, R.; Respaud, M.; Tocuti, G. Ultrafine magnetic particles: A DIET-proxy in organic rich sediments? Front. Earth Sci. 2021, 8, 608387. [Google Scholar] [CrossRef]
- Manga, M.; Boutikos, P.; Semiyaga, S.; Olabinjo, O.; Muoghalu, C.C. Biochar and its potential application for the improvement of the anaerobic digestion process: A critical review. Energies 2022, 16, 4051. [Google Scholar] [CrossRef]
- Abid, N.; Karray, F.; Kallel, I.; Slim, M.; Barakat, A.; Mhiri, N.; Chamkha, M.; Sayadi, S. Role of biochar in anaerobic microbiome enrichment and methane production enhancement during olive mill wastewater biomethanization. Front. Bioeng. Biotechnol. 2023, 10, 1100533. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, J.; Senthil Kumar, P.; Zhou, M.; Yu, J.; Lichtfouse, E. Enhanced methane production by granular activated carbon: A review. Fuel 2022, 320, 123903. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, H.; Liu, L.; Yan, Z.; Chui, C.; Yang, N.; Wang, C.; Shen, G.; Chen, Q. Enhancing methane production in anaerobic digestion of food waste using co-pyrolysis biochar derived from digestate and rice straw. Molecules 2024, 30, 1766. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ren, T.; Yin, M.; Lu, K.; Xu, H.; Huang, X.; Zhang, X. Enhanced anaerobic wastewater treatment by a binary electroactive material: Pseudocapacitance/conductance-mediated microbial interspecies electron transfer. Environ. Sci. Technol. 2023, 57, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Hamid, F.; Pariatamby, A.; Suhaimi, N.; Razali, N.; Ling, K.; Mohan, P. Bioaugmentation-assisted bioremediation and biodegradation mechanisms for PCB in contaminated environments: A review on sustainable clean-up technologies. J. Environ. Chem. Eng. 2023, 11, 110055. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; He, Y.; Ji, R. Biodegradation of phenolic pollutants and bioaugmentation strategies: A review of current knowledge and future perspectives. J. Hazard. Mater. 2024, 469, 131971. [Google Scholar] [CrossRef]
- Sharma, S.; Pathania, S.; Bhagta, S.; Kaushal, N.; Bhardwaj, S.; Bhatia, R.K.; Walia, A. Microbial remediation of polluted environment by using recombinant E. coli: A review. Biotechnol. Environ. 2024, 1, 8. [Google Scholar] [CrossRef]
- Cai, Z.; Karunakaran, E.; Pandhal, J. Bottom-up construction and screening of algae-bacteria consortia for pollutant biodegradation. Front. Microbiol. 2024, 15, 1349016. [Google Scholar] [CrossRef]
- Cao, Z.; Yan, W.; Ding, M.; Yuan, Y. Construction of microbial consortia for microbial degradation of complex compounds. Front. Bioeng. Biotechnol. 2022, 10, 830184. [Google Scholar] [CrossRef]
- Khan, M.F. Fungi for sustainable pharmaceutical remediation: Enzymatic innovations, challenges, and applications—A review. Processes 2025, 13, 1034. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, J. Engineering microbiomes for enhanced bioremediation. PLoS Biol. 2024, 22, e3002951. [Google Scholar] [CrossRef]
- Renganathan, P.; Puente, E.O.; Sukhanova, N.V.; Gaysina, L.A. Hydroponics with microalgae and cyanobacteria: Emerging trends and opportunities in modern agriculture. BioTech 2024, 13, 27. [Google Scholar] [CrossRef]
- Martínez-Ruiz, F.E.; Andrade-Bustamante, G.; Holguín-Peña, R.J.; Renganathan, P.; Gaysina, L.A.; Sukhanova, N.V.; Puente, E.O. Microalgae as functional food ingredients: Nutritional benefits, challenges, and regulatory considerations for safe consumption. Biomass 2025, 5, 25. [Google Scholar] [CrossRef]
- Andrade-Bustamante, G.; Martínez-Ruiz, F.E.; Ortega-García, J.; Renganathan, P.; Gaysina, L.A.; Mahendhiran, M.; Puente, E.O. Microalgae-based functional foods: A blue-green revolution in sustainable nutrition and health. Appl. Microbiol. 2025, 5, 39. [Google Scholar] [CrossRef]
- Chakraborty, S.; Talukdar, A.; Dey, S.; Bhattacharya, S. Role of fungi, bacteria and microalgae in bioremediation of emerging pollutants with special reference to pesticides, heavy metals and pharmaceuticals. Discover Environ. 2025, 3, 91. [Google Scholar] [CrossRef]
- Shivaram, K.B.; Bhatt, P.; Applegate, B.; Simsek, H. Bacteriophage-based biocontrol technology to enhance the efficiency of wastewater treatment and reduce targeted bacterial biofilms. Sci. Total Environ. 2023, 862, 160723. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Montoya, M.D.; Salazar-Ospina, L.; Jiménez, J.N. Combating environmental antimicrobial resistance using bacteriophage cocktails targeting β-lactam-resistant high-risk clones of Klebsiella pneumoniae and Escherichia coli in wastewater: A strategy for treatment and reuse. Water 2025, 17, 2236. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Liang, W. The application of the CRISPR-Cas system in antibiotic resistance. Infect. Drug Resist. 2022, 15, 4155–4167. [Google Scholar] [CrossRef]
- Shim, K.Y.; Shin, H.; Yeo, I.C.; Kim, K.R.; Kwak, I.S.; Jeong, C.B. Environmental DNA surveillance of biocontamination in a drinking water treatment plant. J. Hazard. Mater. 2023, 456, 131656. [Google Scholar] [CrossRef]
- Sui, Q.; Chen, Y.; Yu, D.; Wang, T.; Hai, Y.; Zhang, J.; Chen, M.; Wei, Y. Fates of intracellular and extracellular antibiotic resistance genes and microbial community structures in typical swine wastewater treatment processes. Environ. Int. 2019, 133, 105146. [Google Scholar] [CrossRef]
- Ali, O.S.; Hozayen, W.G.; Almutairi, A.S.; Edris, S.; Karkashan, A.; Abulfaraj, A.A.; Attar, R.; Ouf, A.A.; Abbas, B.; Mahmoud, H.M. The assessment of the risk ranking and mobility potential associated with environmental resistomes in wastewater using metagenomic assembly. Sustainability 2021, 14, 14292. [Google Scholar] [CrossRef]
- Majeed, H.J.; Riquelme, M.V.; Davis, B.C.; Gupta, S.; Angeles, L.; Aga, D.S.; Garner, E.; Pruden, A.; Vikesland, P.J. Evaluation of metagenomic-enabled antibiotic resistance surveillance at a conventional wastewater treatment plant. Front. Microbiol. 2021, 12, 657954. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bao, N.; Yan, Z.; Yuan, Z.; Wang, G.; Xia, F. Degradation of antibiotic resistance genes by VADER with CRISPR-Cas immunity. Appl. Environ. Microbiol. 2023, 89, e00053-23. [Google Scholar] [CrossRef] [PubMed]
- Gencay, Y.E.; Jasinskytė, D.; Robert, C.; Semsey, S.; Martínez, V.; Petersen, A.Ø.; Brunner, K.; Salazar, A.; Turcu, I.C.; Eriksen, M.K.; et al. Engineered phage with antibacterial CRISPR–Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. 2024, 42, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, T. Metagenomic and metatranscriptomic analysis of microbial community structure and gene expression of activated sludge. PLoS ONE 2012, 7, e38183. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, Y.; Song, X.; Hallerman, E.; Peng, L.; Dong, D.; Ma, T.; Zhai, J.; Li, W. Ammonia-oxidizing bacteria and archaea within biofilters of a commercial recirculating marine aquaculture system. AMB Express 2018, 8, 42. [Google Scholar] [CrossRef]
- Cerruti, M.; Guo, B.; Delatolla, R.; De Jonge, N.; Van Steenwijk, A.H.; Kadota, P.; Lawson, C.E.; Mao, T.; Oosterkamp, M.J.; Sabba, F.; et al. Plant-wide systems microbiology for the wastewater industry. Environ. Sci. Water Res. Technol. 2021, 7, 1687–1706. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, C.; Cao, H.; Song, J.; Gong, B.; Li, L.; Wang, L.; He, Y.; Liang, M.; Lin, J.; et al. Microbial functional assemblages predicted by the FAPROTAX analysis are impacted by physicochemical properties, but C, N and S cycling genes are not in mangrove soil in the Beibu Gulf, China. Ecol. Indic. 2022, 139, 108887. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Wang, D.; Li, Y.; Cai, M.; Tian, Y.; Pan, Y.; Chen, X.; Zhang, Q.; Li, A. Optimization of sulfate reduction and methanogenesis via phase separation in a two-phase internal circulation reactor for the treatment of high-sulfate organic wastewater. Water Res. 2024, 260, 121918. [Google Scholar] [CrossRef]
- Dueholm, M.K.; Nierychlo, M.; Andersen, K.S.; Rudkjøbing, V.; Knutsson, S.; Albertsen, M.; Nielsen, P.H. MiDAS 4: A global catalogue of full-length 16S rRNA gene sequences and taxonomy for studies of bacterial communities in wastewater treatment plants. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Wu, L.; Ning, D.; Zhang, B.; Li, Y.; Zhang, P.; Shan, X.; Zhang, Q.; Brown, M.R.; Li, Z.; Van Nostrand, J.D.; et al. Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat. Microbiol. 2019, 4, 1183–1195. [Google Scholar] [CrossRef]
- Yilmaz, P.; Kottmann, R.; Field, D.; Knight, R.; Cole, J.R.; Amaral-Zettler, L.; Gilbert, J.A.; Karsch-Mizrachi, I.; Johnston, A.; Cochrane, G.; et al. Minimum information about a marker gene sequence (MIMARKS) and minimum information about any (x) sequence (MIxS) specifications. Nat. Biotechnol. 2011, 29, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Kim, J.; Wilson, F.P.; Bocher, B.T.; Liu, W.T. Coupling growth kinetics modeling with machine learning reveals microbial immigration impacts and identifies key environmental parameters in a biological wastewater treatment process. Microbiome 2019, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, J.; Oh, S. Machine learning reveals the complex ecological interplay of microbiome in a full-scale membrane bioreactor wastewater treatment plant. Environ. Res. 2023, 222, 115366. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Yu, C.; Truong, D.; Susilo, B.; Hu, A.; Sun, Q. Predicting microbial species in a river based on physicochemical properties by bio-inspired metaheuristic optimized machine learning. Sustainability 2018, 11, 6889. [Google Scholar] [CrossRef]
- Khashaba, N.H.; Ettouney, R.S.; Abdelaal, M.M.; Ashour, F.H.; El-Rifai, M.A. Artificial neural network modeling of biochar enhanced anaerobic sewage sludge digestion. J. Environ. Chem. Eng. 2022, 10, 107988. [Google Scholar] [CrossRef]
- Tong, X.; Goh, S.G.; Mohapatra, S.; Tran, N.H.; You, L.; Zhang, J.; He, Y.; Gin, K.Y. Predicting antibiotic resistance and assessing the risk burden from antibiotics: A holistic modeling framework in a tropical reservoir. Environ. Sci. Technol. 2024, 58, 6781–6792. [Google Scholar] [CrossRef]
- Gu, Y.; Li, B.; Zhong, X.; Liu, C.; Ma, B. Bacterial community composition and function in a tropical municipal wastewater treatment plant. Water 2021, 14, 1537. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Xu, R.; Wang, Y.; Chen, L.; Wei, C. Machine learning modeling for the prediction of phosphorus and nitrogen removal efficiency and screening of crucial microorganisms in wastewater treatment plants. Sci. Total Environ. 2024, 907, 167730. [Google Scholar] [CrossRef]
- Cai, W.; Long, F.; Wang, Y.; Liu, H.; Guo, K. Enhancement of microbiome management by machine learning for biological wastewater treatment. Microb. Biotechnol. 2021, 14, 59–62. [Google Scholar] [CrossRef]
- Tyagi, I.; Tyagi, K.; Ahamad, F.; Bhutiani, R.; Kumar, V. Assessment of bacterial community structure, associated functional role, and water health in full-scale municipal wastewater treatment plants. Toxics 2024, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Xu, J.; Kang, W.; Feng, R.; Hu, X. Ensemble learning model identifies adaptation classification and turning points of river microbial communities in response to heatwaves. Glob. Chang. Biol. 2023, 29, 6988–7000. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Zhang, W.; Li, R.; Bai, Y.; Chang, J. En-WBF: A novel ensemble learning approach to wastewater quality prediction based on weighted BoostForest. Water 2023, 16, 1090. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; De Vrieze, J.; Li, C.; Fang, X.; Li, X. Functional conservation of microbial communities determines composition predictability in anaerobic digestion. ISME J. 2023, 17, 1920–1930. [Google Scholar] [CrossRef]
- Cheng, H.; Cheng, D.; Mao, J.; Lu, T.; Hong, P.Y. Identification and characterization of core sludge and biofilm microbiota in anaerobic membrane bioreactors. Environ. Int. 2019, 133, 105165. [Google Scholar] [CrossRef]
- Seth, N.; Vats, S.; Lakhanpaul, S.; Arafat, Y.; Bansal, M.; Babu, C.R. Microbial community diversity of an integrated constructed wetland used for treatment of sewage. Front. Microbiol. 2024, 15, 1355718. [Google Scholar] [CrossRef]
- Banti, D.C.; Mitrakas, M.; Samaras, P. Membrane fouling controlled by adjustment of biological treatment parameters in step-aerating MBR. Membranes 2021, 11, 553. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, A.; Leyva-Diaz, J.C.; Muñoz-Palazon, B.; Rivadeneyra, M.A.; Hurtado-Martinez, M.; Martin-Ramos, D.; Gonzalez-Martinez, A.; Poyatos, J.M.; Gonzalez-Lopez, J. Biofouling formation and bacterial community structure in hybrid moving bed biofilm reactor-membrane bioreactors: Influence of salinity concentration. Water 2018, 10, 1133. [Google Scholar] [CrossRef]
- Mallah, N.B.; Shah, A.A.; Pirzada, A.M.; Ali, I.; Khan, M.I.; Jatoi, A.S.; Ullman, J.L.; Mahar, R.B. Advanced control strategies of membrane fouling in wastewater treatment: A review. Processes 2024, 12, 2681. [Google Scholar] [CrossRef]
- Biological Waste Expert. Blog Archives November 2024. Available online: https://www.biologicalwasteexpert.com/blog/archives/11-2024 (accessed on 16 June 2025).
- Oh, H.S.; Yeon, K.M.; Yang, C.S.; Kim, S.R.; Lee, C.H.; Park, S.Y.; Han, J.Y.; Lee, J.K. Control of membrane biofouling in MBR for wastewater treatment by quorum quenching bacteria encapsulated in microporous membrane. Environ. Sci. Technol. 2012, 46, 4877–4884. [Google Scholar] [CrossRef]
- Acharya, K.; Blackburn, A.; Mohammed, J.; Haile, A.T.; Hiruy, A.M.; Werner, D. Metagenomic water quality monitoring with a portable laboratory. Water Res. 2020, 184, 116112. [Google Scholar] [CrossRef]
- Ivshina, I.; Bazhutin, G.; Tyumina, E. Rhodococcus strains as a good biotool for neutralizing pharmaceutical pollutants and obtaining therapeutically valuable products: Through the past into the future. Front. Microbiol. 2022, 13, 967127. [Google Scholar] [CrossRef]
- Fish, K.E.; Clarizia, L.; Meegoda, J. Microplastics in aquatic environments: A review of recent advances. J. Environ. Eng. Sci. 2023, 18, 138–156. [Google Scholar] [CrossRef]
- Da Silva, M.R.; Souza, K.S.; Motteran, F.; De Araújo, L.C.; Singh, R.; Bhadouria, R.; De Oliveira, M.B. Exploring biodegradative efficiency: A systematic review on the main microplastic-degrading bacteria. Front. Microbiol. 2024, 15, 1360844. [Google Scholar] [CrossRef]
- Youssef, Y.A.; Abuarab, M.E.; Mahrous, A.; Mahmoud, M. Enhanced degradation of ibuprofen in an integrated constructed wetland-microbial fuel cell: Treatment efficiency, electrochemical characterization, and microbial community dynamics. RSC Adv. 2023, 13, 29809–29818. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, M.C.; Maugeri, A.; Favara, G.; Mastra, C.L.; San Lio, R.M.; Barchitta, M.; Agodi, A. The impact of wastewater on antimicrobial resistance: A scoping review of transmission pathways and contributing factors. Antibiotics 2025, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Yang, J.; Kim, S.; Park, J.; Kim, M.; Park, W. Occurrence of antibiotic resistance genes and multidrug-resistant bacteria during wastewater treatment processes. Sci. Total Environ. 2022, 811, 152331. [Google Scholar] [CrossRef] [PubMed]
- Talat, A.; Bashir, Y.; Khalil, N.; Brown, C.L.; Gupta, D.; Khan, A.U. Antimicrobial resistance transmission in the environmental settings through traditional and UV-enabled advanced wastewater treatment plants: A metagenomic insight. Environ. Microbiome 2025, 20, 27. [Google Scholar] [CrossRef]
- Tiwari, A.; Jaén-Gil, A.; Karavaeva, A.; Gomiero, A.; Ásmundsdóttir, Á.M.; Silva, M.J.; Salmivirta, E.; Tran, T.T.; Sarekoski, A.; Cook, J.; et al. Antibiotic resistance genes, antibiotic residues, and microplastics in influent and effluent wastewater from treatment plants in Norway, Iceland, and Finland. medRxiv 2025, 2025-02. [Google Scholar] [CrossRef]
- Che, Y.; Xu, X.; Yang, Y.; Břinda, K.; Hanage, W.; Yang, C.; Zhang, T. High-resolution genomic surveillance elucidates a multilayered hierarchical transfer of resistance between WWTP- and human/animal-associated bacteria. Microbiome 2022, 10, 16. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, H.; Gao, R.; Zhu, Y.; Rensing, C. Organic compounds stimulate horizontal transfer of antibiotic resistance genes in mixed wastewater treatment systems. Chemosphere 2017, 184, 53–61. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, B.; Ren, S.; Liu, X.; Wang, Y. Regulation of quorum sensing for the manipulation of conjugative transfer of antibiotic resistance genes in wastewater treatment system. Water Res. 2024, 253, 121222. [Google Scholar] [CrossRef]
- Kalli, M.; Noutsopoulos, C.; Mamais, D. The fate and occurrence of antibiotic-resistant bacteria and antibiotic resistance genes during advanced wastewater treatment and disinfection: A review. Water 2022, 15, 2084. [Google Scholar] [CrossRef]
- European Union. Directive 2001/18/EC on the Deliberate Release into the Environment of Genetically Modified Organisms. Available online: https://eur-lex.europa.eu/eli/dir/2001/18/oj (accessed on 18 June 2025).
- European Union. Summary of the Directive 2001/18/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=LEGISSUM:sa0015 (accessed on 23 June 2025).
- Pires, J.; Santos, R.; Monteiro, S. Antibiotic resistance genes in bacteriophages from wastewater treatment plant and hospital wastewaters. Sci. Total Environ. 2023, 892, 164708. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ondon, B.S.; Ho, S.; Jiang, J.; Li, F. Antibiotic resistant bacteria and genes in wastewater treatment plants: From occurrence to treatment strategies. Sci. Total Environ. 2022, 838, 156544. [Google Scholar] [CrossRef]
- Li, X.; Wu, S.; Dong, Y.; Fan, H.; Bai, Z.; Zhuang, X. Engineering microbial consortia towards bioremediation. Water 2020, 13, 2928. [Google Scholar] [CrossRef]
- Adrian, E.; Georgina, A.; Yair, S. Multifaceted applications of synthetic microbial communities: Advances in biomedicine, bioremediation, and industry. Microbiol. Res. 2024, 15, 1709–1727. [Google Scholar] [CrossRef]
- Teufel, M.; Klein, C.A.; Mager, M.; Sobetzko, P. A multifunctional system for genome editing and large-scale interspecies gene transfer. Nat. Commun. 2022, 13, 3430. [Google Scholar] [CrossRef]
- Ruan, Z.; Chen, K.; Cao, W.; Meng, L.; Yang, B.; Xu, M.; Xing, Y.; Li, P.; Freilich, S.; Chen, C.; et al. Engineering natural microbiomes toward enhanced bioremediation by microbiome modeling. Nat. Commun. 2024, 15, 4694. [Google Scholar] [CrossRef]
- Ramírez-Vargas, C.A.; Prado, A.; Arias, C.A.; Carvalho, P.N.; Esteve-Núñez, A.; Brix, H. Microbial electrochemical technologies for wastewater treatment: Principles and evolution from microbial fuel cells to bioelectrochemical-based constructed wetlands. Water 2018, 10, 1128. [Google Scholar] [CrossRef]
- Yin, Q.; Zhu, X.; Zhan, G.; Bo, T.; Yang, Y.; Tao, Y.; He, X.; Li, D.; Yan, Z. Enhanced methane production in an anaerobic digestion and microbial electrolysis cell coupled system with co-cultivation of Geobacter and Methanosarcina. J. Environ. Sci. 2016, 42, 210–214. [Google Scholar] [CrossRef]
- Qin, X.; Lu, X.; Cai, T.; Niu, C.; Han, Y.; Zhang, Z.; Zhu, X.; Zhen, G. Magnetite-enhanced bioelectrochemical stimulation for biodegradation and biomethane production of waste activated sludge. Sci. Total Environ. 2021, 789, 147859. [Google Scholar] [CrossRef] [PubMed]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Application of bioelectrochemical system and magnetite nanoparticles on the anaerobic digestion of sewage sludge: Effect of electrode configuration. Catalysts 2022, 12, 642. [Google Scholar] [CrossRef]
- Liu, D.; Geppert, F.; Na Ayudthaya, S.P.; Buisman, C.; Ter Heijne, A. Granular carbon-based electrodes as cathodes in methane-producing bioelectrochemical systems. Front. Bioeng. Biotechnol. 2018, 6, 379308. [Google Scholar] [CrossRef] [PubMed]
- De la Puente, C.; Carrillo-Peña, D.; Pelaz, G.; Morán, A.; Mateos, R. Microbial electrosynthesis for CO2 conversion and methane production: Influence of electrode geometry on biofilm development. Greenhouse Gases Sci. Technol. 2023, 13, 173–185. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, T.; Buisman, C.; Ter Heijne, A. Heat-treated stainless steel felt as a new cathode material in a methane-producing bioelectrochemical system. ACS Sustain. Chem. Eng. 2017, 5, 11346–11353. [Google Scholar] [CrossRef]
- Alevizos, V.; Yue, Z.; Edralin, S.; Xu, C.; Georlimos, N.; Papakostas, G.A. Biomimicry-inspired automated machine learning fit-for-purpose wastewater treatment for sustainable water reuse. Water 2024, 17, 1395. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A. Use, potential, needs, and limits of AI in wastewater treatment applications. Water 2024, 17, 170. [Google Scholar] [CrossRef]
- Huang, W.; Lin, C.; Nguyen, M.K.; Hussain, A.; Bui, T.; Ngo, H.H. A review of biosensor for environmental monitoring: Principle, application, and corresponding achievement of sustainable development goals. Bioengineered 2023, 14, 58. [Google Scholar] [CrossRef]
- Lin, L.; Ju, F. Evaluation of different 16S rRNA gene hypervariable regions and reference databases for profiling engineered microbiota structure and functional guilds in a swine wastewater treatment plant. Interface Focus 2023, 13, 20230012. [Google Scholar] [CrossRef]
- Cui, Y.; Rasul, F.; Jiang, Y.; Zhong, Y.; Zhang, S.; Boruta, T.; Riaz, S.; Daroch, M. Construction of an artificial consortium of Escherichia coli and cyanobacteria for clean indirect production of volatile platform hydrocarbons from CO2. Front. Microbiol. 2022, 13, 965968. [Google Scholar] [CrossRef]
- Hossain, G.S.; Liang, Y.; Foo, J.L.; Chang, M.W. Engineered microbial consortia for next-generation feedstocks. Biotechnol. Notes 2024, 5, 23. [Google Scholar] [CrossRef]
- Sousa, J.F.; Amaro, H.M.; Ribeirinho-Soares, S.; Esteves, A.F.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.M. Native microalgae-bacteria consortia: A sustainable approach for effective urban wastewater bioremediation and disinfection. Microorganisms 2024, 12, 1421. [Google Scholar] [CrossRef]
- Malik, S.; Dhasmana, A.; Preetam, S.; Mishra, Y.K.; Chaudhary, V.; Bera, S.P.; Ranjan, A.; Bora, J.; Kaushik, A.; Minkina, T.; et al. Exploring microbial-based green nanobiotechnology for wastewater remediation: A sustainable strategy. Nanomaterials 2022, 12, 4187. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Show, P.L. Microalgal-bacterial consortia as future prospect in wastewater bioremediation, environmental management and bioenergy production. Indian J. Microbiol. 2021, 61, 262. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, A.; Zhuang, G. Construction of environmental synthetic microbial consortia: Based on engineering and ecological principles. Front. Microbiol. 2022, 13, 829717. [Google Scholar] [CrossRef] [PubMed]
| Contaminant Type | Key Microbial Taxa/Genera | Major Degradation Genes/Pathways | Analytical/Computational Tools Used | Reference |
|---|---|---|---|---|
| Pesticides | Proteobacteria, Actinomycetota, Thauera | opd, mpd, atzA/B/D/F, chd, hdx, hdl-1 | PICRUSt2 | [48,49,50,51] |
| Hydrocarbons | Pseudomonas, Rhodococcus, Betaproteobacteria, Sulfuritalea, Ottowia, Thauera, Hyphomicrobium | Genes involved in benzoate, toluene, and polycyclic aromatic hydrocarbon (PAH) pathways | PICRUSt2, Shotgun Metagenomics | [50,52,53,54] |
| Endocrine-disrupting compounds/heavy metals | Proteobacteria, Verrucomicrobia, Cyanobacteria | Xenobiotic degradation gene clusters | PICRUSt2 | [55,56] |
| Micropollutants (e.g., pharmaceuticals: diclofenac, venlafaxine) | Thauera, Hyphomicrobium | Not specifically identified (linked through removal efficiency correlations) | 16S rRNA gene sequencing, correlation network analysis | [48,50,57] |
| Microplastics | Klebsiella, Pseudomonas, Sphingomonas, Xanthomonas, Acinetobacter | Plastic-degrading enzymes (e.g., PETase, esterases) | 16S rRNA gene sequencing | [49] |
| Surfactants | Aeromonas, sulfur-metabolizing bacteria | Genes involved in carbohydrate and sulfur metabolism | Comparative genomics | [54] |
| Nutrients | Diverse taxa including immobilized microbial consortia | Nitrogen and phosphorus cycling genes (e.g., amoA, nirK, phoX) | Cell immobilization studies, functional genomics | [51] |
| Pathogenic microbial agents | Acinetobacter, total coliforms, fecal coliforms | Not applicable | Activated sludge systems, stabilization ponds | [52] |
| Dissolved organic matter | Community hubs: rapid-growing taxa; peripherals: slow-growing recalcitrant degraders | Genes for redox reactions, C–N and C–S bond transformation pathways | Comparative genomics, reactomics | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renganathan, P.; Gaysina, L.A.; García Gutiérrez, C.; Rueda Puente, E.O.; Sainz-Hernández, J.C. Harnessing Engineered Microbial Consortia for Xenobiotic Bioremediation: Integrating Multi-Omics and AI for Next-Generation Wastewater Treatment. J. Xenobiot. 2025, 15, 133. https://doi.org/10.3390/jox15040133
Renganathan P, Gaysina LA, García Gutiérrez C, Rueda Puente EO, Sainz-Hernández JC. Harnessing Engineered Microbial Consortia for Xenobiotic Bioremediation: Integrating Multi-Omics and AI for Next-Generation Wastewater Treatment. Journal of Xenobiotics. 2025; 15(4):133. https://doi.org/10.3390/jox15040133
Chicago/Turabian StyleRenganathan, Prabhaharan, Lira A. Gaysina, Cipriano García Gutiérrez, Edgar Omar Rueda Puente, and Juan Carlos Sainz-Hernández. 2025. "Harnessing Engineered Microbial Consortia for Xenobiotic Bioremediation: Integrating Multi-Omics and AI for Next-Generation Wastewater Treatment" Journal of Xenobiotics 15, no. 4: 133. https://doi.org/10.3390/jox15040133
APA StyleRenganathan, P., Gaysina, L. A., García Gutiérrez, C., Rueda Puente, E. O., & Sainz-Hernández, J. C. (2025). Harnessing Engineered Microbial Consortia for Xenobiotic Bioremediation: Integrating Multi-Omics and AI for Next-Generation Wastewater Treatment. Journal of Xenobiotics, 15(4), 133. https://doi.org/10.3390/jox15040133