Environmental Arsenic Exposure, Biomarkers and Lung Function in Children from Yaqui Communities in Sonora, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Recruitment and Sample Selection
2.2. Interviews
2.3. Environmental Sample Collection
Soil Samples
2.4. Drinking Water Samples
2.5. Biological Sampling
Urine Collection
2.6. Blood Collection
2.7. Determination of Total Arsenic
Dust Samples
2.8. Drinking Water
2.9. Urine
2.10. Determination of Lung Biomarkers Levels in Serum
2.11. Spirometry Tests
2.12. Statistical Analysis
3. Results
3.1. Anthropometric Characteristics
3.2. Environmental Arsenic
3.3. Urinary Arsenic, MMP-9 and CC16, and Spirometric Indices for Lung Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Arsenic, Metals, Fibres, and Dusts; IARC: Lyon, France, 2011; Volume 100C, p. 527. [Google Scholar]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Chau, B.; Witten, M.L.; Cromey, D.; Chen, Y.; Lantz, R.C. Lung developmental is altered after inhalation exposure to various concentrations of calcium arsenate. Toxicol. Appl. Pharmacol. 2021, 432, 115754. [Google Scholar] [CrossRef] [PubMed]
- Witten, M.L.; Chau, B.; Sáez, E.; Boitano, S.; Lantz, R.C. Early life inhalation exposure to mine tailings dust affects lung development. Toxicol. Appl. Pharmacol. 2019, 365, 124–132. [Google Scholar] [CrossRef]
- Beamer, P.; Klimecki, W.; Loh, M.; Van Horne, Y.; Sugeng, A.; Lothrop, N.; Billheimer, D.; Guerra, S.; Lantz, R.C.; Canales, R.A.; et al. Association of children’s urinary CC16 levels with arsenic concentrations in multiple environmental media. Int. J. Environ. Res. Public Health 2016, 13, 521. [Google Scholar] [CrossRef]
- García-Rico, L.; Meza-Figueroa, D.; Gandolfi, J.A.; Del Rivero, C.I.; Martínez-Cinco, M.A.; Meza-Montenegro, M.M. Health Risk Assessment and Urinary Excretion of Children Exposed to Arsenic through Drinking Water and Soils in Sonora, Mexico. Biol. Trace Elem. Res. 2019, 187, 9–21. [Google Scholar] [CrossRef]
- Wang, B.; Lin, C.; Zhang, X.; Xu, D.; Cheng, H.; Wang, Q.; Liu, X.; Ma, J. Effects of geography, age, and gender on Chinese children’s soil ingestion rate. HERA Int. J. 2018, 24, 1983–1989. [Google Scholar] [CrossRef]
- Middleton, D.R.; Watts, M.J.; Beriro, D.J.; Hamilton, E.M.; Leonardi, G.S.; Fletcher, T.; Close, R.M.; Polya, D.A. Arsenic in residential soil and household dust in Cornwall, South West England: Potential human exposure and the influence of historical mining. Environ. Sci. Process Impacts 2017, 19, 517–527. [Google Scholar] [CrossRef]
- Csavina, J.; Field, J.; Taylor, M.P.; Gao, S.; Landázuri, A.; Betterton, E.A.; Sáez, A.E. A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Sci. Total Environ. 2012, 433, 58–73. [Google Scholar] [CrossRef]
- Benhaddya, M.L.; Boukhelkhal, A.; Halis, Y.; Hadjel, M. Human health risks associated with metals from urban soil and road dust in an oilfield area of Southeastern Algeria. Arch. Environ. Contam. Toxicol. 2016, 70, 556–571. [Google Scholar] [CrossRef]
- Nwabueze, I.E.; Jane, A.E.; John, R.D. An Estimation of Daily Intake of Potentially Toxic Elements from Urban Dust of Abakaliki, Nigeria. ChemSearch J. 2018, 9, 38–42. [Google Scholar]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y.; Hua, X. Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environ. Int. 2019, 128, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Loosmore, G.A.; Hunt, J.R. Dust resuspension without saltation. J. Geophys. Res. 2000, 105, 20663–20672. [Google Scholar] [CrossRef] [PubMed]
- Vega-Millán, C.B.; Dévora-Figueroa, A.G.; Burgess, J.L.; Beamer, P.I.; Furlong, M.; Lantz, R.C.; Meza-Figueroa, D.; O’ Rourke, M.K.; García-Rico, L.; Meza-Escalante, E.R.; et al. Inflammation biomarkers associated with arsenic exposure by drinking water and respiratory outcomes in indigenous children from three Yaqui villages in southern Sonora, México. Environ. Sci. Pollut. Res. Int. 2021, 28, 34355–34366. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Chemical Agents and Related Occupations; IARC Monographs on the Identification of Carcinogenic Hazards to Humans; IARC: Lyon, France, 2012; Volume 100 Pt F, pp. 9–562. [Google Scholar]
- Signes-Pastor, A.J.; Díaz-Coto, S.; Martinez-Camblor, P.; Carey, M.; Soler-Blasco, R.; Garcı’a-Villarino, M.; Fernández-Somoano, A.; Julvez, J.; Carrasco, P.; Lertxundi, A.; et al. Arsenic exposure and respiratory outcomes during childhood in the INMA study. PLoS ONE 2022, 17, e0274215. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Martinez-Camblor, P.; Baker, E.; Madan, J.; Guill, M.F.; Karagas, M.R. Prenatal exposure to arsenic and lung function in children from the New Hampshire Birth Cohort Study. Environ. Int. 2021, 155, 106673. [Google Scholar] [CrossRef]
- Ahmed, S.; Akhtar, E.; Roy, A.; von Ehrenstein, O.S.; Vahter, M.; Wagatsuma, Y.; Raqib, P. Arsenic exposure alters lung function and airway inflammation in children: A cohort study in rural Bangladesh. Environ. Int. 2017, 101, 108–116. [Google Scholar] [CrossRef]
- Olivas-Calderón, E.; Recio-Vega, R.; Gandolfi, A.J.; Lantz, R.C.; González-Cortes, T.; Gonzalez-De Alba, C.; Froines, J.R.; Espinosa-Fematt, J.A. Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicol. Appl. Pharmacol. 2015, 287, 161–167. [Google Scholar] [CrossRef]
- Ramsey, K. Arsenic and Respiratory Disease. In Handbook of Arsenic Toxicology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 335–347. ISBN 9780124186880. [Google Scholar] [CrossRef]
- Sanchez, T.R.; Perzanowski, M.; Graziano, J.H. Inorganic arsenic and respiratory health, from early life exposure to sex-specific effects: A systematic review. Environ. Res. 2016, 147, 537–555. [Google Scholar] [CrossRef]
- Henderson, M.W.; Madenspacher, J.H.; Whitehead, G.S.; Thomas, S.Y.; Aloor, J.J.; Gowdy, K.M.; Fessler, M.B. Effects of Orally Ingested Arsenic on Respiratory Epithelial Permeability to Bacteria and Small Molecules in Mice. Environ. Health Perspect. 2017, 125, 097024. [Google Scholar] [CrossRef]
- Lantz, R.C.; Lynch, B.J.; Boitano, S.; Poplin, G.S.; Littau, S.; Tsaprailis, G.; Burgess, J.L. Pulmonary biomarkers based on alterations in protein expression after exposure to arsenic. Environ. Health Perspect. 2007, 115, 586–591. [Google Scholar] [CrossRef]
- Hinton, D.E.; Baumann, P.C.; Gardner, G.R.; Hawkins, W.E.; Hendricks, J.D.; Murchelano, R.A.; Okihiro, M.S. Histopathologic biomarkers. In Biomarkers; CRC Press: Boca Raton, FL, USA, 1992; pp. 155–210. [Google Scholar]
- Zhou, Y.; Mu, G.; Liu, Y.L.; Ma, J.; Wang, B.; Shi, T.; Tan, A.; Yuan, J.; Chen, W. Urinary polycyclic aromatic hydrocarbon metabolites, Club cell secretory protein and lung function. Environ. Int. 2018, 111, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Josyula, A.B.; Poplin, G.S.; Kurzius-Spencer, M.; McClellen, H.E.; Kopplin, M.J.; Stürup, S.; Clark Lantz, R.; Burgess, J.L. Environmental arsenic exposure and sputum metalloproteinase concentrations. Environ. Res. 2006, 102, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Kurzius-Spencer, M.; O’rourke, M.K.; Littau, S.R.; Roberge, J.; Meza-Montenegro, M.M.; Gutiérrez-Millán, I.E.; Harris, R.B. Environmental arsenic exposure and serum matrix metalloproteinase-9. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 163. [Google Scholar] [CrossRef]
- Miyoshi, S.; Katayama, H.; Matsubara, M.; Kato, T.; Hamaguchi, N.; Yamaguchi, O. Prediction of Spirometric Indices Using Forced Oscillometric Indices in Patients with Asthma, COPD, and Interstitial Lung Disease. Int. J. Chron. Obs. Pulmon. Dis. 2020, 15, 1565–1575. [Google Scholar] [CrossRef]
- NMX-AA-132-SCFI-2016; Muestreo de Suelos Para la Identificación y la Cuantificación de Metales y Metaloides, y Manejo de la Muestra. Secretaría De Economía: Mexico City, Mexico, 2017.
- Meza, M.M.; Kopplin, M.J.; Burgess, J.L.; Gandolfi, A.J. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ. Res. 2004, 96, 119–126. [Google Scholar] [CrossRef]
- USEPA. EPA. Method 6200 Field Portable X-Ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soil and Sediment. United States Environmental Protection Agency. 2007. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-6200-field-portable-x-ray-fluorescence-spectrometry-determination (accessed on 6 February 2025).
- American Thoracic Society (ATS). Standardization of Spirometry, 1994 Update. Am. J. Respir. Crit. Care Med. 1995, 152, 1107–1136. [Google Scholar] [CrossRef]
- Hankinson, J.L.; Odencrantz, J.R.; Fedan, K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999, 159, 179–187. [Google Scholar] [CrossRef]
- Fletcher, C.M.; Elmes, P.C.; Fairbairn, A.S.; Wood, C.H. The Significance of Respiratory Symptoms and the Diagnosis of Chronic Bronchitis in a Working Population. Br. Med. J. 1959, 2, 257–266. [Google Scholar] [CrossRef]
- Celli, B.R.; MacNee, W.; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur. Respir. J. 2004, 23, 932–946, Erratum in Eur. Respir. J. 2006, 27, 242. [Google Scholar] [CrossRef]
- NOM-147-SEMARNAT/SSA1–2004; Norma Oficial Mexicana, Que Establece Criterios Para Determinar las Concentraciones de Remediación de Suelos Contaminados por Arsénico, Bario, Berilio, Cadmio, Cromo Hexavalente, Mercurio, Níquel, Plata, Plomo, Selenio, Talio y/o Vanadio. Mexican Ministry of Environment and Natural Resources: Mexico City, Mexico; Ministry of Health: Mexico City, Mexico, 2007.
- Gabarrón, M.; Faz, A.; Acosta, J.A. Use of multivariable and redundancy analysis to assess the behavior of metals and arsenic in urban soil and road dust affected by metallic mining as a base for risk assessment. J. Environ. Manag. 2018, 206, 192–201. [Google Scholar] [CrossRef]
- Alamdar, A.; Ali Musstjab, A.S.E.S.; Ali, S.W.; Sohail, M.; Bhowmik, A.K.; Cincinelli, A.; Subhani, M.; Ghaffar, B.; Ullah, R.; Huang, Q.; et al. Human Arsenic exposure via dust across the different ecological zones of Pakistan. Ecotoxicol. Environ. Saf. 2016, 126, 219–227. [Google Scholar] [CrossRef] [PubMed]
- García-Rico, L.; Meza-Figueroa, D.; Beamer, P.I.; Burgess, J.L.; O’Rourke, M.K.; Lantz, C.R.; Furlong, M.; Martinez-Cinco, M.; Mondaca-Fernandez, I.; Balderas-Cortes, J.J.; et al. Serum matrix metalloproteinase-9 in children exposed to arsenic from playground dust at elementary schools in Hermosillo, Sonora, Mexico. Environ. Geochem. Health 2020, 42, 499–511. [Google Scholar] [CrossRef] [PubMed]
- NOM-127-SSA1-2021; Agua Para Uso y Consumo Humano. Límites Permisibles de la Calidad del Agua. Norma Oficial Mexicana: Mexico City, Mexico, 2022.
- Dauphiné, D.C.; Ferreccio, C.; Guntur, S.; Yuan, Y.; Hammond, K.; Balmes, J.; Smith, A.H.; Steinmaus, C. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: Preliminary findings. Int. Arch. Occup. Environ. Health 2011, 84, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Parvez, F.; Chen, Y.; Yunus, M.; Olopade, C.; Segers, S.; Slavkovich, V.; Argos, M.; Hasan, R.; Ahmed, A.; Islam, T.; et al. Arsenic exposure and impaired lung function. Findings from a large population-based prospective cohort study. Am. J. Respir. Crit. Care Med. 2013, 188, 813–819. [Google Scholar] [CrossRef]
- Das, D.; Bindhani, B.; Mukherjee, B.; Saha, H.; Biswas, P.; Dutta, K.; Prasad, P.; Sinha, D.; Ray, M.R. Chronic low-level arsenic exposure reduces lung function in male population without skin lesions. Int. J. Public. Health 2014, 59, 655–663. [Google Scholar] [CrossRef]
- Steinmaus, C.; Ferreccio, C.; Acevedo, J.; Balmes, J.R.; Liaw, J.; Troncoso, P.; Dauphiné, D.C.; Nardone, A.; Smith, A.H. High risks of lung disease associated with early-life and moderate lifetime arsenic exposure in northern Chile. Toxicol. Appl. Pharmacol. 2016, 313, 10–15. [Google Scholar] [CrossRef]
- Prasad, P.; Sarkar, N.; Sinha, D. Effect of low- and high-level groundwater arsenic on peripheral blood and lung function of exposed rural women. Regul. Toxicol. Pharmacol. 2020, 115, 104684. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Zou, Z.; Zheng, F.; Zhang, A. Human arsenic exposure and lung function impairment in coal-burning areas in Guizhou, China. Ecotoxicol. Environ. Saf. 2020, 190, 110174. [Google Scholar] [CrossRef]
- Parvez, F.; Chen, Y.; Brandt-Rauf, P.W.; Bernard, A.; Dumont, X.; Slavkovich, V.; Argos, M.; D’Armiento, J.; Foronjy, R.; Hasan, M.R.; et al. Nonmalignant respiratory effects of chronic arsenic exposure from drinking water among never-smokers in Bangladesh. Environ. Health Perspect. 2008, 116, 190–195. [Google Scholar] [CrossRef]
- von Ehrenstein, O.S.; Mazumder, D.N.; Yuan, Y.; Samanta, S.; Balmes, J.; Sil, A.; Ghosh, N.; Hira-Smith, M.; Haque, R.; Purushothamam, R.; et al. Decrements in lung function related to arsenic in drinking water in West Bengal, India. Am. J. Epidemiol. 2005, 162, 533–541. [Google Scholar] [CrossRef]
- Nafees, A.A.; Kazi, A.; Fatmi, Z.; Irfan, M.; Ali, A.; Kayama, F. Lung function decrement with arsenic exposure to drinking groundwater along River Indus: A comparative cross-sectional study. Environ. Geochem. Health 2011, 33, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.; Sanchez, T.R.; Grau-Perez, M.; Yeh, F.; Francesconi, K.A.; Goessler, W.; George, C.M.; Heaney, C.; Best, L.G.; Umans, J.G.; et al. Low-moderate arsenic exposure and respiratory in American Indian communities in the Strong Heart Study. Environ. Health 2019, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Huang, X.; Zhang, C.; Liu, C.; Cui, X.; Zhou, Y.; Sun, H.; Qiu, G.; Guo, H.; He, M.; et al. The dose-response association of urinary metals with altered pulmonary function and risks of restrictive and obstructive lung diseases: A population-based study in China. BMJ Open 2015, 21, 5. [Google Scholar] [CrossRef]
- Shih, Y.H.; Argos, M.; Turyk, M.E. Urinary arsenic concentration, airway inflammation, and lung function in the U.S. adult population. Environ. Res. 2019, 175, 308–315. [Google Scholar] [CrossRef]
- De, B.K.; Majumdar, D.; Sen, S.; Guru, S.; Kundu, S. Pulmonary involvement in chronic arsenic poisoning from drinking contaminated ground-water. J. Assoc. Physicians India 2004, 52, 395–400. [Google Scholar] [PubMed]
- Hays, A.M.; Srinivasan, D.; Witten, M.L.; Carter, D.E.; Lantz, R.C. Arsenic and cigarette smoke synergistically increase DNA oxidation in the lung. Toxicol. Pathol. 2006, 34, 396–404. [Google Scholar] [CrossRef]
- Lantz, R.C.; Hays, A.M. Role of oxidative stress in arsenic-induced toxicity. Drug Metab. Rev. 2006, 38, 791–804. [Google Scholar] [CrossRef]
- Lantz, R.C.; Chau, B.; Sarihan, P.; Witten, M.L.; Pivniouk, V.I.; Chen, G.J. In utero and postnatal exposure to arsenic alters pulmonary structure and function. Toxicol. Appl. Pharmacol. 2009, 235, 105–113. [Google Scholar] [CrossRef]
- Broeckaert, F.; Bernard, A. Clara cell secretory protein (CC16): Characteristics and perspectives as lung peripheral biomarkers. Clin. Exp. Allergy 2000, 30, 469–475. [Google Scholar] [CrossRef]
- Okada, Y.; Gonoji, Y.; Naka, K.; Tomita, K.; Nakanishi, I.; Iwata, K.; Yamashita, K.; Hayakawa, T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J. Biol. Chem. 1992, 267, 21712–21719. [Google Scholar] [CrossRef]
- Parks, W.C.; Shapiro, S.D. Matrix metalloproteinases in lung biology. Respir. Res. 2000, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Miyazaki, N.; Oashi, K.; Tanaka, S.; Ohmichi, M.; Abe, S. Sputum matrix metalloproteinase-9: Tissue inhibitor of metalloproteinase-1 ratio in acute asthma. J. Allergy Clin. Immunol. 2000, 105, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.J.; Senior, R.M. Matrix metalloproteinase-9 in lung remodeling. Am. J. Respir. Cell Mol. Biol. 2003, 28, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.E.; Liguori, A.E.; Zong, Y.; Lantz, R.C.; Burgess, J.L.; Boitano, S. Arsenic upregulates MMP-9 and inhibits wound repair in human airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L293–L302. [Google Scholar] [CrossRef]
- Vignola, A.M.; Paganin, F.; Capieu, L.; Scichilone, N.; Bellia, M.; Maakel, L.; Bellia, P.; Godard, P.; Bousquet, J.; Chanez, P. Airway remodelling assessed by sputum and high-resolution computed tomography in asthma and COPD. Eur. Respir. J. 2004, 24, 910–917. [Google Scholar] [CrossRef]
- Koç, M.; Ediger, D.; Budak, F.; Karadağ, M.; Oral, H.B.; Uzaslan, E.; Ege, E.; Gözü, R.O. Matrix Metalloproteinase-9 (MMP-9) Elevated in Serum but not in Bronchial Lavage Fluid in Patients with Lung Cancer. Tumori. J. 2006, 92, 149–154. [Google Scholar] [CrossRef]
- Christopoulou, M.-E.; Papakonstantinou, E.; Stolz, D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 3786. [Google Scholar] [CrossRef]
- Lo, C.Y.; Huang, H.Y.; He, J.R.; Huang, T.T.; Heh, C.C.; Sheng, T.F.; Chung, K.F.; Kuo, H.P.; Wang, C.H. Increased matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio in smokers with airway hyperresponsiveness and accelerated lung function decline. Int. J. Chron. Obstr. Pulmon Dis. 2018, 13, 1135–1144. [Google Scholar] [CrossRef]
- Kurzius-Spencer, M.; Harris, R.B.; Hartz, V.; Roberge, J.; Hsu, C.H.; O’Rourke, M.K.; Burgess, J.L. Relation of dietary inorganic arsenic to serum matrix metalloproteinase-9 (MMP-9) at different threshold concentrations of tap water arsenic. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 445–451. [Google Scholar] [CrossRef]
- Khan, M.A.; Hira-Smith, M.; Ahmed, S.I.; Yunus, M.; Hasan, S.M.T.; Liaw, J.; Balmes, J.; Raqib, R.; Yuan, Y.; Kalman, D.; et al. Prospective cohort study of respiratory effects at ages 14 to 26 following early life exposure to arsenic in drinking water. Environ. Epidemiol. 2020, 4, e089. [Google Scholar] [CrossRef]
- Recio-Vega, R.; Gonzalez-Cortes, T.; Olivas-Calderon, E.; Lantz, R.C.; Gandolfi, A.J.; Gonzalez-De Alba, C. In utero and early childhood exposure to arsenic decreases lung function in children. J. Appl. Toxicol. 2015, 35, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Milton, A.H.; Hasan, Z.; Rahman, A.; Rahman, M. Non-cancer effects of chronic arsenicosis in Bangladesh: Preliminary results. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. 2003, 38, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Milton, A.H.; Rahman, M. Respiratory effects and arsenic contaminated well water in Bangladesh. Int. J. Environ. Health Res. 2002, 12, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S.F.; Li, Z.; Korrick, S.A.; Spiegelman, D.; Enelow, R.; Nadeau, K.; Baker, E.; Karagas, M.R. Infant infections and respiratory symptoms in relation to in utero arsenic exposure in a U.S. cohort. Environ. Health Perspect. 2016, 124, 840–847. [Google Scholar] [CrossRef]
- Mazumder, D.N.; R Haque, N.; Ghosh, B.K.; De, A.; Santra, D. Chakraborti, and AH. Smith. Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India. Int. J. Epidemiol. 2000, 29, 1047–1052. [Google Scholar] [CrossRef]
- Smith, A.H.; Lopiper, P.A.; Bates, M.N.; Steinmaus, C.M. Arsenic epidemiology and drinking water standards. Science 2002, 296, 2145–2146. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Kimmel, C.A.; Correa, A.; Eskenazi, B. Children’s health and the environment: Public health issues and challenges for risk assessment. Environ. Health Perspect. 2004, 112, 257–265. [Google Scholar] [CrossRef]
| Variable Mean, sd | Pótam (n = 69) | Vícam (n = 51) | Cócorit (n = 55) | Total (n = 175) | * p-Value |
|---|---|---|---|---|---|
| Age (years) | 9.28, 1.71 | 10.25, 2.86 | 9.95, 2.30 | 9.77, 2.31 | 0.0556 |
| Gender% [n] | 0.9893 | ||||
| Girls | 53.62 (37) | 54.90 (28) | 54.55 (30) | 54.29 (95) | |
| Boys | 46.38 (32) | 45.10 (23) | 45.45 (25) | 45.71 (80) | |
| BMI (kg/m2) | 18.11, 4.03 | 19.30, 4.52 | 19.26, 4.56 | 18.82, 4.36 | 0.2208 |
| Residence time (Years) | 9.28, 1.71 | 10.25, 2.86 | 9.95, 2.30 | 9.77, 2.31 | 0.0556 |
| Passive smoking % (n) | 0.0002 * | ||||
| No | 78.26 (54) | 90.20 (46) | 56.36 (31) | 74.86 (131) | |
| Yes | 21.74 (15) | 9.80 (5) | * 43.64 (24) | 25.14 (44) | |
| Arsenic in dust (mg/kg) | 13.77, 3.37 | 13.79, 6.03 | * 20.25, 6.74 | 15.81, 3.01 | <0.050 * |
| Variable Median (IQR) | Pótam (n = 69) | Vícam (n = 51) | Cócorit (n = 55) | Total (n = 175) | * p-Value |
|---|---|---|---|---|---|
| Urinary arsenic (µg/L) | 96.70 * (59.49, 153.46) | 26.35 ** (15.60, 55.75) | 33.75 ** (19.45, 45.06) | 44.13 (25.10, 95.54) | 0.0000 *** |
| CC16 (ng/mL) | 29.20 *** (23.21, 40.35) | 26.02 * (20.50, 33.94) | 38.39 ** (33.29, 53.73) | 32.60 (23.78, 40.35) | 0.0000 *** |
| MMP9 (ng/mL) | 535.16 *** (407.26, 722.73) | 437.18 * (331.17, 550.82) | 322.03 ** (130.09, 526.69) | 469.51 (317.37, 605.37) | 0.0013 *** |
| FVC (L) | 2.23 *** (1.98, 2.82) | 2.26 (1.66, 2.89) | 2.16 (1.65, 3.08) | 2.23 (1.84, 2.89) | 0.6553 |
| FEV1 (L) | 1.83 (1.62, 2.36) | 1.96 (1.56, 2.46) | 1.90 (1.49, 2.52) | 1.85 (1.52, 2.43) | 0.8944 |
| FEV1/FVC ratio | 83.03 (77.55, 87.19) | 85.93 * (82.47, 91.16) | 85.78 * (82.27, 90.04) | 84.82 (80.77, 89.35) | 0.0004 *** |
| Variable | Parameter | Person Coefficient | p-Value |
|---|---|---|---|
| * Urinary arsenic (µg/L) | FVC (L) | −0.0031 | 0.9674 |
| %predicted FVC | 0.0186 | 0.8071 | |
| FEV1 (L) | −0.0475 | 0.5322 | |
| %predictedFEV1 | −0.0676 | 0.3741 | |
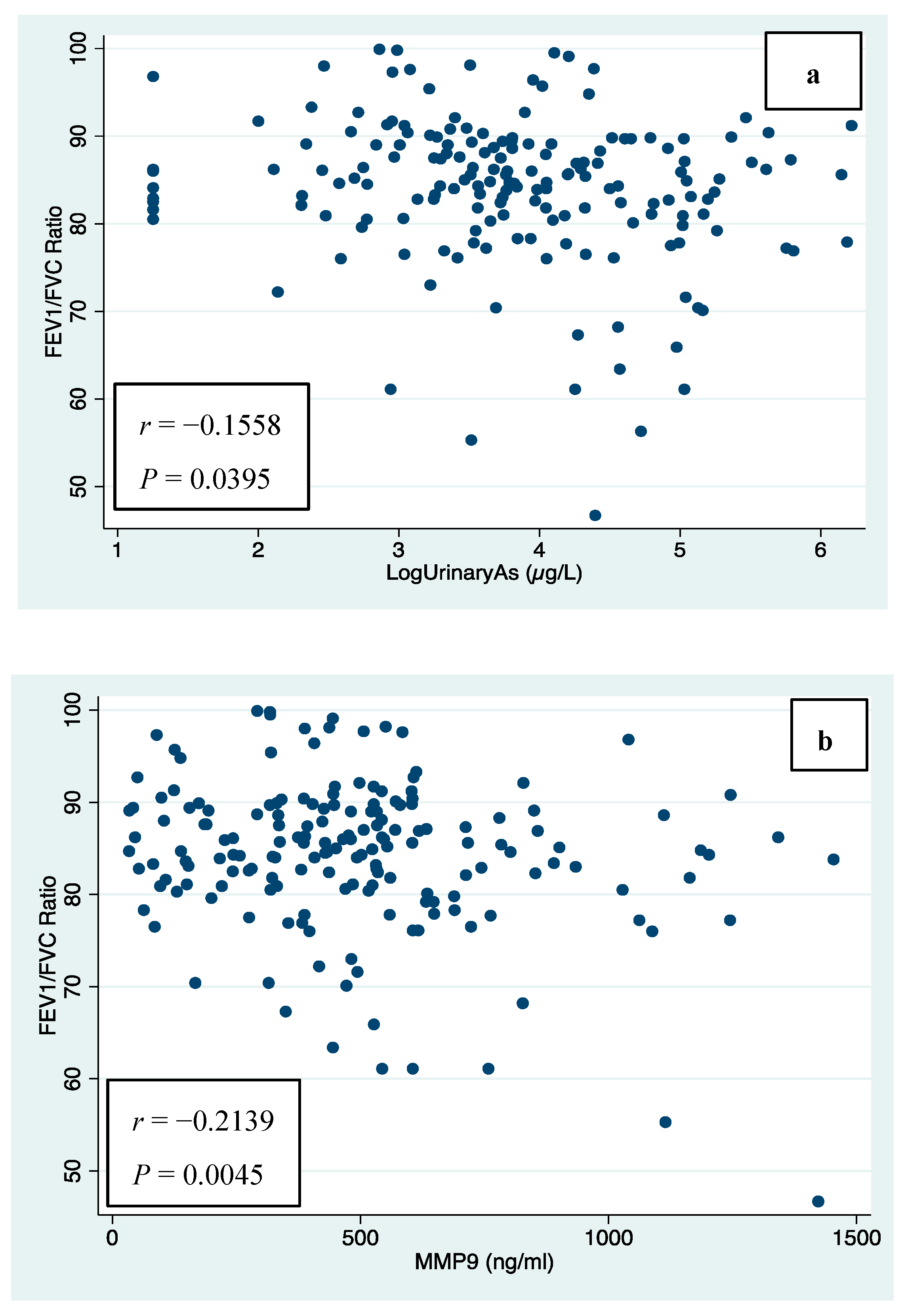
| FEV1/FVC ratio | −0.1558 | 0.0395 | |
| Arsenic levels in water (ug/L) | FVC (L) | 0.0642 | 0.3984 |
| %predicted FVC | 0.2176 | 0.0038 | |
| FEV1 (L) | −0.0353 | 0.6431 | |
| %predicted FEV1 | 0.0311 | 0.6833 | |
| FEV1/FVC ratio | −0.2862 | 0.0001 | |
| Arsenic levels in dust (mg/kg) | FVC (L) | −0.0255 | 0.7372 |
| %predicted FVC | −0.0862 | 0.2565 | |
| FEV1 (L) | 0.0301 | 0.6929 | |
| %predicted FEV1 | 0.0182 | 0.8111 | |
| FEV1/FVC ratio | 0.1492 | 0.0488 | |
| MMP-9 (ng/mL) | FVC (L) | 0.1121 | 0.1395 |
| %predicted FVC | 0.2382 | 0.0015 | |
| FEV1 (L) | 0.0339 | 0.6560 | |
| %predicted FEV1 | 0.0939 | 0.2163 | |
| FEV1/FVC ratio | −0.2139 | 0.0045 | |
| CC16 (ng/mL) | FVC (L) | −0.1170 | 0.1230 |
| %predicted FVC | −0.0535 | 0.4819 | |
| FEV1 (L) | −0.0918 | 0.2268 | |
| %predicted FEV1 | −0.0187 | 0.8059 | |
| FEV1/FVC ratio | 0.0797 | 0.2946 |
| Spirometric Indices Coefficient β (95%CI) p-Value | Arsenic Levels in Drinking Water (µg/L) | Arsenic Levels in Dust (mg/kg) | Urinary Arsenic (µg/L) | MMP-9 (ng/mL) | CC16 (ng/mL) |
|---|---|---|---|---|---|
| FVC (L) | 0.306 (0.126, 0.486) 0.0008 * | −0.040 (−0.086, 0.006) 0.0885 | 0.251 (−0.023, 0.526) 0.0729 | 0.200 (0.001, 0.398) 0.0488 * | −0.033 (−0.136, 0.070) 0.5260 |
| % of predicted FVC | nd | nd | 0.791 (−1.30, 2.88) 0.0010 | 0.012 (0.005, 0.019) 0.0000 | −0.018 (−0.292, 0.056) 0.0006 |
| FEV1(L) | 0.165 (−0.067, 0.397) 0.1632 | −0.012 (−0.070, 0.046) 0.6816 | 0.175 (−0.172, 0.523) 0.3235 | 0.108 (−0.145, 0.360) 0.4030 | −0.001 (−0.131, 0.128) 0.9836 |
| % of predicted FEV1 | nd | nd | −0.723 (−2.70, 1.23) 0.0152 | 0.004 (−0.003, 0.011) 0.0105 | −0.052 (−0.216, 0.112) 0.0161 |
| FEV1/FVC ratio | −0.027 (−0.039, −0.015) 0.0000 * | 0.004 (0.001, 0.007) 0.0076 * | −0.022 (−0.041, −0.003) 0.0222 * | −0.017 (−0.030, −0.003) 0.0167 * | 0.004 (−0.003, 0.011) 0.3120 |
| Variables | Predicted Values | Percentages (n) | * U-arsenic (µg/L) Median, (IQR) ** p-Value | MMP-9 (ng/mL) Median, (IQR) ** p-Value | CC16 (ng/mL) Median, (IQR) ** p-Value |
|---|---|---|---|---|---|
| FEV1 | ≥80 | 60.6% (106) | 43.3 (21.8, 95.5) | 446.0 (256.7, 584.5) | 34.6 (24.9, 44.9) |
| <80 | 39.4% (69) | 45.6 (25.8, 91.5) 0.3816 | 506.4 (334.2, 648.3) 0.1001 | 29.0 (21.3, 37.4) 0.0148 | |
| FVC | ≥80 | 73.1% (128) | 42.0 (20.23, 77.74) | 481.2 (303.3, 633.4) | 32.2 (23.7, 40) |
| <80 | 26.9% (47) | 43.6 (25.5, 91.3) 0.9338 | 428.6 (317.5, 579.7) 0.2658 | 33.3 (24.4, 45.9) 0.4856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dévora-Figueroa, A.G.; Estrada-Vargas, A.; Burgess, J.L.; Beamer, P.I.; Guillen-Rodríguez, J.M.; García-Rico, L.; Villa-Guillen, D.E.; Mondaca-Fernández, I.; Meza-Montenegro, M.M. Environmental Arsenic Exposure, Biomarkers and Lung Function in Children from Yaqui Communities in Sonora, Mexico. J. Xenobiot. 2025, 15, 115. https://doi.org/10.3390/jox15040115
Dévora-Figueroa AG, Estrada-Vargas A, Burgess JL, Beamer PI, Guillen-Rodríguez JM, García-Rico L, Villa-Guillen DE, Mondaca-Fernández I, Meza-Montenegro MM. Environmental Arsenic Exposure, Biomarkers and Lung Function in Children from Yaqui Communities in Sonora, Mexico. Journal of Xenobiotics. 2025; 15(4):115. https://doi.org/10.3390/jox15040115
Chicago/Turabian StyleDévora-Figueroa, Ana G., Anaid Estrada-Vargas, Jefferey L. Burgess, Paloma I. Beamer, José M. Guillen-Rodríguez, Leticia García-Rico, Diana Evelyn Villa-Guillen, Iram Mondaca-Fernández, and Maria M. Meza-Montenegro. 2025. "Environmental Arsenic Exposure, Biomarkers and Lung Function in Children from Yaqui Communities in Sonora, Mexico" Journal of Xenobiotics 15, no. 4: 115. https://doi.org/10.3390/jox15040115
APA StyleDévora-Figueroa, A. G., Estrada-Vargas, A., Burgess, J. L., Beamer, P. I., Guillen-Rodríguez, J. M., García-Rico, L., Villa-Guillen, D. E., Mondaca-Fernández, I., & Meza-Montenegro, M. M. (2025). Environmental Arsenic Exposure, Biomarkers and Lung Function in Children from Yaqui Communities in Sonora, Mexico. Journal of Xenobiotics, 15(4), 115. https://doi.org/10.3390/jox15040115







