Cardioprotective Effects of SAR Through Attenuating Cardiac-Specific Markers, Inflammatory Markers, Oxidative Stress, and Anxiety in Rats Challenged with 5-Fluorouracil
Abstract
1. Introduction
2. Methodology
- Negative control (NC) group: They were treated orally with 1 mL of distilled water (D.W.) by oral feeding tube for 10 days.
- 5-Florouracil (5-FU) group: They were treated the same as the negative control group, but on day 10 they received (150 mg/kg) as a single intraperitoneal (I.P.) dose.
- N-acetyl cysteine (NAC) group: Treated with 100 mg/kg NAC daily orally for 10 days. This group’s treatment is a standard cardioprotective agent known for its well-established antioxidant, anti-inflammatory, and cytoprotective properties [22].
- Low dose of SAR group: Treated with 0.5 mg/kg SAR orally for 10 days.
- High dose of SAR: Treated with 5 mg/kg SAR orally for 10 days.
2.1. Biochemical Tests
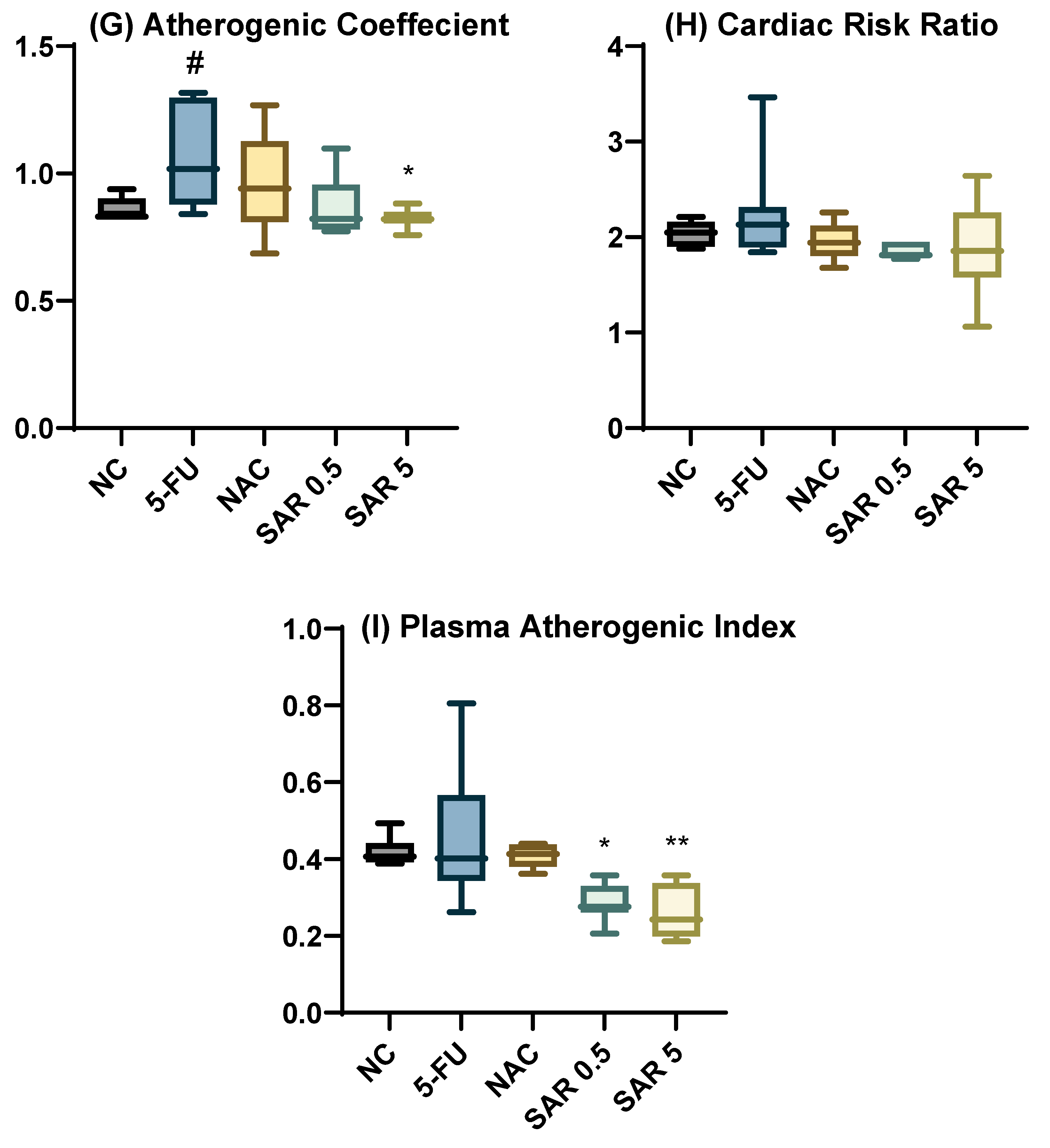
2.2. Determination of Atherogenic Indices
2.3. Evaluation of Locomotor Behavior and Anxiety Test in Rats
Open Field Test
2.4. Histopathological Protocol
2.5. Semiquantitative Histomorphometry Analysis of Myocardial Toxicity
2.6. Statistical Analysis
3. Results
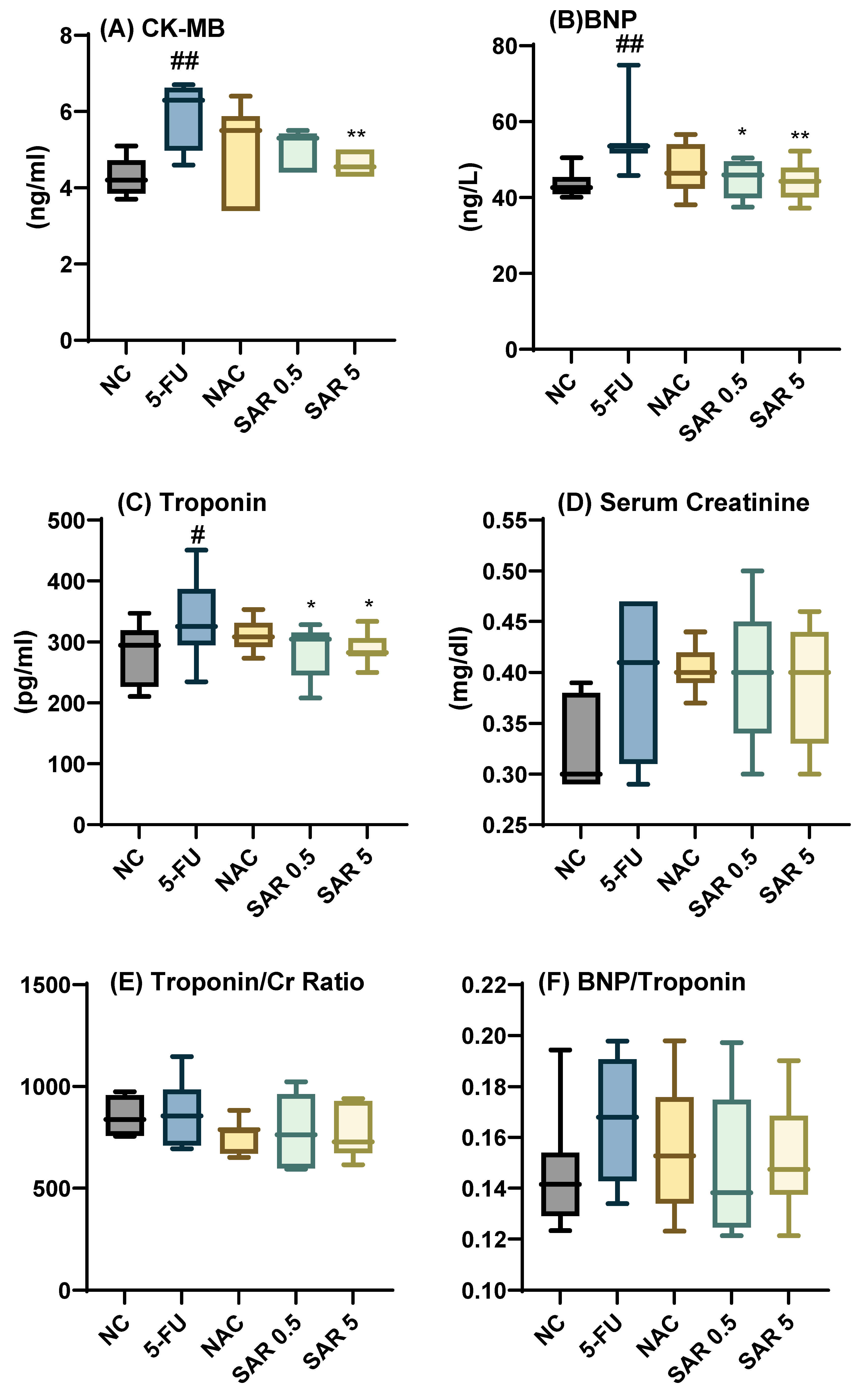
3.1. Impact of Different Doses of SAR on Cardiac-Specific Biomarkers, and Cardiac Indices
3.2. Impact of Different Doses of SAR on Weight Change, AST, ALT, LDH, and ALP
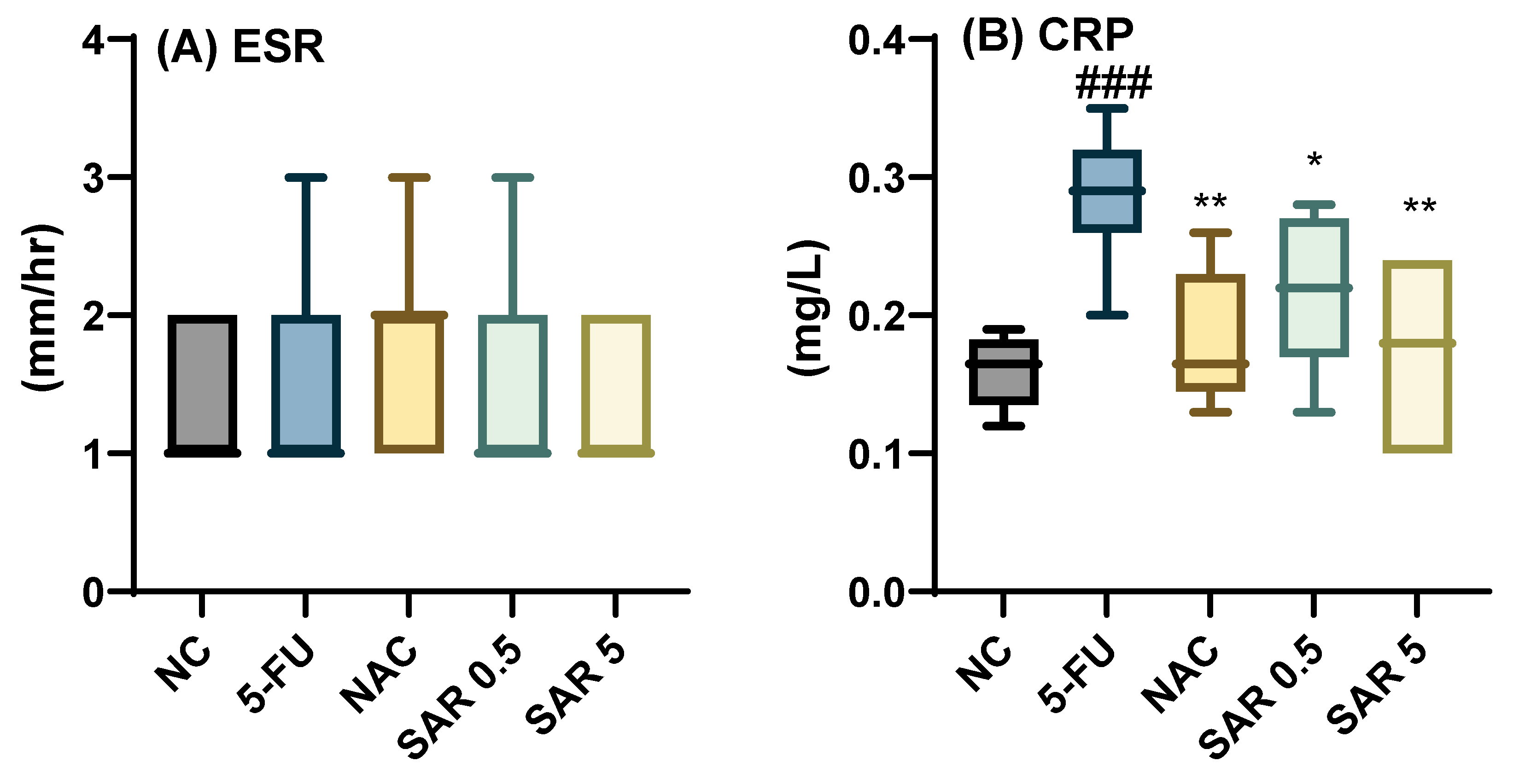
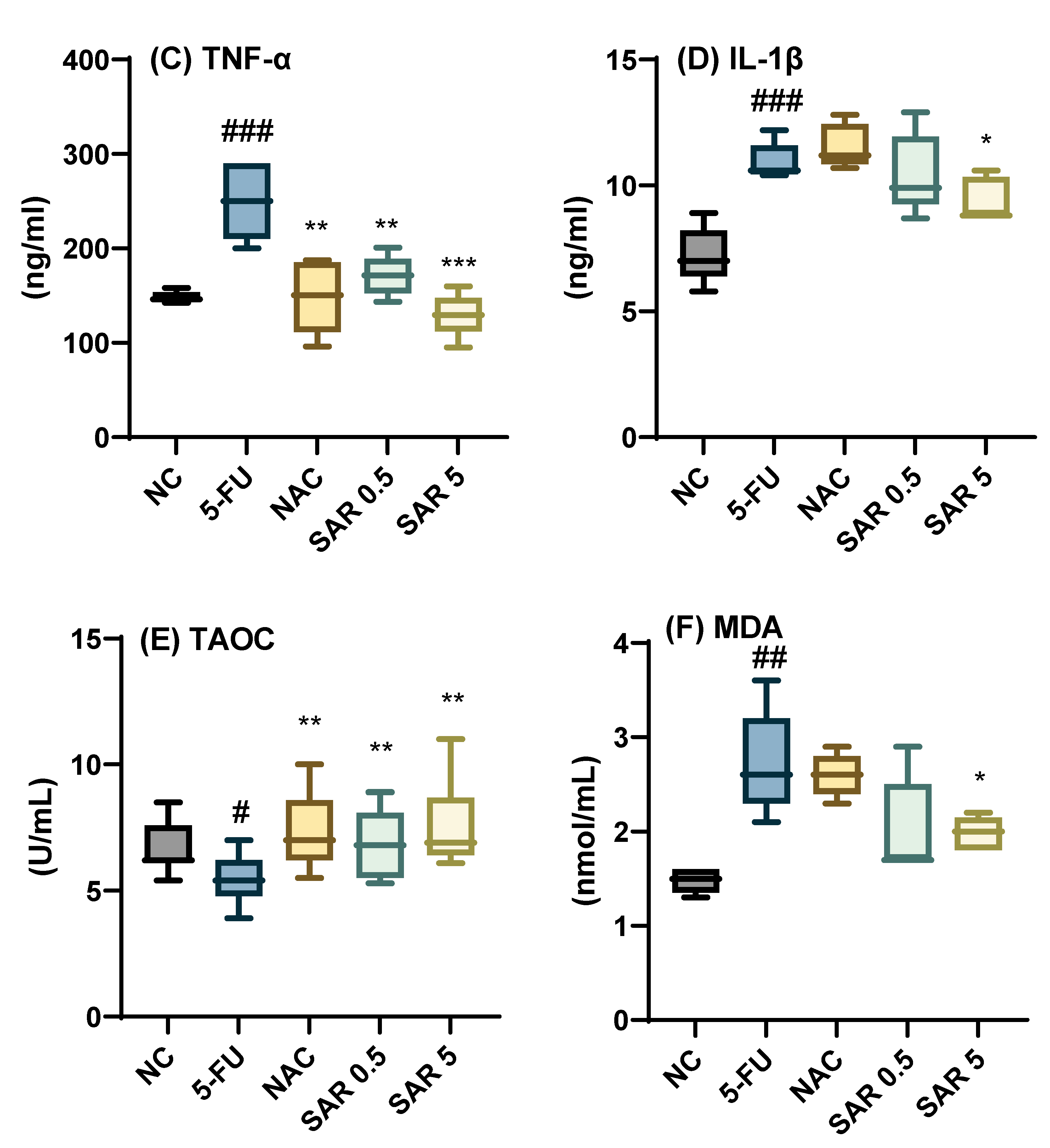
3.3. Impact of Different Doses of SAR on Inflammatory and Oxidative Stress Biomarkers
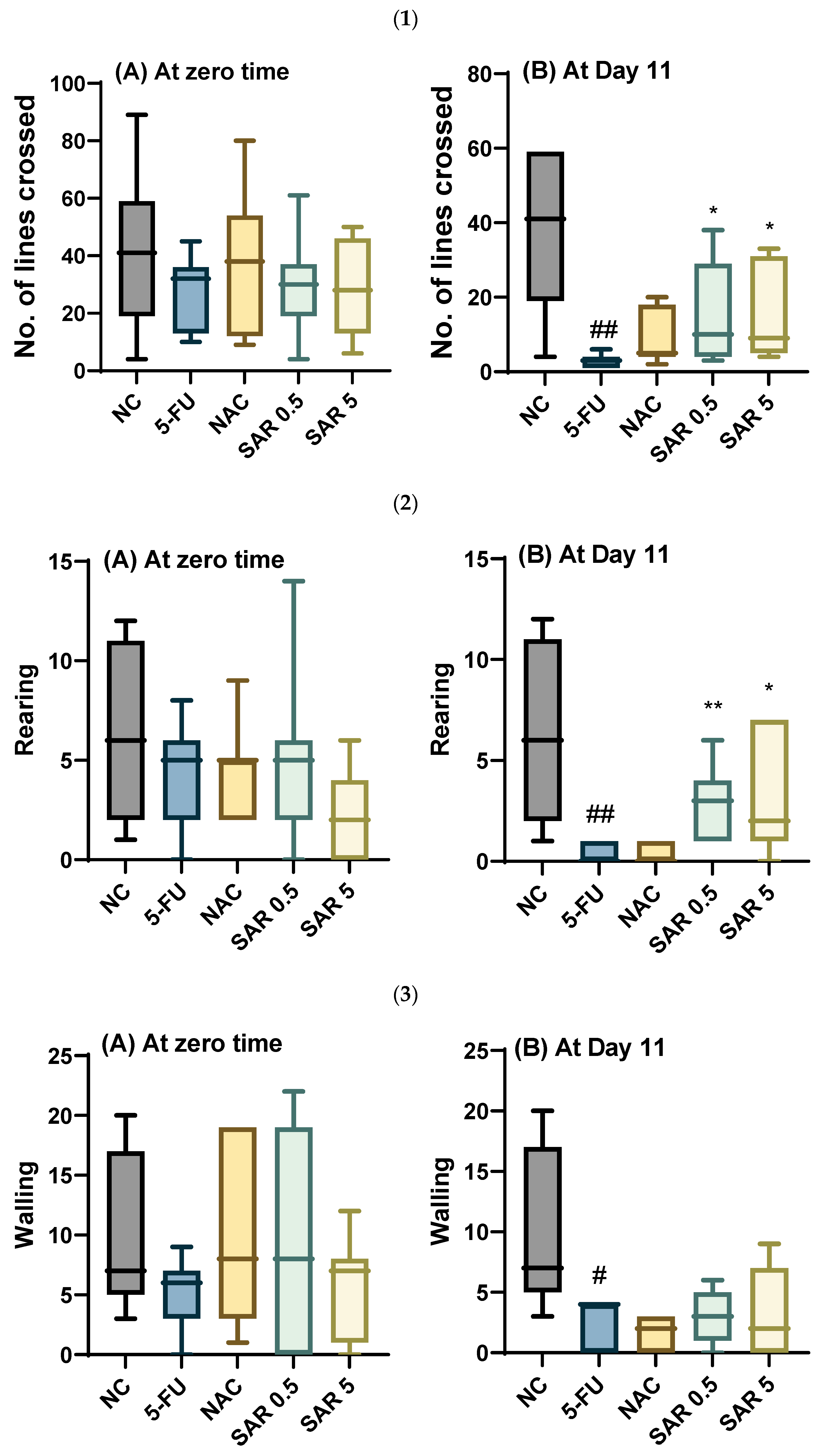
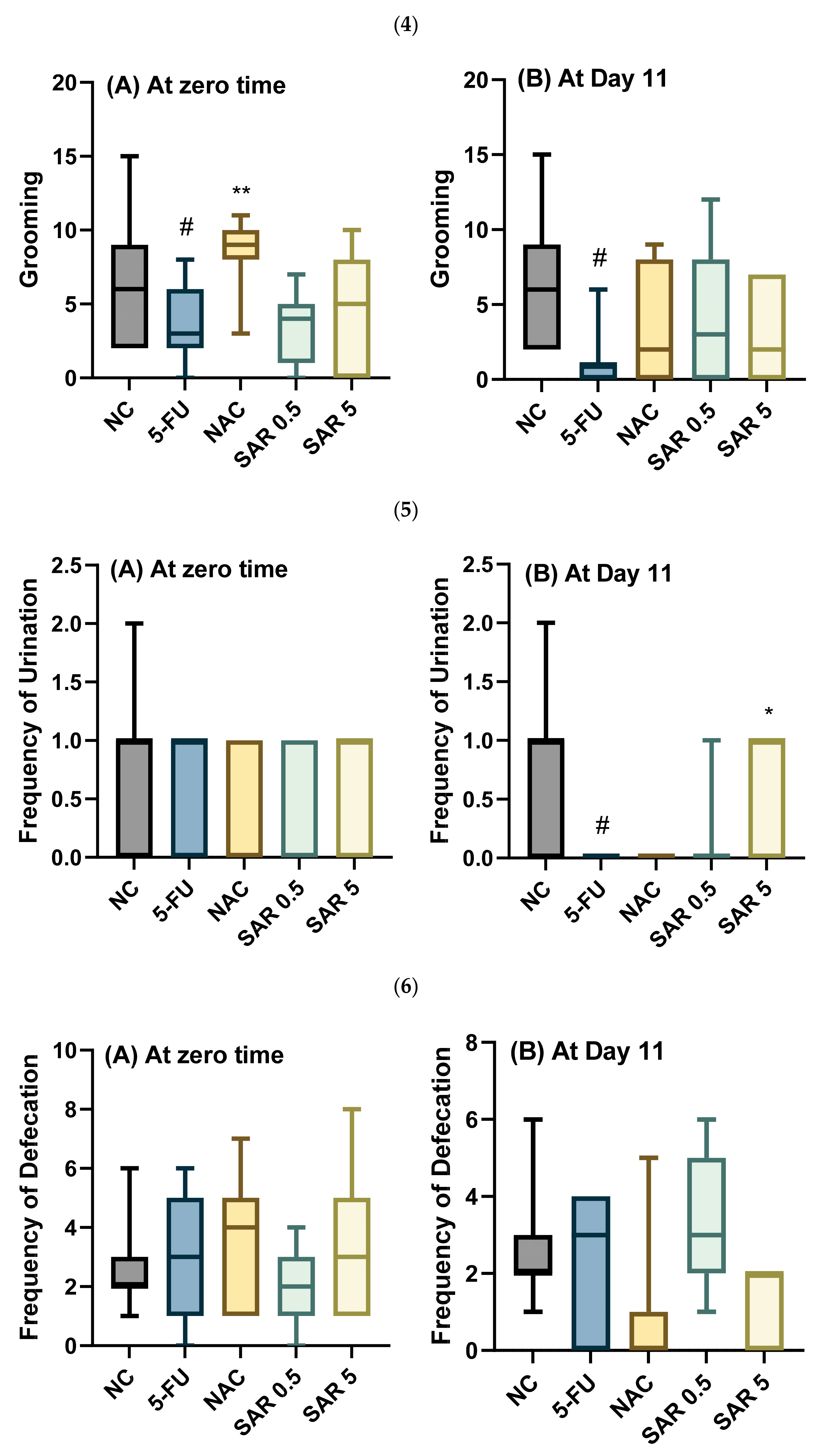
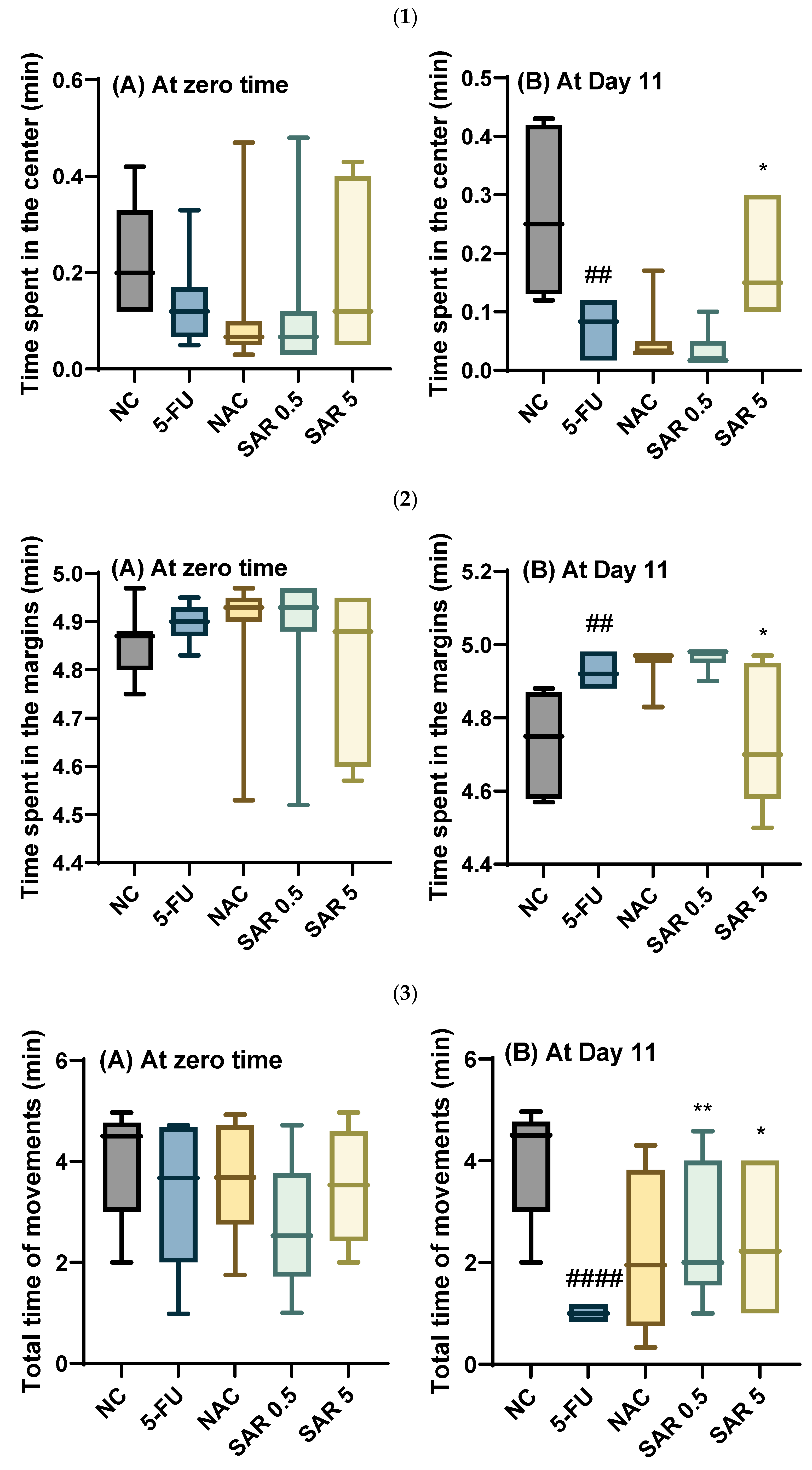
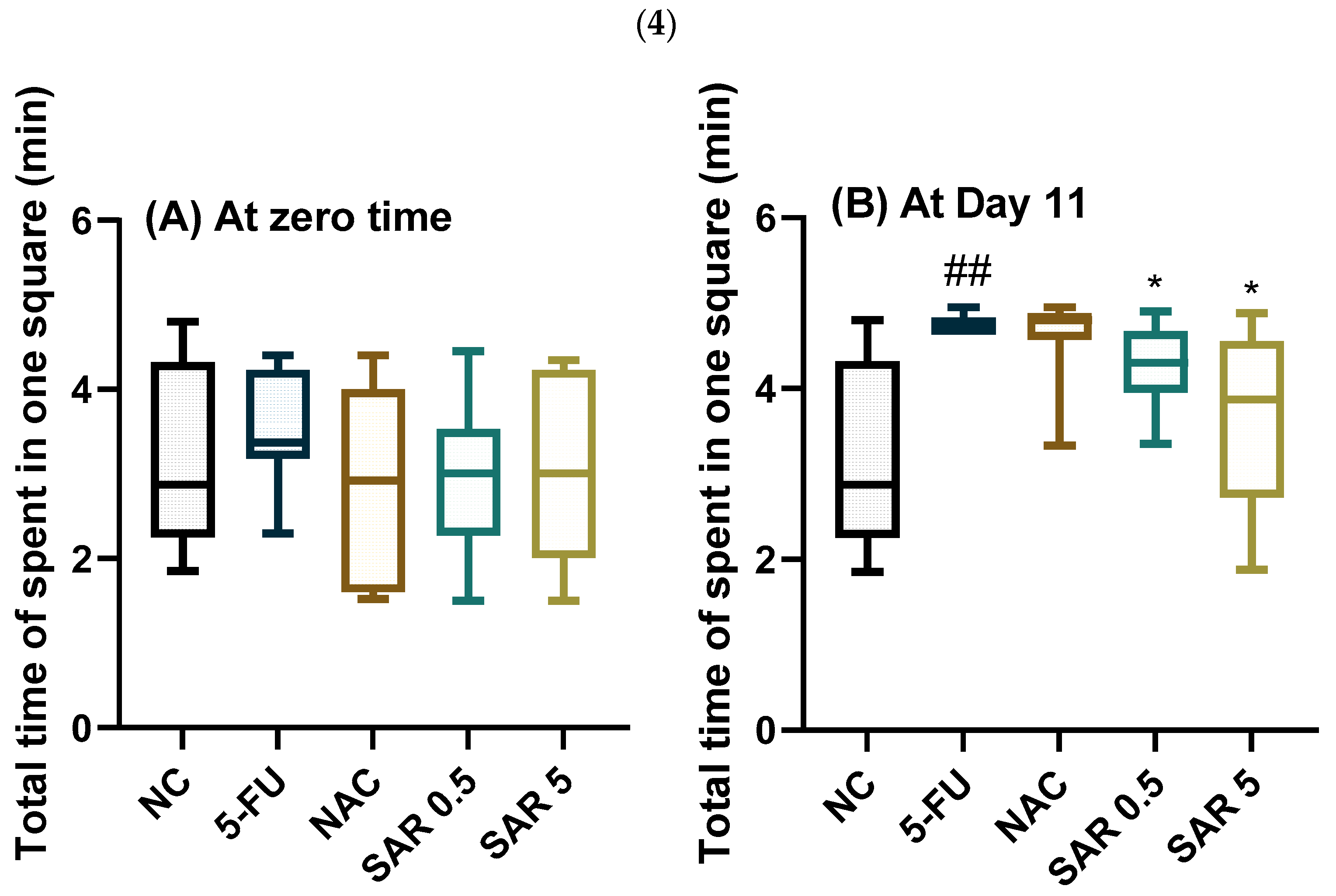
3.4. Impact of Different Doses of SAR on Anxiety
3.4.1. Number of Lines Crossed, Rearing, Walling, Grooming, and Frequencies of Urination and Defecation
3.4.2. Time Spent in the Center, Time Spent in the Margins, Total Time of Movement, and Total Time Spent in One Square
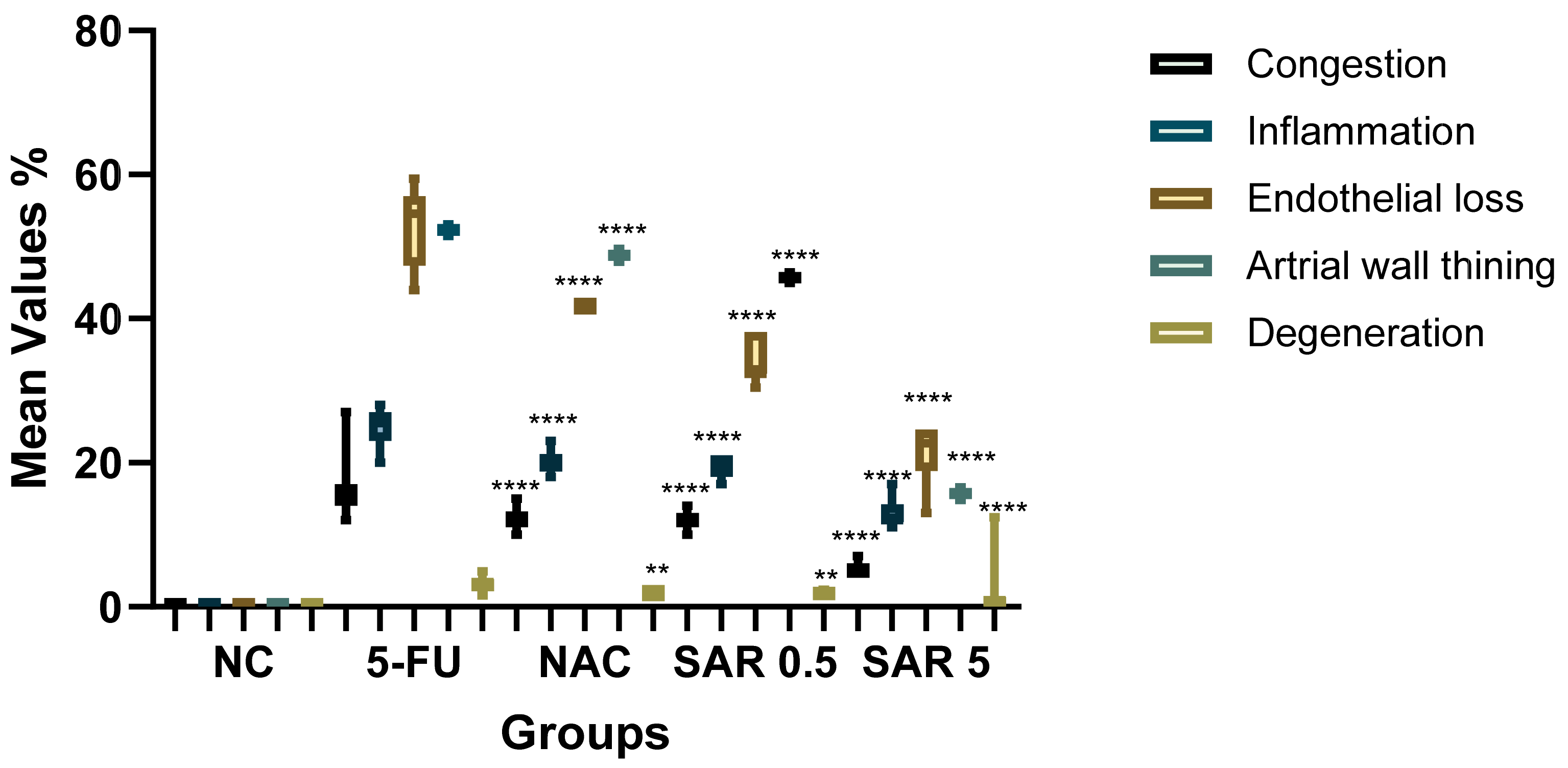
3.5. Histopathological Finding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, E.T.H.; Bickford, C.L. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J. Am. Coll Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; De azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F.; et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012, 23 (Suppl. 7), vii155–vii166. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.B.; Leung, K.T.; Poon, E.N. Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2022, 23, 1912. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.A.; Abtar, A.N.; Othman, H.H.; Aziz, T.A. Effects of quercetin, sitagliptin alone or in combination in testicular toxicity induced by doxorubicin in rats. Drug Des. Devel Ther. 2019, 13, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, N.; Rosenthal, A.; Dabas, N.; Kropotova, Y.; Lippman, M.; Bishopric, N.H. Trastuzumab-induced cardiotoxicity: A review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. Breast Cancer Res. Treat. 2021, 188, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Polk, A.; Vaage-Nilsen, M.; Vistisen, K.; Nielsen, D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 2013, 39, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.H.; Ahmed, Z.A.; Aziz, T.A. Effect of Telmisartan and Quercetin in 5 Fluorouracil-Induced Renal Toxicity in Rats. J. Inflamm. Res. 2022, 15, 6113. [Google Scholar] [CrossRef] [PubMed Central]
- Alter, P.; Herzum, M.; Soufi, M.; Schaefer, J.; Maisch, B. Cardiotoxicity of 5-fluorouracil. Cardiovasc. Hematol. Agents Med. Chem. 2006, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M. Cardiovascular and Cardioprotective Agents in 2021. Curr. Pharm. Des. 2021, 27, 2915–2916. [Google Scholar] [CrossRef] [PubMed]
- Hamaamin, K.S.; Aziz, T.A. Doxorubicin-Induced Cardiotoxicity: Mechanisms and Management. Al-Rafidain J. Med. Sci. 2022, 3, 87–97. [Google Scholar] [CrossRef]
- Goyal, V.; Bews, H.; Cheung, D.; Premecz, S.; Mandal, S.; Shaikh, B.; Best, R.; Bhindi, R.; Chaudhary, R.; Ravandi, A.; et al. The Cardioprotective Role of N-Acetyl Cysteine Amide in the Prevention of Doxorubicin and Trastuzumab-Mediated Cardiac Dysfunction. Can. J. Cardiol. 2016, 32, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Miriyala, S.; Panchatcharam, M. PPARγ and Its Role in Cardiovascular Diseases. PPAR Res. 2017, 2017, 6404638. [Google Scholar] [CrossRef] [PubMed Central]
- Hamblin, M.; Chang, L.; Fan, Y.; Zhang, J.; Chen, Y.E. PPARs and the Cardiovascular System. Antioxid. Redox Signal 2009, 11, 1415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Honda, A.; Kamata, S.; Satta, C.; Machida, Y.; Uchii, K.; Terasawa, K.; Nemoto, A.; Oyama, T.; Ishii, I. Structural Basis for Anti-non-alcoholic Fatty Liver Disease and Diabetic Dyslipidemia Drug Saroglitazar as a PPAR α/γ Dual Agonist. Biol. Pharm. Bull. 2021, 44, 1210. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Parmar, D.; Sheikh, F.; Sarin, S.K.; Cisneros, L.; Gawrieh, S.; Momin, T.; Duseja, A.; Sanyal, A.J. Saroglitazar, a Dual PPAR α/γ Agonist, Improves Atherogenic Dyslipidemia in Patients With Non-Cirrhotic Nonalcoholic Fatty Liver Disease: A Pooled Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2597–2605.e2. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.Q.; Jing, Z.; Liu, X.; Feng, X.Y.; Liu, Y.Y.; Wang, S.Q.; Xu, W.R.; Liu, J.W.; Cheng, X.C. Virtual identification of novel PPARα/γ dual agonists by scaffold hopping of saroglitazar. J. Biomol. Struct. Dyn. 2018, 36, 3496–3512. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.R.; Giri, S.R.; Trivedi, C.; Bhoi, B.; Rath, A.; Vanage, G.; Vyas, P.; Ranvir, R.; Patel, P.R. Saroglitazar, a novel PPARα/γ agonist with predominant PPARα activity, shows lipid-lowering and insulin-sensitizing effects in preclinical models. Pharmacol. Res. Perspect. 2015, 3, e00136. [Google Scholar] [CrossRef]
- Peerapanyasut, W.; Kobroob, A.; Palee, S.; Chattipakorn, N.; Wongmekiat, O. N-Acetylcysteine Attenuates the Increasing Severity of Distant Organ Liver Dysfunction after Acute Kidney Injury in Rats Exposed to Bisphenol A. Antioxidants 2019, 8, 497. [Google Scholar] [CrossRef]
- Smaga, I.; Frankowska, M.; Filip, M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br. J. Pharmacol. 2021, 178, 2569–2594. [Google Scholar] [CrossRef]
- Young, M.E.; Laws, F.A.; Goodwin, G.W.; Taegtmeyer, H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J. Biol. Chem. 2001, 276, 44390–44395. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Liu, H.; Yang, Y.; Yang, Y.; Jiao, Y.; Tay, F.R.; Chen, J. Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects. Oxid. Med. Cell. Longev. 2018, 2018, 2835787. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.A. Cardioprotective Effect of Quercetin and Sitagliptin in Doxorubicin-Induced Cardiac Toxicity in Rats. Cancer Manag. Res. 2021, 13, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, R.; Kavitha, S. Atherogenic indices in stroke patients: A retrospective study. Iran. J. Neurol. 2017, 16, 78. [Google Scholar] [PubMed Central]
- Harro, J. (Ed.) Psychiatric Vulnerability, Mood, and Anxiety Disorders; Humana: New York, NY, USA, 2023; p. 190. [Google Scholar] [CrossRef]
- Nku, C.O.; Oghale, G.O.; Ajiwhen, I.O. Locomotor Behaviour and Anxiety in the Open Field and Light/Dark Box in CD1 Mice Treated with Aspirin, Cataflam and Ethanolic Extract of Cannabis sativa. J. Adv. Med. Med. Res. 2015, 6, 563–572. [Google Scholar] [CrossRef]
- Lee, W.-S.; Kim, J. Peroxisome proliferator-activated receptors and the heart: Lessons from the past and future directions. PPAR Res. 2015, 2015, 271983. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhang, C.; Zou, Y.; Kang, L.; Song, L. Role of Biomarkers of Myocardial Injury to Predict Adverse Outcomes in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Qual. Outcomes 2024, 17, E010243. [Google Scholar] [CrossRef]
- Peela, J.; Jarari, A.; Hai, A.; Rawal, A.; Kolla, S.; Sreekumar, S.; Khurana, L.; Sidhanathi, N. Cardiac Biomarkers: The Troponins and CK-MB. Ibnosina J. Med. Biomed. Sci. 2010, 2, 190–197. [Google Scholar] [CrossRef]
- Babuin, L.; Jaffe, A.S. Troponin: The biomarker of choice for the detection of cardiac injury. CMAJ Can. Med. Assoc. J. 2005, 173, 1191. [Google Scholar] [CrossRef] [PubMed Central]
- Nadar, S.K.; Shaikh, M.M. Biomarkers in Routine Heart Failure Clinical Care. Card. Fail. Rev. 2019, 5, 50. [Google Scholar] [CrossRef] [PubMed Central]
- Finck, B.N. The PPAR regulatory system in cardiac physiology and disease. Cardiovasc. Res. 2007, 73, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Moore, G.G. 5-Fluorouracil-associated severe hypertriglyceridaemia with positive rechallenge Case report. BMJ Case Rep. 2023, 16, 254871. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.T.; Baumfalk, D.R.; Parr, S.K.; Butenas, A.L.E.; Scheuermann, B.C.; Turpin, V.R.G.; Behnke, B.J.; Hashmi, M.H.; Ade, C.J. Impaired microvascular reactivity in patients treated with 5-fluorouracil chemotherapy regimens: Potential role of endothelial dysfunction. IJC Heart Vasc. 2023, 49, 101300. [Google Scholar] [CrossRef]
- Joshi, S.R. Saroglitazar for the treatment of dyslipidemia in diabetic patients. Expert. Opin. Pharmacother. 2015, 16, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Ryu, J.; Kim, H.J.; Song, J.W.; Jeon, J.H.; Lee, D.H.; Oh, D.J.; Gweon, D.-G.; Oh, W.-Y.; Yoo, H.; et al. Therapeutic Effects of Targeted PPARɣ Activation on Inflamed High-Risk Plaques Assessed by Serial Optical Imaging In Vivo. Theranostics 2018, 8, 45. [Google Scholar] [CrossRef] [PubMed Central]
- Li, A.C.; Binder, C.J.; Gutierrez, A.; Brown, K.K.; Plotkin, C.R.; Pattison, J.W.; Valledor, A.F.; Davis, R.A.; Willson, T.M.; Witztum, J.L.; et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J. Clin. Investig. 2004, 114, 1564. [Google Scholar] [CrossRef] [PubMed Central]
- Li, A.C.; Glass, C.K. PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J. Lipid. Res. 2004, 45, 2161–2173. [Google Scholar] [CrossRef]
- Canbaz, H.; Görmel, S. Prognostic value of troponin/creatinine ratio in patients with chest pain. Turk. J. Thorac. Cardiovasc. Surg. 2023, 31, 11. [Google Scholar] [CrossRef] [PubMed Central]
- Brennan, E.; Saith, S.; Marmur, J.; Kahn, A.; Khan, A.; Adeniran, O.; Syed, R.; Waqas, A.; Mucha, T.; Amador, F.; et al. A-67 | Assessment of BNP/Troponin Ratio in Patients Undergoing Cardiac Catheterization. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101543. [Google Scholar] [CrossRef]
- Baghshani, H.; LotfiGhahramanloo, M. Evaluation of lead-induced cardiac toxicity in mice by measurement of selected biochemical as well as oxidative indices. Comp. Clin. Path. 2020, 29, 1165–1171. [Google Scholar] [CrossRef]
- Park, P.G.; Song, J.J.; Park, Y.B.; Lee, S.W. Clinical application of low erythrocyte sedimentation rate/high C-reactive protein to antineutrophil cytoplasmic antibody-associated vasculitis. J. Clin. Lab. Anal. 2022, 36, e24237. [Google Scholar] [CrossRef]
- Patel, H.; Giri, P.; Patel, P.; Singh, S.; Gupta, L.; Patel, U.; Modi, N.; Shah, K.; Jain, M.R.; Srinivas, N.R.; et al. Preclinical evaluation of saroglitazar magnesium, a dual PPAR-α/γ agonist for treatment of dyslipidemia and metabolic disorders. Xenobiotica 2018, 48, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, A.I.; Baiz, H.Q.; Ahmed, Z.A.; Othman, H.H.; Aziz, T.A. Healing Effects of Saroglitazar Gel in Thermally Induced Burn in Rats. Al-Anbar Med. J. 2024, 20, 230–237. [Google Scholar] [CrossRef]
- Rubio-Ruíz, M.E.; Plata-Corona, J.C.; Soria-Castro, E.; Díaz-Juárez, J.A.; Sánchez-Aguilar, M. Pleiotropic Effects of Peroxisome Proliferator-Activated Receptor Alpha and Gamma Agonists on Myocardial Damage: Molecular Mechanisms and Clinical Evidence-A Narrative Review. Cells 2024, 13, 1488. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.Z.; Ivashchenko, C.Y.; Usher, M.G.; Mortensen, R.M. PPAR-γ in the Cardiovascular System. PPAR Res. 2008, 2008, 745804. [Google Scholar] [CrossRef] [PubMed]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, X. Effect of rosiglitazone on inflammatory cytokines and oxidative stress after intensive insulin therapy in patients with newly diagnosed type 2 diabetes. Diabetol. Metab. Syndr. 2019, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, R.; Monroy, M.A.; Daly, J.M. 15-Deoxy-Delta12,14-prostaglandin J2 (15d-PGJ2) mediates repression of TNF-alpha by decreasing levels of acetylated histone H3 and H4 at its promoter. Biochem. Biophys. Res. Commun. 2007, 359, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shi, L.; Xin, W.; Xu, J.; Xu, J.; Li, Q.; Xu, Z.; Wang, J.; Wang, G.; Yao, W.; et al. Activation of PPARγ inhibits pro-inflammatory cytokines production by upregulation of miR-124 in vitro and in vivo. Biochem. Biophys. Res. Commun. 2017, 486, 726–731. [Google Scholar] [CrossRef]
- Akbari, R.; Behdarvand, T.; Afarin, R.; Yaghooti, H.; Jalali, M.T.; Mohammadtaghvaei, N. Saroglitazar improved hepatic steatosis and fibrosis by modulating inflammatory cytokines and adiponectin in an animal model of non-alcoholic steatohepatitis. BMC Pharmacol. Toxicol. 2021, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, N.; Afarin, R.; Shakerian, E.; Bavarsad, S.S.; Mohammadtaghvaei, N. The effect of saraglitazar on TGF-β-induced smad3 phosphorylation and expression of genes related to liver fibrosis in LX2 cell line. Mol. Biol. Rep. 2024, 51, 541. [Google Scholar] [CrossRef] [PubMed]
- Tavaglione, F.; Loomba, R. Emerging Combination of Saroglitazar and Vitamin E for the Treatment of NAFLD and NASH. J. Clin. Exp. Hepatol. 2024, 14, 101449. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- de Koning, M.S.L.Y.; Emmens, J.E.; Romero-Hernández, E.; Bourgonje, A.R.; Assa, S.; Figarska, S.M.; Cleland, J.G.F.; Samani, N.J.; Ng, L.L.; Lang, C.C.; et al. Systemic oxidative stress associates with disease severity and outcome in patients with new-onset or worsening heart failure. Clin. Res. Cardiol. 2023, 112, 1056–1066. [Google Scholar] [CrossRef]
- Panda, P.; Verma, H.K.; Lakkakula, S.; Merchant, N.; Kadir, F.; Rahman, S.; Jeffree, M.S.; Lakkakula, B.V.K.S.; Rao, P.V. Biomarkers of Oxidative Stress Tethered to Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 9154295. [Google Scholar] [CrossRef]
- Abbas, A.; Blandon, J.; Rude, J.; Elfar, A.; Mukherjee, D. PPAR-γ Agonist in Treatment of Diabetes: Cardiovascular Safety Considerations. Cardiovasc. Hematol. Agents Med. Chem. 2012, 10, 124–134. [Google Scholar] [CrossRef]
- Wu, K.K. Peroxisome Proliferator-Activated Receptors Protect against Apoptosis via 14-3-3. PPAR Res. 2010, 2010, 417646. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Deciphering the molecular pathways of saroglitazar: A dual PPAR α/γ agonist for managing metabolic NAFLD. Metabolism 2024, 155, 155912. [Google Scholar] [CrossRef]
- Panigrahy, S.R.; Pradhan, S.; Maharana, C.S. Amelioration of oxidative stress and neuroinflammation by Saroglitazar, a dual PPARα/γ agonist in MES induced epileptic rats. Biomed. Pharmacol. J. 2019, 12, 1985–1991. [Google Scholar] [CrossRef]
- Banozic, A.; Grkovic, I.; Puljak, L.; Sapunar, D. Behavioral Changes Following Experimentally-Induced Acute Myocardial Infarction in Rats. Int. Heart J. 2014, 55, 169–177. [Google Scholar] [CrossRef]
- Schoemaker, R.G.; Smits, J.F.M. Behavioral changes following chronic myocardial infarction in rats. Physiol. Behav. 1994, 56, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Was, H.; Borkowska, A.; Bagues, A.; Tu, L.; Liu, J.Y.H.; Lu, Z.; Rudd, J.A.; Nurgali, K.; Abalo, R. Mechanisms of Chemotherapy-Induced Neurotoxicity. Front. Pharmacol. 2022, 13, 750507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quintão, N.L.M.; Santin, J.R.; Stoeberl, L.C.; Corrêa, T.P.; Melato, J.; Costa, R. Pharmacological Treatment of Chemotherapy-Induced Neuropathic Pain: PPARγ Agonists as a Promising Tool. Front. Neurosci. 2019, 13, 907. [Google Scholar] [CrossRef] [PubMed Central]
- Beheshti, F.; Hosseini, M.; Hashemzehi, M.; Soukhtanloo, M.; Asghari, A. The effects of PPAR-γ agonist pioglitazone on anxiety and depression-like behaviors in lipopolysaccharide injected rats. Toxin Rev. 2021, 40, 1223. [Google Scholar] [CrossRef]
- Westlund, K.N.; Zhang, M. Building and Testing PPARγ Therapeutic ELB00824 with an Improved Therapeutic Window for Neuropathic Pain. Molecules 2020, 25, 1120. [Google Scholar] [CrossRef] [PubMed]
- Chintalgattu, V.; Harris, G.S.; Akula, S.M.; Katwa, L.C. PPAR-gamma agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblasts. Cardiovasc. Res. 2007, 74, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Zhang, H.G. Transcription Factors Involved in the Development and Prognosis of Cardiac Remodeling. Front. Pharmacol. 2022, 13, 828549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Luo, Z.; Wang, P.; Sun, J.; Yu, H.; Cao, T.; Ni, Y.; Chen, J.; Yan, Z.; Liu, D.; et al. Rosiglitazone Restores Endothelial Dysfunction in a Rat Model of Metabolic Syndrome through PPARγ- and PPARδ-Dependent Phosphorylation of Akt and eNOS. PPAR Res. 2011, 2011, 291656. [Google Scholar] [CrossRef] [PubMed Central]
- Ho, C.C.; Wen, P.C.; Yu, W.C.; Hu, Y.W.; Yang, C.C. Pre-existing chronic kidney disease and hypertension increased the risk of car-diotoxicity among colorectal cancer patients treated with anticancer drugs. J. Chin. Med. Assoc. 2021, 84, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Belger, C.; Abrahams, C.; Imamdin, A.; Lecour, S. Doxorubicin-induced cardiotoxicity and risk factors. Int. J. Cardiol. Heart Vasc. 2023, 50, 101332. [Google Scholar] [CrossRef] [PubMed]

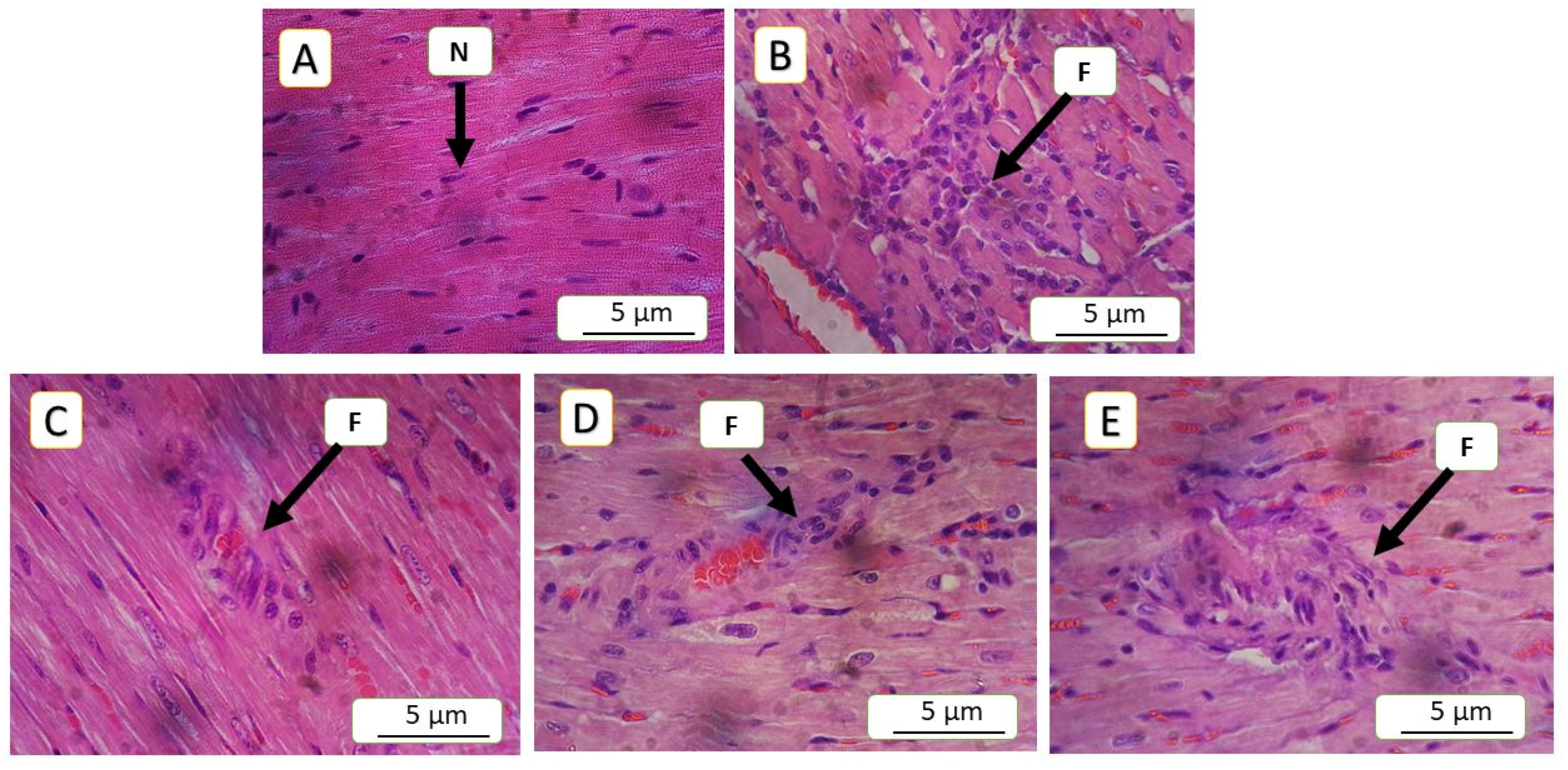
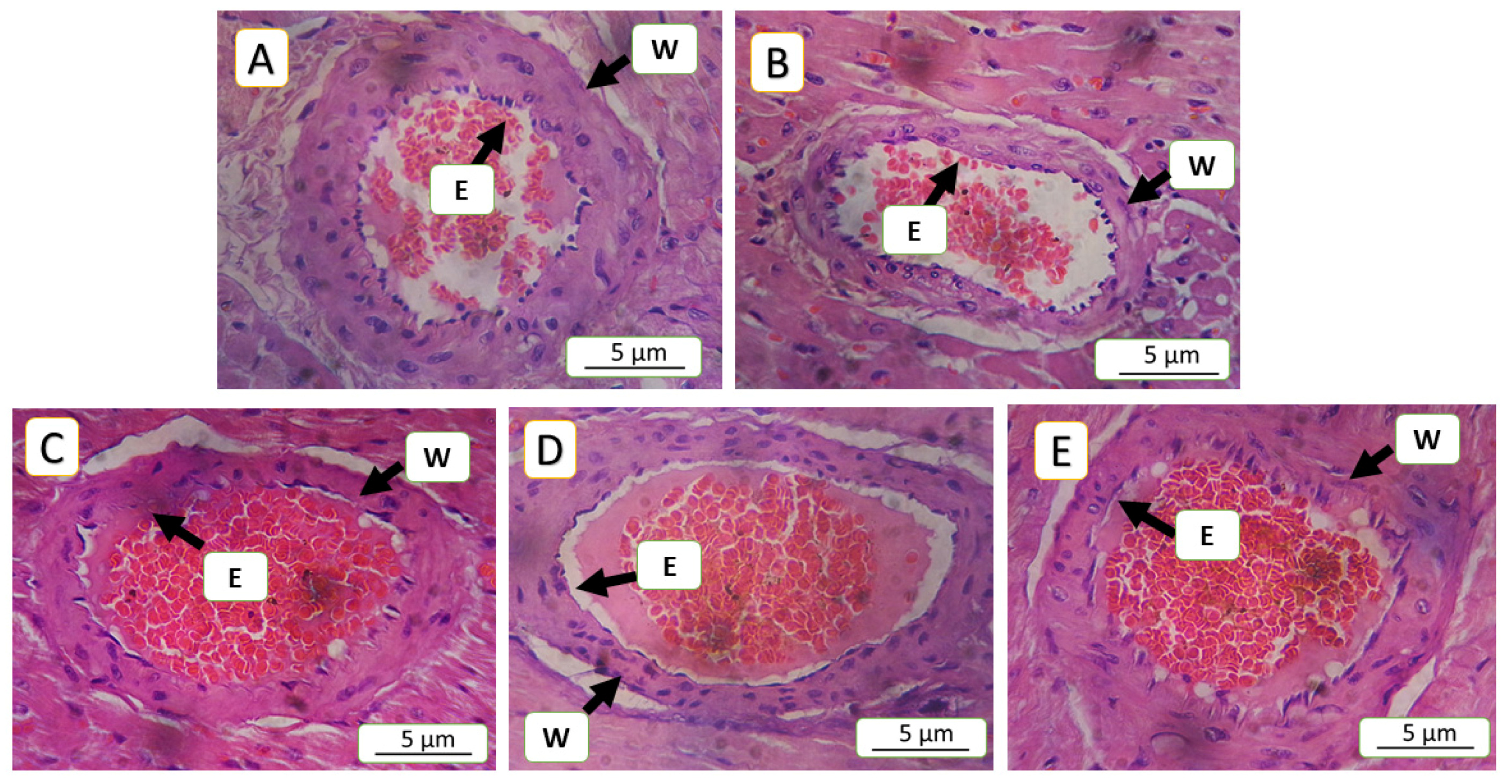

| Lesions | Interpretation | Score | Grade |
|---|---|---|---|
| Congestion | Absence | 0 | NO |
| 5–10% congestion | 1 | Mild | |
| 10–15% congestion | 2 | Moderate | |
| >15% congestion | 3 | Sever | |
| Inflammation | Absence | 0 | NO |
| 5–15% inflammatory cells | 1 | Mild | |
| 15–25% inflammatory cells | 2 | Moderate | |
| >25% inflammatory cells | 3 | Sever | |
| Endothelial loss | Absence | 0 | NO |
| <25% Endothelial loss | 1 | Mild | |
| 25–50% Endothelial loss | 2 | Moderate | |
| >50% Endothelial loss | 3 | Sever | |
| Arterial wall | Absence | 0 | NO |
| <25% thinning | 1 | Mild | |
| 25–50% thinning | 2 | Moderate | |
| >50% thinning | 3 | Sever | |
| Degeneration | Absence | 0 | NO |
| <1.5% degenerated area | 1 | Mild | |
| 1.5–2% degenerated area | 2 | Moderate | |
| >3% degenerated area | 3 | Sever |
| Parameters | NC | 5-FU | NAC | SAR 0.5 | SAR 5 |
|---|---|---|---|---|---|
| Δ Weight | 42.14 ± 14.6 | 42.14 ± 15.7 | 27.14 ± 20 | 18.5 ± 16.7 * | 32.86 ± 17.9 |
| AST | 113.7 ± 10.92 | 155.9 ± 19.1 ### | 136.1 ± 8 * | 133.1 ± 16.2 * | 130.6 ± 9.5 * |
| ALT | 47.23 ± 9.7 | 56.8 ± 3.1 # | 53.3 ± 8.5 | 56.8 ± 9.3 | 49 ± 4.3 ** |
| AST/ALT | 2.44 ± 0.5 | 2.85 ± 0.3 | 2.69 ± 0.47 | 2.37 ± 0.2 * | 2.6 ± 0.2 |
| ALP | 267.8 ± 59 | 341 ± 27 # | 283.8 ± 58 * | 248 ± 69 ** | 272 ± 35 ** |
| LDH | 273 ± 72 | 550 ± 96 ### | 342 ± 29 ** | 429 ± 66 | 465 ± 89 |
| Groups | Congestion | Inflammation | Endothelial Loss | Arterial Wall Thinning | Degeneration | Score | Grade |
|---|---|---|---|---|---|---|---|
| NC | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 00 ± 0.00 | 0 | No Lesion |
| 5-FU (150 mg/kg) | 16.45 ± 4 | 25.14 ± 2.34 | 52.89 ± 1.5 | 52.32 ± 0.48 | 3.20 ± 0.95 | 3 | Severe |
| NAC (100 mg/kg) | 12.18 ± 1.46 * | 20.28 ± 1.72 * | 41.72 ± 2.21 * | 48.79 ± 0.3 | 1.89 ± 0.14 * | 2 | Moderate |
| SAR (0.5 mg/kg) | 12.04 ± 1.17 * | 19.50 ± 1 * | 34.02 ± 1.57 * | 45.70 ± 0.48 | 1.84 ± 0.28 * | 2 | Moderate |
| SAR (5 mg/kg) | 5.18 ± 1 ** | 12.92 ± 1.81 ** | 21.32 ± 2.82 ** | 15.71 ± 0.34 ** | 1.21 ± 0.43 * | 1 | Mild |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, R.H.; Aziz, T.A. Cardioprotective Effects of SAR Through Attenuating Cardiac-Specific Markers, Inflammatory Markers, Oxidative Stress, and Anxiety in Rats Challenged with 5-Fluorouracil. J. Xenobiot. 2025, 15, 130. https://doi.org/10.3390/jox15040130
Rasheed RH, Aziz TA. Cardioprotective Effects of SAR Through Attenuating Cardiac-Specific Markers, Inflammatory Markers, Oxidative Stress, and Anxiety in Rats Challenged with 5-Fluorouracil. Journal of Xenobiotics. 2025; 15(4):130. https://doi.org/10.3390/jox15040130
Chicago/Turabian StyleRasheed, Roza Haroon, and Tavga Ahmed Aziz. 2025. "Cardioprotective Effects of SAR Through Attenuating Cardiac-Specific Markers, Inflammatory Markers, Oxidative Stress, and Anxiety in Rats Challenged with 5-Fluorouracil" Journal of Xenobiotics 15, no. 4: 130. https://doi.org/10.3390/jox15040130
APA StyleRasheed, R. H., & Aziz, T. A. (2025). Cardioprotective Effects of SAR Through Attenuating Cardiac-Specific Markers, Inflammatory Markers, Oxidative Stress, and Anxiety in Rats Challenged with 5-Fluorouracil. Journal of Xenobiotics, 15(4), 130. https://doi.org/10.3390/jox15040130







