Effects of Docosahexaenoic Acid on Prostate Cancer
Abstract
1. Introduction
2. Overview of Molecular Aspects, Metabolism, and Sources
3. DHA Levels in Normal and PCa Cells: Evidence for a Tumor Suppressor?
4. Epidemiologic Findings on DHA Association with PCa Risk
5. Experimental Evidence on DHA’s Effect Against PCa
5.1. Androgen Signaling
5.2. Nuclear Receptors
5.3. Metabolism
5.4. Modulation of Cell Death
5.5. Modulation of Prostate Tumor Microenvironment
5.6. Combination of DHA with Other Therapies
| GENE | DHA EFFECT | MODEL (S) | FUNCTION | REFERENCE |
|---|---|---|---|---|
| AKT1 | ↓ | LNCaP, DU145 | 1, 2, 4 | [178] |
| AR | ↓ | DU145 | 1–6 | [86] |
| ATF3 | ↑ | PC3 | 2 | [22] |
| BAX | ↑ | DU145 | 2 | [157] |
| CASP1 | ↑ | DU145 | 2 | [157] |
| CASP3 | ↑ | DU145 | 2 | [157] |
| CASP9 | ↑ | DU145 | 2 | [157] |
| CCNA2 | ↓ | PC3 | 1 | [22] |
| CCND2 | ↑ | PC3 | 1 | [22] |
| CIDEA | ↑ | DU145 | 2 | [157] |
| DRG-1 | ↓ | LNCaP | 6 | [86] |
| ERRFI1 | ↑ | PNT1A | 1, 4, 5 | [9] |
| FADD | ↓ | LNCaP, DU145 | 2 | [178] |
| FASN | ↓ | PC3 | 3 | [22] |
| FKBP51 | ↓ | LNCaP cells | 1, 3, 5 | [86] |
| FOS | ↓ | PNT1A, 22Rv1 | 1 | [9] |
| HDAC5 | ↑ | 22Rv1 | 1, 3 | [9] |
| IL6 | ↑ | PC3 | 3 | [22] |
| IL10 | ↑ | PC3 | 3 | [22] |
| KLK3 | ↓ | LNCaP | 7 | [86] |
| LTA | ↑ | DU145 | 2 | [157] |
| ODC | ↓ | LNCaP cells | 2, 5 | [86] |
| PPARG | ↑ | PNT1A | 1, 4 | [9] |
| PPARGC1A | ↓ | PNT1A, PC3 | 1, 4 | [9] |
| PPARGC1B | ↑ | 22Rv1 | 1, 4 | [9] |
| MAX | ↓ | LNCaP, DU145 | 1, 2 | [178] |
| MAP2K4 | ↓ | LNCaP, DU145 | 1, 2, 6 | [178] |
| NKX3.1 | ↓ | LNCaP | 1, 6 | [86] |
| NR0B1 | ↑↓ | PNT1A, 22Rv1, PC3 | 4, 5, 7 | [9] |
| PTGS2 | ↑ | PC3 | 3 | [22] |
| RIPK1 | ↓ | LNCaP, DU145 | 2 | [178] |
| RORA | ↑ | 22Rv1 | 4, 5 | [9] |
| TNFRSF11A | ↓↑ | LNCaP, DU145 | 2, 3, 6 | [178] |
| TMPRSS2 | ↓ | LNCaP | 1 | [86] |
| TP53 | ↑ | DU145 | 2, 6 | [157] |
| TRAF3 | ↓ | LNCaP, DU145 | 2, 3 | [178] |
| WAF/CIP1 | ↑ | PC3 | 1 | [22] |
6. Conclusions, Limitations, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- Ali, F.F.; Rifaai, R.A. Preventive effect of omega-3 fatty acids in a rat model of stress-induced liver injury. J. Cell. Physiol. 2019, 234, 11960–11968. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; Nabavi, S.M. Omega-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev. 2015, 34, 359–380. [Google Scholar] [CrossRef]
- Black, H.S.; Rhodes, L.E. Potential Benefits of Omega-3 Fatty Acids in Non-Melanoma Skin Cancer. J. Clin. Med. 2016, 5, 23. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Redegeld, F.; Asghari, M.H.; Mosaffa, N.; Mortaz, E. Long-chain polyunsaturated omega-3 fatty acids reduce multiple myeloma exosome-mediated suppression of NK cell cytotoxicity. DARU J. Pharm. Sci. 2020, 28, 647–659. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Nimiya, Y.; Lee, K.S.S.; Sanidad, K.; Qi, W.; Sukamtoh, E.; Park, Y.; Liu, Z.; Zhang, G. ω-3 Polyunsaturated fatty acids and their cytochrome P450-derived metabolites suppress colorectal tumor development in mice. J. Nutr. Biochem. 2017, 48, 29–35. [Google Scholar] [CrossRef]
- Tamarindo, G.H.; Góes, R.M. Docosahexaenoic acid differentially modulates the cell cycle and metabolism- related genes in tumor and pre-malignant prostate cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158766. [Google Scholar] [CrossRef]
- Calder, P.C. Docosahexaenoic Acid. Ann. Nutr. Metab. 2016, 69 (Suppl. 1), 7–21. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef]
- Giltay, E.J.; Gooren, L.J.G.; Toorians, A.W.F.T.; Katan, M.B.; Zock, P.L. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 2004, 80, 1167–1174. [Google Scholar] [CrossRef]
- Bilodeau, J.-F.; Gevariya, N.; Larose, J.; Robitaille, K.; Roy, J.; Oger, C.; Galano, J.-M.; Bergeron, A.; Durand, T.; Fradet, Y.; et al. Long chain omega-3 fatty acids and their oxidized metabolites are associated with reduced prostate tumor growth. Prostaglandins Leukot. Essent. Fat. Acids 2021, 164, 102215. [Google Scholar] [CrossRef]
- AbuMweis, S.; Abu Omran, D.; Al-Shami, I.; Jew, S. The ratio of eicosapentaenoic acid to docosahexaenoic acid as a modulator for the cardio-metabolic effects of omega-3 supplements: A meta-regression of randomized clinical trials. Complement. Ther. Med. 2021, 57, 102662. [Google Scholar] [CrossRef]
- Shang, T.; Liu, L.; Zhou, J.; Zhang, M.; Hu, Q.; Fang, M.; Wu, Y.; Yao, P.; Gong, Z. Protective effects of various ratios of DHA/EPA supplementation on high-fat diet-induced liver damage in mice. Lipids Health Dis. 2017, 16, 65. [Google Scholar] [CrossRef]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef]
- Bieri, J.G.; Prival, E.L. Lipid composition of testes from various species. Comp. Biochem. Physiol. 1965, 15, 275–282. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Wei, L.; Zhang, H.; Zhang, J.; Zhou, X.; Zhu, S.; Du, Y.; Su, R.; Fang, C.; et al. DHA supplementation and pregnancy complications. J. Transl. Med. 2023, 21, 394. [Google Scholar] [CrossRef]
- Roqueta-Rivera, M.; Stroud, C.K.; Haschek, W.M.; Akare, S.J.; Segre, M.; Brush, R.S.; Agbaga, M.-P.; Anderson, R.E.; Hess, R.A.; Nakamura, M.T. Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male delta-6 desaturase-null mice. J. Lipid Res. 2010, 51, 360–367. [Google Scholar] [CrossRef]
- Roqueta-Rivera, M.; Abbott, T.L.; Sivaguru, M.; Hess, R.A.; Nakamura, M.T. Deficiency in the omega-3 fatty acid pathway results in failure of acrosome biogenesis in mice. Biol. Reprod. 2011, 85, 721–732. [Google Scholar] [CrossRef]
- Eser, P.O.; Vanden Heuvel, J.P.; Araujo, J.; Thompson, J.T. Marine- and plant-derived ω-3 fatty acids differentially regulate prostate cancer cell proliferation. Mol. Clin. Oncol. 2013, 1, 444–452. [Google Scholar] [CrossRef]
- Shin, S.; Jing, K.; Jeong, S.; Kim, N.; Song, K.-S.; Heo, J.-Y.; Park, J.-H.; Seo, K.-S.; Han, J.; Park, J.-I.; et al. The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. BioMed Res. Int. 2013, 2013, 568671. [Google Scholar] [CrossRef]
- Tamarindo, G.H.; Ribeiro, D.L.; Gobbo, M.G.; Guerra, L.H.A.; Rahal, P.; Taboga, S.R.; Gadelha, F.R.; Góes, R.M. Melatonin and docosahexaenoic acid decrease proliferation of PNT1A prostate benign cells via modulation of mitochondrial bioenergetics and ROS production. Oxid. Med. Cell. Longev. 2019, 2019, 5080798. [Google Scholar] [CrossRef]
- Tamarindo, G.H.; Ribeiro, C.F.; Silva, A.D.T.; Castro, A.; Caruso, Í.P.; Souza, F.P.; Taboga, S.R.; Loda, M.; Góes, R.M. The polyunsaturated fatty acid docosahexaenoic affects mitochondrial function in prostate cancer cells. Cancer Metab. 2024, 12, 24. [Google Scholar] [CrossRef]
- Meng, H.; Shen, Y.; Shen, J.; Zhou, F.; Shen, S.; Das, U.N. Effect of n-3 and n-6 unsaturated fatty acids on prostate cancer (PC-3) and prostate epithelial (RWPE-1) cells in vitro. Lipids Health Dis. 2013, 12, 160. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef]
- Plourde, M.; Cunnane, S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef]
- Zhang, T.; Naudin, S.; Hong, H.G.; Albanes, D.; Männistö, S.; Weinstein, S.J.; Moore, S.C.; Stolzenberg-Solomon, R.Z. Dietary Quality and Circulating Lipidomic Profiles in 2 Cohorts of Middle-Aged and Older Male Finnish Smokers and American Populations. J. Nutr. 2023, 153, 2389–2400. [Google Scholar] [CrossRef]
- Loukil, I.; Mutch, D.M.; Plourde, M. Genetic association between FADS and ELOVL polymorphisms and the circulating levels of EPA/DHA in humans: A scoping review. Genes Nutr. 2024, 19, 11. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Moniri, N.H. Free-fatty acid receptor-4 (GPR120): Cellular and molecular function and its role in metabolic disorders. Biochem. Pharmacol. 2016, 110–111, 1–15. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Oh, D.Y.; Walenta, E. Omega-3 Fatty Acids and FFAR4. Front. Endocrinol. 2014, 5, 115. [Google Scholar] [CrossRef]
- Wang, J.; Hong, Y.; Shao, S.; Zhang, K.; Hong, W. FFAR1-and FFAR4-dependent activation of Hippo pathway mediates DHA-induced apoptosis of androgen-independent prostate cancer cells. Biochem. Biophys. Res. Commun. 2018, 506, 590–596. [Google Scholar] [CrossRef]
- Watt, M.J.; Clark, A.K.; Selth, L.A.; Haynes, V.R.; Lister, N.; Rebello, R.; Porter, L.H.; Niranjan, B.; Whitby, S.T.; Lo, J.; et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci. Transl. Med. 2019, 11, eaau5758. [Google Scholar] [CrossRef]
- Wu, J.; Luo, J.; He, Q.; Zhang, F.; Shi, C.; Zhao, J.; Li, C.; Deng, W. CD36 molecule and AMP-activated protein kinase signaling drive docosahexaenoic acid-induced lipid remodeling in goat mammary epithelial cells. Int. J. Biol. Macromol. 2025, 311, 144076. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef]
- Miao, Z.; Chen, G.-D.; Huo, S.; Fu, Y.; Wu, M.-Y.; Xu, F.; Jiang, Z.; Tang, J.; Gou, W.; Xiao, C.; et al. Interaction of n-3 polyunsaturated fatty acids with host CD36 genetic variant for gut microbiome and blood lipids in human cohorts. Clin. Nutr. 2022, 41, 1724–1734. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.-Y.; Sokolowska, M.; Eberlein, M.; Alsaaty, S.; Martinez-Anton, A.; Logun, C.; Qi, H.-Y.; Shelhamer, J.H. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A2 via GPR120 receptor to produce prostaglandin E2 and plays an anti-inflammatory role in macrophages. Immunology 2014, 143, 81–95. [Google Scholar] [CrossRef]
- Sánchez-Reyes, O.B.; Romero-Ávila, M.T.; Castillo-Badillo, J.A.; Takei, Y.; Hirasawa, A.; Tsujimoto, G.; Villalobos-Molina, R.; García-Sáinz, J.A. Free fatty acids and protein kinase C activation induce GPR120 (free fatty acid receptor 4) phosphorylation. Eur. J. Pharmacol. 2014, 723, 368–374. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as nuclear receptors for nutrient and energy metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef]
- Cheng, S.; Qian, K.; Wang, Y.; Wang, G.; Liu, X.; Xiao, Y.; Wang, X. PPARγ inhibition regulates the cell cycle, proliferation and motility of bladder cancer cells. J. Cell. Mol. Med. 2019, 23, 3724–3736. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.-D. The role of ppars in disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef]
- Suzuki, H.; Manabe, S.; Wada, O.; Crawford, M.A. Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. Int. J. Vitam. Nutr. Res. 1997, 67, 272–278. [Google Scholar]
- Tamarindo, G.H.; Ribeiro, C.F.; Rodrigues, S.; Góes, R.M.; Loda, M. DHA suppresses hormone-sensitive and castration-resistant prostate cancer growth by decreasing de novo lipogenesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2025, 1870, 159634. [Google Scholar] [CrossRef]
- Kuda, O. Bioactive metabolites of docosahexaenoic acid. Biochimie 2017, 136, 12–20. [Google Scholar] [CrossRef]
- Poorani, R.; Bhatt, A.N.; Dwarakanath, B.S.; Das, U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2016, 785, 116–132. [Google Scholar] [CrossRef]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef]
- Wendell, S.G.; Golin-Bisello, F.; Wenzel, S.; Sobol, R.W.; Holguin, F.; Freeman, B.A. 15-Hydroxyprostaglandin dehydrogenase generation of electrophilic lipid signaling mediators from hydroxy ω-3 fatty acids. J. Biol. Chem. 2015, 290, 5868–5880. [Google Scholar] [CrossRef]
- Morisseau, C.; Inceoglu, B.; Schmelzer, K.; Tsai, H.-J.; Jinks, S.L.; Hegedus, C.M.; Hammock, B.D. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J. Lipid Res. 2010, 51, 3481–3490. [Google Scholar] [CrossRef]
- Gu, Z.; Wu, J.; Wang, S.; Suburu, J.; Chen, H.; Thomas, M.J.; Shi, L.; Edwards, I.J.; Berquin, I.M.; Chen, Y.Q. Polyunsaturated fatty acids affect the localization and signaling of PIP3/AKT in prostate cancer cells. Carcinogenesis 2013, 34, 1968–1975. [Google Scholar] [CrossRef]
- Zadra, G.; Ribeiro, C.F.; Chetta, P.; Ho, Y.; Cacciatore, S.; Gao, X.; Syamala, S.; Bango, C.; Photopoulos, C.; Huang, Y.; et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 631–640. [Google Scholar] [CrossRef]
- De Piano, M.; Manuelli, V.; Zadra, G.; Otte, J.; Edqvist, P.-H.D.; Pontén, F.; Nowinski, S.; Niaouris, A.; Grigoriadis, A.; Loda, M.; et al. Lipogenic signalling modulates prostate cancer cell adhesion and migration via modification of Rho GTPases. Oncogene 2020, 39, 3666–3679. [Google Scholar] [CrossRef]
- Zadra, G.; Photopoulos, C.; Loda, M. The fat side of prostate cancer. Biochim. Biophys. Acta 2013, 1831, 1518–1532. [Google Scholar] [CrossRef]
- Labbé, D.P.; Zadra, G.; Yang, M.; Reyes, J.M.; Lin, C.Y.; Cacciatore, S.; Ebot, E.M.; Creech, A.L.; Giunchi, F.; Fiorentino, M.; et al. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat. Commun. 2019, 10, 4358. [Google Scholar] [CrossRef]
- Tousignant, K.D.; Rockstroh, A.; Poad, B.L.J.; Talebi, A.; Young, R.S.E.; Taherian Fard, A.; Gupta, R.; Zang, T.; Wang, C.; Lehman, M.L.; et al. Therapy-induced lipid uptake and remodeling underpin ferroptosis hypersensitivity in prostate cancer. Cancer Metab. 2020, 8, 11. [Google Scholar] [CrossRef]
- Kotrikadze, N.; Alibegashvili, M.; Zibzibadze, M.; Abashidze, N.; Chigogidze, T.; Managadze, L.; Artsivadze, K. Activity and content of antioxidant enzymes in prostate tumors. Exp. Oncol. 2008, 30, 244–247. [Google Scholar]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef]
- Cavazos, D.A.; Price, R.S.; Apte, S.S.; deGraffenried, L.A. Docosahexaenoic acid selectively induces human prostate cancer cell sensitivity to oxidative stress through modulation of NF-κB. Prostate 2011, 71, 1420–1428. [Google Scholar] [CrossRef]
- Lin, S.-C.; Tsai, Y.-C.; Chen, Y.-L.; Lin, H.-K.; Huang, Y.-C.; Lin, Y.-S.; Cheng, Y.-S.; Chen, H.-Y.; Li, C.-J.; Lin, T.-Y.; et al. Un-methylation of NUDT21 represses docosahexaenoic acid biosynthesis contributing to enzalutamide resistance in prostate cancer. Drug Resist. Updates 2024, 77, 101144. [Google Scholar] [CrossRef]
- Zhao, Z.; Reinstatler, L.; Klaassen, Z.; Xu, Y.; Yang, X.; Madi, R.; Terris, M.K.; Qian, S.Y.; Kelavkar, U.; Moses, K.A. The Association of Fatty Acid Levels and Gleason Grade among Men Undergoing Radical Prostatectomy. PLoS ONE 2016, 11, e0166594. [Google Scholar] [CrossRef]
- Brasky, T.M.; Darke, A.K.; Song, X.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 2013, 105, 1132–1141. [Google Scholar] [CrossRef]
- Brasky, T.M.; Till, C.; White, E.; Neuhouser, M.L.; Song, X.; Goodman, P.; Thompson, I.M.; King, I.B.; Albanes, D.; Kristal, A.R. Serum phospholipid fatty acids and prostate cancer risk: Results from the prostate cancer prevention trial. Am. J. Epidemiol. 2011, 173, 1429–1439. [Google Scholar] [CrossRef]
- Berg, T.; Johansen, L.; Brekke, I.B. Insulin potentiates cholecystokinin (CCK)-induced secretion of pancreatic kallikrein. Acta Physiol. Scand. 1985, 123, 89–95. [Google Scholar] [CrossRef]
- Ritch, C.R.; Wan, R.L.; Stephens, L.B.; Taxy, J.B.; Huo, D.; Gong, E.M.; Zagaja, G.P.; Brendler, C.B. Dietary fatty acids correlate with prostate cancer biopsy grade and volume in Jamaican men. J. Urol. 2007, 177, 97–101; discussion 101. [Google Scholar] [CrossRef]
- DeFina, L.F.; Bassett, M.H.; Finley, C.E.; Barlow, C.E.; Willis, B.L.; Cooper, T.; Clark, S.M.; Harris, W.S.; Radford, N.B. Association between omega-3 fatty acids and serum prostate-specific antigen. Nutr. Cancer 2016, 68, 58–62. [Google Scholar] [CrossRef]
- Sorongon-Legaspi, M.K.; Chua, M.; Sio, M.C.; Morales, M. Blood level omega-3 Fatty acids as risk determinant molecular biomarker for prostate cancer. Prostate Cancer 2013, 2013, 875615. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Stampfer, M.J.; Michaud, D.S.; Augustsson, K.; Colditz, G.C.; Willett, W.C.; Giovannucci, E.L. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am. J. Clin. Nutr. 2004, 80, 204–216. [Google Scholar] [CrossRef]
- Norrish, A.E.; Skeaff, C.M.; Arribas, G.L.; Sharpe, S.J.; Jackson, R.T. Prostate cancer risk and consumption of fish oils: A dietary biomarker-based case-control study. Br. J. Cancer 1999, 81, 1238–1242. [Google Scholar] [CrossRef]
- Fu, Y.-Q.; Zheng, J.-S.; Yang, B.; Li, D. Effect of individual omega-3 fatty acids on the risk of prostate cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Epidemiol. 2015, 25, 261–274. [Google Scholar] [CrossRef]
- Brouwer, I.A. Omega-3 PUFA: Good or bad for prostate cancer? Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 97–99. [Google Scholar] [CrossRef]
- Chua, M.E.; Sio, M.C.D.; Sorongon, M.C.; Dy, J.S. Relationship of dietary intake of omega-3 and omega-6 Fatty acids with risk of prostate cancer development: A meta-analysis of prospective studies and review of literature. Prostate Cancer 2012, 2012, 826254. [Google Scholar] [CrossRef]
- Dinwiddie, M.T.; Terry, P.D.; Whelan, J.; Patzer, R.E. Omega-3 Fatty Acid Consumption and Prostate Cancer: A Review of Exposure Measures and Results of Epidemiological Studies. J. Am. Coll. Nutr. 2016, 35, 452–468. [Google Scholar] [CrossRef]
- Massie, C.E.; Lynch, A.; Ramos-Montoya, A.; Boren, J.; Stark, R.; Fazli, L.; Warren, A.; Scott, H.; Madhu, B.; Sharma, N.; et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011, 30, 2719–2733. [Google Scholar] [CrossRef]
- Pelekanou, V.; Castanas, E. Androgen control in prostate cancer. J. Cell. Biochem. 2016, 117, 2224–2234. [Google Scholar] [CrossRef]
- Shafi, A.A.; Yen, A.E.; Weigel, N.L. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol. Ther. 2013, 140, 223–238. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, W.; Zhang, J.; Li, H. Androgen receptor: What we know and what we expect in castration-resistant prostate cancer. Int. Urol. Nephrol. 2018, 50, 1753–1764. [Google Scholar] [CrossRef]
- Mitsuhashi, N.; Mizuno, M.; Miyagawa, A.; Kato, J. Inhibitory effect of fatty acids on the binding of androgen receptor and R1881. Endocrinol. Jpn. 1988, 35, 93–96. [Google Scholar] [CrossRef]
- Hu, Z.; Qi, H.; Zhang, R.; Zhang, K.; Shi, Z.; Chang, Y.; Chen, L.; Esmaeili, M.; Baniahmad, A.; Hong, W. Docosahexaenoic acid inhibits the growth of hormone-dependent prostate cancer cells by promoting the degradation of the androgen receptor. Mol. Med. Rep. 2015, 12, 3769–3774. [Google Scholar] [CrossRef]
- Chung, B.H.; Mitchell, S.H.; Zhang, J.S.; Young, C.Y. Effects of docosahexaenoic acid and eicosapentaenoic acid on androgen-mediated cell growth and gene expression in LNCaP prostate cancer cells. Carcinogenesis 2001, 22, 1201–1206. [Google Scholar] [CrossRef]
- Friedrichs, W.; Ruparel, S.B.; Marciniak, R.A.; deGraffenried, L. Omega-3 fatty acid inhibition of prostate cancer progression to hormone independence is associated with suppression of mTOR signaling and androgen receptor expression. Nutr. Cancer 2011, 63, 771–777. [Google Scholar] [CrossRef]
- Wang, C.; Luo, F.; Zhou, Y.; Du, X.; Shi, J.; Zhao, X.; Xu, Y.; Zhu, Y.; Hong, W.; Zhang, J. The therapeutic effects of docosahexaenoic acid on oestrogen/androgen-induced benign prostatic hyperplasia in rats. Exp. Cell Res. 2016, 345, 125–133. [Google Scholar] [CrossRef]
- Degeorges, A.; Hoffschir, F.; Cussenot, O.; Gauville, C.; Le Duc, A.; Dutrillaux, B.; Calvo, F. Recurrent cytogenetic alterations of prostate carcinoma and amplification of c-myc or epidermal growth factor receptor in subclones of immortalized PNT1 human prostate epithelial cell line. Int. J. Cancer 1995, 62, 724–731. [Google Scholar] [CrossRef]
- Akinsete, J.A.; Ion, G.; Witte, T.R.; Hardman, W.E. Consumption of high ω-3 fatty acid diet suppressed prostate tumorigenesis in C3(1) Tag mice. Carcinogenesis 2012, 33, 140–148. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Suburu, J.; Gu, Z.; Cai, J.; Axanova, L.S.; Cramer, S.D.; Thomas, M.J.; Perry, D.L.; Edwards, I.J.; et al. Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer. Carcinogenesis 2012, 33, 404–412. [Google Scholar] [CrossRef]
- Amaro, G.M.; da Silva, A.D.T.; Tamarindo, G.H.; Lamas, C.d.A.; Taboga, S.R.; Cagnon, V.H.A.; Góes, R.M. Differential effects of omega-3 PUFAS on tumor progression at early and advanced stages in TRAMP mice. Prostate 2022, 82, 1491–1504. [Google Scholar] [CrossRef]
- Stoykova, G.E.; Schlaepfer, I.R. Lipid metabolism and endocrine resistance in prostate cancer, and new opportunities for therapy. Int. J. Mol. Sci. 2019, 20, 2626. [Google Scholar] [CrossRef]
- Jacob, A.; Raj, R.; Allison, D.B.; Myint, Z.W. Androgen receptor signaling in prostate cancer and therapeutic strategies. Cancers 2021, 13, 5417. [Google Scholar] [CrossRef]
- Davies, A.; Conteduca, V.; Zoubeidi, A.; Beltran, H. Biological Evolution of Castration-resistant Prostate Cancer. Eur. Urol. Focus 2019, 5, 147–154. [Google Scholar] [CrossRef]
- Daniels, V.A.; Luo, J.; Paller, C.J.; Kanayama, M. Therapeutic approaches to targeting androgen receptor splice variants. Cells 2024, 13, 104. [Google Scholar] [CrossRef]
- Rusca, A.; Di Stefano, A.F.D.; Doig, M.V.; Scarsi, C.; Perucca, E. Relative bioavailability and pharmacokinetics of two oral formulations of docosahexaenoic acid/eicosapentaenoic acid after multiple-dose administration in healthy volunteers. Eur. J. Clin. Pharmacol. 2009, 65, 503–510. [Google Scholar] [CrossRef]
- Jiang, M.; Fernandez, S.; Jerome, W.G.; He, Y.; Yu, X.; Cai, H.; Boone, B.; Yi, Y.; Magnuson, M.A.; Roy-Burman, P.; et al. Disruption of PPARgamma signaling results in mouse prostatic intraepithelial neoplasia involving active autophagy. Cell Death Differ. 2010, 17, 469–481. [Google Scholar] [CrossRef]
- Hartley, A.; Ahmad, I. The role of PPARγ in prostate cancer development and progression. Br. J. Cancer 2023, 128, 940–945. [Google Scholar] [CrossRef]
- Elix, C.; Pal, S.K.; Jones, J.O. The role of peroxisome proliferator-activated receptor gamma in prostate cancer. Asian J. Androl. 2018, 20, 238–243. [Google Scholar]
- Tew, B.Y.; Hong, T.B.; Otto-Duessel, M.; Elix, C.; Castro, E.; He, M.; Wu, X.; Pal, S.K.; Kalkum, M.; Jones, J.O. Vitamin K epoxide reductase regulation of androgen receptor activity. Oncotarget 2017, 8, 13818–13831. [Google Scholar] [CrossRef]
- Strand, D.W.; Jiang, M.; Murphy, T.A.; Yi, Y.; Konvinse, K.C.; Franco, O.E.; Wang, Y.; Young, J.D.; Hayward, S.W. PPARγ isoforms differentially regulate metabolic networks to mediate mouse prostatic epithelial differentiation. Cell Death Dis. 2012, 3, e361. [Google Scholar] [CrossRef]
- Ahmad, I.; Mui, E.; Galbraith, L.; Patel, R.; Tan, E.H.; Salji, M.; Rust, A.G.; Repiscak, P.; Hedley, A.; Markert, E.; et al. Sleeping Beauty screen reveals Pparg activation in metastatic prostate cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 8290–8295. [Google Scholar] [CrossRef]
- Chang, S.-N.; Lee, J.M.; Oh, H.; Kim, U.; Ryu, B.; Park, J.-H. Troglitazone inhibits the migration and invasion of PC-3 human prostate cancer cells by upregulating E-cadherin and glutathione peroxidase 3. Oncol. Lett. 2018, 16, 5482–5488. [Google Scholar] [CrossRef]
- Moss, P.E.; Lyles, B.E.; Stewart, L.V. The PPARγ ligand ciglitazone regulates androgen receptor activation differently in androgen-dependent versus androgen-independent human prostate cancer cells. Exp. Cell Res. 2010, 316, 3478–3488. [Google Scholar] [CrossRef]
- Qin, L.; Gong, C.; Chen, A.-M.; Guo, F.-J.; Xu, F.; Ren, Y.; Liao, H. Peroxisome proliferator-activated receptor γ agonist rosiglitazone inhibits migration and invasion of prostate cancer cells through inhibition of the CXCR4/CXCL12 axis. Mol. Med. Rep. 2014, 10, 695–700. [Google Scholar] [CrossRef]
- Almahmoud, S.; Elix, C.C.; Jones, J.O.; Hopkins, C.R.; Vennerstrom, J.L.; Zhong, H.A. Virtual screening and biological evaluation of PPARγ antagonists as potential anti-prostate cancer agents. Bioorg. Med. Chem. 2021, 46, 116368. [Google Scholar] [CrossRef]
- Bernardo, A.; Giammarco, M.L.; De Nuccio, C.; Ajmone-Cat, M.A.; Visentin, S.; De Simone, R.; Minghetti, L. Docosahexaenoic acid promotes oligodendrocyte differentiation via PPAR-γ signalling and prevents tumor necrosis factor-α-dependent maturational arrest. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1013–1023. [Google Scholar] [CrossRef]
- Geng, L.; Zhou, W.; Liu, B.; Wang, X.; Chen, B. DHA induces apoptosis of human malignant breast cancer tissues by the TLR-4/PPAR-α pathways. Oncol. Lett. 2018, 15, 2967–2977. [Google Scholar] [CrossRef]
- Zand, H.; Rhimipour, A.; Bakhshayesh, M.; Shafiee, M.; Nour Mohammadi, I.; Salimi, S. Involvement of PPAR-gamma and p53 in DHA-induced apoptosis in Reh cells. Mol. Cell. Biochem. 2007, 304, 71–77. [Google Scholar] [CrossRef]
- Samokhvalov, V.; Zlobine, I.; Jamieson, K.L.; Jurasz, P.; Chen, C.; Lee, K.S.S.; Hammock, B.D.; Seubert, J.M. PPARδ signaling mediates the cytotoxicity of DHA in H9c2 cells. Toxicol. Lett. 2015, 232, 10–20. [Google Scholar] [CrossRef]
- Hwang, J.-K.; Yu, H.-N.; Noh, E.-M.; Kim, J.-M.; Hong, O.-Y.; Youn, H.J.; Jung, S.H.; Kwon, K.-B.; Kim, J.-S.; Lee, Y.-R. DHA blocks TPA-induced cell invasion by inhibiting MMP-9 expression via suppression of the PPAR-γ/NF-κB pathway in MCF-7 cells. Oncol. Lett. 2017, 13, 243–249. [Google Scholar] [CrossRef]
- Gionfriddo, G.; Plastina, P.; Augimeri, G.; Catalano, S.; Giordano, C.; Barone, I.; Morelli, C.; Giordano, F.; Gelsomino, L.; Sisci, D.; et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer. Cells 2020, 9, 174. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Astakhova, A.A.; Goriainov, S.V.; Sergeeva, M.G. Comparison of PPAR ligands as modulators of resolution of inflammation, via their influence on cytokines and oxylipins release in astrocytes. Int. J. Mol. Sci. 2020, 21, 9577. [Google Scholar] [CrossRef]
- O’Flaherty, J.T.; Hu, Y.; Wooten, R.E.; Horita, D.A.; Samuel, M.P.; Thomas, M.J.; Sun, H.; Edwards, I.J. 15-lipoxygenase metabolites of docosahexaenoic acid inhibit prostate cancer cell proliferation and survival. PLoS ONE 2012, 7, e45480. [Google Scholar] [CrossRef]
- Puhr, M.; Hoefer, J.; Eigentler, A.; Ploner, C.; Handle, F.; Schaefer, G.; Kroon, J.; Leo, A.; Heidegger, I.; Eder, I.; et al. The glucocorticoid receptor is a key player for prostate cancer cell survival and a target for improved antiandrogen therapy. Clin. Cancer Res. 2018, 24, 927–938. [Google Scholar] [CrossRef]
- Arora, V.K.; Schenkein, E.; Murali, R.; Subudhi, S.K.; Wongvipat, J.; Balbas, M.D.; Shah, N.; Cai, L.; Efstathiou, E.; Logothetis, C.; et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013, 155, 1309–1322. [Google Scholar] [CrossRef]
- Eigentler, A.; Handle, F.; Schanung, S.; Degen, A.; Hackl, H.; Erb, H.H.H.; Fotakis, G.; Hoefer, J.; Ploner, C.; Jöhrer, K.; et al. Glucocorticoid treatment influences prostate cancer cell growth and the tumor microenvironment via altered glucocorticoid receptor signaling in prostate fibroblasts. Oncogene 2024, 43, 235–247. [Google Scholar] [CrossRef]
- Cao, H.; Li, M.-Y.; Li, G.; Li, S.-J.; Wen, B.; Lu, Y.; Yu, X. Retinoid X Receptor α Regulates DHA-Dependent Spinogenesis and Functional Synapse Formation In Vivo. Cell Rep. 2020, 31, 107649. [Google Scholar] [CrossRef]
- Hiebl, V.; Ladurner, A.; Latkolik, S.; Dirsch, V.M. Natural products as modulators of the nuclear receptors and metabolic sensors LXR, FXR and RXR. Biotechnol. Adv. 2018, 36, 1657–1698. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Roskams, T.; Joniau, S.; Van Poppel, H.; Oyen, R.; Baert, L.; Heyns, W.; Verhoeven, G. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int. J. Cancer 2002, 98, 19–22. [Google Scholar] [CrossRef]
- Rossi, S.; Graner, E.; Febbo, P.; Weinstein, L.; Bhattacharya, N.; Onody, T.; Bubley, G.; Balk, S.; Loda, M. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol. Cancer Res. 2003, 1, 707–715. [Google Scholar]
- Scaglia, N.; Tyekucheva, S.; Zadra, G.; Photopoulos, C.; Loda, M. De novo fatty acid synthesis at the mitotic exit is required to complete cellular division. Cell Cycle 2014, 13, 859–868. [Google Scholar] [CrossRef]
- Fiorentino, M.; Zadra, G.; Palescandolo, E.; Fedele, G.; Bailey, D.; Fiore, C.; Nguyen, P.L.; Migita, T.; Zamponi, R.; Di Vizio, D.; et al. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab. Investig. 2008, 88, 1340–1348. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Ulrix, W.; Heyns, W.; Verhoeven, G. Coordinate regulation of lipogenic gene expression by androgens: Evidence for a cascade mechanism involving sterol regulatory element binding proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 12975–12980. [Google Scholar] [CrossRef]
- Huang, W.-C.; Li, X.; Liu, J.; Lin, J.; Chung, L.W.K. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol. Cancer Res. 2012, 10, 133–142. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Rider, L.; Rodrigues, L.U.; Gijón, M.A.; Pac, C.T.; Romero, L.; Cimic, A.; Sirintrapun, S.J.; Glodé, L.M.; Eckel, R.H.; et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol. Cancer Ther. 2014, 13, 2361–2371. [Google Scholar] [CrossRef]
- Liu, Y.; Zuckier, L.S.; Ghesani, N.V. Dominant uptake of fatty acid over glucose by prostate cells: A potential new diagnostic and therapeutic approach. Anticancer. Res. 2010, 30, 369–374. [Google Scholar]
- Tousignant, K.D.; Rockstroh, A.; Taherian Fard, A.; Lehman, M.L.; Wang, C.; McPherson, S.J.; Philp, L.K.; Bartonicek, N.; Dinger, M.E.; Nelson, C.C.; et al. Lipid Uptake Is an Androgen-Enhanced Lipid Supply Pathway Associated with Prostate Cancer Disease Progression and Bone Metastasis. Mol. Cancer Res. 2019, 17, 1166–1179. [Google Scholar] [CrossRef]
- Narita, S.; Nara, T.; Sato, H.; Koizumi, A.; Huang, M.; Inoue, T.; Habuchi, T. Research Evidence on High-Fat Diet-Induced Prostate Cancer Development and Progression. J. Clin. Med. 2019, 8, 597. [Google Scholar] [CrossRef]
- Tamarindo, G.H.; Gobbo, M.G.; Taboga, S.R.; Almeida, E.A.; Góes, R.M. Melatonin ameliorates degenerative alterations caused by age in the rat prostate and mitigates high-fat diet damages. Cell Biol. Int. 2021, 45, 92–106. [Google Scholar] [CrossRef]
- Ribeiro, D.L.; Pinto, M.E.; Maeda, S.Y.; Taboga, S.R.; Góes, R.M. High fat-induced obesity associated with insulin-resistance increases FGF-2 content and causes stromal hyperplasia in rat ventral prostate. Cell Tissue Res. 2012, 349, 577–588. [Google Scholar] [CrossRef]
- Silva, S.A.; Gobbo, M.G.; Pinto-Fochi, M.E.; Rafacho, A.; Taboga, S.R.; Almeida, E.A.; Góes, R.M.; Ribeiro, D.L. Prostate hyperplasia caused by long-term obesity is characterized by high deposition of extracellular matrix and increased content of MMP-9 and VEGF. Int. J. Exp. Pathol. 2015, 96, 21–30. [Google Scholar] [CrossRef]
- Pytlowanciv, E.Z.; Pinto-Fochi, M.E.; Reame, V.; Gobbo, M.G.; Ribeiro, D.L.; Taboga, S.R.; Góes, R.M. Differential ontogenetic exposure to obesogenic environment induces hyperproliferative status and nuclear receptors imbalance in the rat prostate at adulthood. Prostate 2016, 76, 662–678. [Google Scholar] [CrossRef]
- Ribeiro, D.L.; Pinto, M.E.; Rafacho, A.; Bosqueiro, J.R.; Maeda, S.Y.; Anselmo-Franci, J.A.; Taboga, S.R.; Góes, R.M. High-fat diet obesity associated with insulin resistance increases cell proliferation, estrogen receptor, and PI3K proteins in rat ventral prostate. J. Androl. 2012, 33, 854–865. [Google Scholar] [CrossRef]
- Escobar, E.L.O.; Gomes-Marcondes, M.C.C.; Carvalho, H.F. Dietary fatty acid quality affects AR and PPARgamma levels and prostate growth. Prostate 2009, 69, 548–558. [Google Scholar] [CrossRef]
- Tang, N.-T.; Snook, R.D.; Brown, M.D.; Haines, B.A.; Ridley, A.; Gardner, P.; Denbigh, J.L. Fatty-Acid Uptake in Prostate Cancer Cells Using Dynamic Microfluidic Raman Technology. Molecules 2020, 25, 1652. [Google Scholar] [CrossRef]
- Huang, L.-H.; Chung, H.-Y.; Su, H.-M. Docosahexaenoic acid reduces sterol regulatory element binding protein-1 and fatty acid synthase expression and inhibits cell proliferation by inhibiting pAkt signaling in a human breast cancer MCF-7 cell line. BMC Cancer 2017, 17, 890. [Google Scholar] [CrossRef]
- Tamarindo, G.H. Docosahexaenoic Acid Effects on Cell Metabolism in Prostate Cancer Initiation and Progression. Ph.D. Thesis, Institute of Biology, State University of Campinas, Campinas, São Paulo, Brazil, 2022. [Google Scholar]
- Marín de Mas, I.; Aguilar, E.; Zodda, E.; Balcells, C.; Marin, S.; Dallmann, G.; Thomson, T.M.; Papp, B.; Cascante, M. Model-driven discovery of long-chain fatty acid metabolic reprogramming in heterogeneous prostate cancer cells. PLoS Comput. Biol. 2018, 14, e1005914. [Google Scholar] [CrossRef]
- Bailey, A.P.; Koster, G.; Guillermier, C.; Hirst, E.M.A.; MacRae, J.I.; Lechene, C.P.; Postle, A.D.; Gould, A.P. Antioxidant role for lipid droplets in a stem cell niche of drosophila. Cell 2015, 163, 340–353. [Google Scholar] [CrossRef]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar]
- Mao, P.; Nakao, K.; Angrist, A. Human Prostatic Carcinoma: An Electron Microscope Study. Cancer Res. 1996, 26, 955–973. [Google Scholar]
- Panov, A.; Orynbayeva, Z. Bioenergetic and antiapoptotic properties of mitochondria from cultured human prostate cancer cell lines PC-3, DU145 and LNCaP. PLoS ONE 2013, 8, e72078. [Google Scholar] [CrossRef]
- Grupp, K.; Jedrzejewska, K.; Tsourlakis, M.C.; Koop, C.; Wilczak, W.; Adam, M.; Quaas, A.; Sauter, G.; Simon, R.; Izbicki, J.R.; et al. High mitochondria content is associated with prostate cancer disease progression. Mol. Cancer 2013, 12, 145. [Google Scholar] [CrossRef]
- Burch, T.C.; Rhim, J.S.; Nyalwidhe, J.O. Mitochondria Biogenesis and Bioenergetics Gene Profiles in Isogenic Prostate Cells with Different Malignant Phenotypes. BioMed Res. Int. 2016, 2016, 1785201. [Google Scholar] [CrossRef]
- Schöpf, B.; Weissensteiner, H.; Schäfer, G.; Fazzini, F.; Charoentong, P.; Naschberger, A.; Rupp, B.; Fendt, L.; Bukur, V.; Giese, I.; et al. OXPHOS remodeling in high-grade prostate cancer involves mtDNA mutations and increased succinate oxidation. Nat. Commun. 2020, 11, 1487. [Google Scholar] [CrossRef]
- Higuchi, M.; Kudo, T.; Suzuki, S.; Evans, T.T.; Sasaki, R.; Wada, Y.; Shirakawa, T.; Sawyer, J.R.; Gotoh, A. Mitochondrial DNA determines androgen dependence in prostate cancer cell lines. Oncogene 2006, 25, 1437–1445. [Google Scholar] [CrossRef]
- Ghosh, P.; Vidal, C.; Dey, S.; Zhang, L. Mitochondria targeting as an effective strategy for cancer therapy. Int. J. Mol. Sci. 2020, 21, 3363. [Google Scholar] [CrossRef]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef]
- Shekari, N.; Javadian, M.; Ghasemi, M.; Baradaran, B.; Darabi, M.; Kazemi, T. Synergistic Beneficial Effect of Docosahexaenoic Acid (DHA) and Docetaxel on the Expression Level of Matrix Metalloproteinase-2 (MMP-2) and MicroRNA-106b in Gastric Cancer. J. Gastrointest. Cancer 2020, 51, 70–75. [Google Scholar] [CrossRef]
- Ding, X.; Ge, L.; Yan, A.; Ding, Y.; Tao, J.; Liu, Q.; Qiao, C. Docosahexaenoic acid serving as sensitizing agents and gefitinib resistance revertants in EGFR targeting treatment. OncoTargets Ther. 2019, 12, 10547–10558. [Google Scholar] [CrossRef]
- Lages, E.B.; Fernandes, R.S.; Silva, J.d.O.; de Souza, Â.M.; Cassali, G.D.; de Barros, A.L.B.; Miranda Ferreira, L.A. Co-delivery of doxorubicin, docosahexaenoic acid, and α-tocopherol succinate by nanostructured lipid carriers has a synergistic effect to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2020, 132, 110876. [Google Scholar] [CrossRef]
- Shan, K.; Feng, N.; Zhu, D.; Qu, H.; Fu, G.; Li, J.; Cui, J.; Chen, H.; Wang, R.; Qi, Y.; et al. Free docosahexaenoic acid promotes ferroptotic cell death via lipoxygenase dependent and independent pathways in cancer cells. Eur. J. Nutr. 2022, 61, 4059–4075. [Google Scholar] [CrossRef]
- Olkhovik, D.M.; Silkina, M.O.; Razumovskaya, A.V.; Klycheva, K.V.; Fatkulin, A.A.; Kulagin, T.A.; Nikulin, S.V. Omega-3 Docosahexaenoic Acid as a Promising Inducer of Ferroptosis: Dynamics of Action in Prostate and Colorectal Cancer Models. Dokl. Biochem. Biophys. 2025, 520, 25–28. [Google Scholar] [CrossRef]
- Sun, Y.; Jia, X.; Hou, L.; Liu, X.; Gao, Q. Involvement of apoptotic pathways in docosahexaenoic acid-induced benefit in prostate cancer: Pathway-focused gene expression analysis using RT2 Profile PCR Array System. Lipids Health Dis. 2017, 16, 59. [Google Scholar] [CrossRef]
- Amaro, G.M.; da Silva, A.D.T.; Martins, L.P.; Taboga, S.R.; Cagnon, V.H.A.; Góes, R.M. Dietary docosahexaenoic acid impairs prostate cancer progression in TRAMP mice in the early stages of disease through modulation of inflammatory microenvironment. J. Mol. Histol. 2025, 56, 194. [Google Scholar] [CrossRef]
- Pakula, H.; Pederzoli, F.; Fanelli, G.N.; Nuzzo, P.V.; Rodrigues, S.; Loda, M. Deciphering the tumor microenvironment in prostate cancer: A focus on the stromal component. Cancers 2024, 16, 3685. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef]
- Monge, P.; Astudillo, A.M.; Pereira, L.; Balboa, M.A.; Balsinde, J. Dynamics of docosahexaenoic acid utilization by mouse peritoneal macrophages. Biomolecules 2023, 13, 1635. [Google Scholar] [CrossRef]
- Wu, S.; Peng, H.; Li, S.; Huang, L.; Wang, X.; Li, Y.; Liu, Y.; Xiong, P.; Yang, Q.; Tian, K.; et al. The ω-3 Polyunsaturated Fatty Acid Docosahexaenoic Acid Enhances NK-Cell Antitumor Effector Functions. Cancer Immunol. Res. 2024, 12, 744–758. [Google Scholar] [CrossRef]
- Liang, P.; Henning, S.M.; Guan, J.; Grogan, T.; Elashoff, D.; Olefsky, J.M.; Cohen, P.; Aronson, W.J. Role of Host GPR120 in Mediating Dietary Omega-3 Fatty Acid Inhibition of Prostate Cancer. J. Natl. Cancer Inst. 2019, 111, 52–59. [Google Scholar] [CrossRef]
- Liang, P.; Henning, S.M.; Schokrpur, S.; Wu, L.; Doan, N.; Said, J.; Grogan, T.; Elashoff, D.; Cohen, P.; Aronson, W.J. Effect of Dietary Omega-3 Fatty Acids on Tumor-Associated Macrophages and Prostate Cancer Progression. Prostate 2016, 76, 1293–1302. [Google Scholar] [CrossRef]
- Liang, P.; Henning, S.M.; Guan, J.; Grogan, T.; Elashoff, D.; Cohen, P.; Aronson, W.J. Effect of dietary omega-3 fatty acids on castrate-resistant prostate cancer and tumor-associated macrophages. Prostate Cancer Prostatic Dis. 2020, 23, 127–135. [Google Scholar] [CrossRef]
- Davidsson, S.; Fiorentino, M.; Andrén, O.; Fang, F.; Mucci, L.A.; Varenhorst, E.; Fall, K.; Rider, J.R. Inflammation, focal atrophic lesions, and prostatic intraepithelial neoplasia with respect to risk of lethal prostate cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2280–2287. [Google Scholar] [CrossRef]
- Rani, A.; Dasgupta, P.; Murphy, J.J. Prostate cancer: The role of inflammation and chemokines. Am. J. Pathol. 2019, 189, 2119–2137. [Google Scholar] [CrossRef]
- Shan, K.; Feng, N.; Cui, J.; Wang, S.; Qu, H.; Fu, G.; Li, J.; Chen, H.; Wang, X.; Wang, R.; et al. Resolvin D1 and D2 inhibit tumour growth and inflammation via modulating macrophage polarization. J. Cell. Mol. Med. 2020, 24, 8045–8056. [Google Scholar] [CrossRef]
- Liang, P.; Henning, S.M.; Grogan, T.; Elashoff, D.; Ye, H.; Cohen, P.; Aronson, W.J. Effects of dietary omega-3 fatty acids on orthotopic prostate cancer progression, tumor associated macrophages, angiogenesis and T-cell activation-dependence on GPR120. Prostate Cancer Prostatic Dis. 2022, 25, 539–546. [Google Scholar] [CrossRef]
- Liang, P.; Henning, S.M.; Grogan, T.; Elashoff, D.; Said, J.; Cohen, P.; Aronson, W.J. Effect of omega-3 fatty acid diet on prostate cancer progression and cholesterol efflux in tumor-associated macrophages-dependence on GPR120. Prostate Cancer Prostatic Dis. 2024, 27, 700–708. [Google Scholar] [CrossRef]
- Pakula, H.; Omar, M.; Carelli, R.; Pederzoli, F.; Fanelli, G.N.; Pannellini, T.; Socciarelli, F.; Van Emmenis, L.; Rodrigues, S.; Fidalgo-Ribeiro, C.; et al. Distinct mesenchymal cell states mediate prostate cancer progression. Nat. Commun. 2024, 15, 363. [Google Scholar] [CrossRef]
- Giannoni, E.; Taddei, M.L.; Morandi, A.; Comito, G.; Calvani, M.; Bianchini, F.; Richichi, B.; Raugei, G.; Wong, N.; Tang, D.; et al. Targeting stromal-induced pyruvate kinase M2 nuclear translocation impairs oxphos and prostate cancer metastatic spread. Oncotarget 2015, 6, 24061–24074. [Google Scholar] [CrossRef]
- Bianchini, F.; Giannoni, E.; Serni, S.; Chiarugi, P.; Calorini, L. 22: 6n-3 DHA inhibits differentiation of prostate fibroblasts into myofibroblasts and tumorigenesis. Br. J. Nutr. 2012, 108, 2129–2137. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, C.-Y.; Kao, C.-L.; Jiang, Y.; Liu, C.-M. Docosahexaenoic acid inhibits lipopolysaccharide-induced metastatic activities by decreasing inflammation on prostate cancer cell. Pharmazie 2019, 74, 675–679. [Google Scholar]
- Kudo, Y.; Nakamura, K.; Tsuzuki, H.; Hirota, K.; Kawai, M.; Takaya, D.; Fukuzawa, K.; Honma, T.; Yoshino, Y.; Nakamura, M.; et al. Docosahexaenoic acid enhances the treatment efficacy for castration-resistant prostate cancer by inhibiting autophagy through Atg4B inhibition. Arch. Biochem. Biophys. 2024, 760, 110135. [Google Scholar] [CrossRef]
- Shaikh, I.A.A.; Brown, I.; Schofield, A.C.; Wahle, K.W.J.; Heys, S.D. Docosahexaenoic acid enhances the efficacy of docetaxel in prostate cancer cells by modulation of apoptosis: The role of genes associated with the NF-kappaB pathway. Prostate 2008, 68, 1635–1646. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Olejarz, W.; Kubiak-Tomaszewska, G.; Ciechanowicz, A.K.; Struga, M. The Effect of Fatty Acids on Ciprofloxacin Cytotoxic Activity in Prostate Cancer Cell Lines-Does Lipid Component Enhance Anticancer Ciprofloxacin Potential? Cancers 2022, 14, 409. [Google Scholar] [CrossRef]
- Lv, H.; Jia, W.; Yang, L.; Dong, P.; Liu, J.; Wang, S.; Li, X.; Hu, J.; Zhao, L.; Shi, Y. Influence of unsaturated fatty acids on the antitumor activity of polymeric conjugates grafted with cabazitaxel against prostate cancer. Biomed. Pharmacother. 2023, 169, 115902. [Google Scholar] [CrossRef]
- Hajjaji, N.; Bougnoux, P. Selective sensitization of tumors to chemotherapy by marine-derived lipids: A review. Cancer Treat. Rev. 2013, 39, 473–488. [Google Scholar] [CrossRef]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.C.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer 2011, 117, 3774–3780. [Google Scholar] [CrossRef]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef]
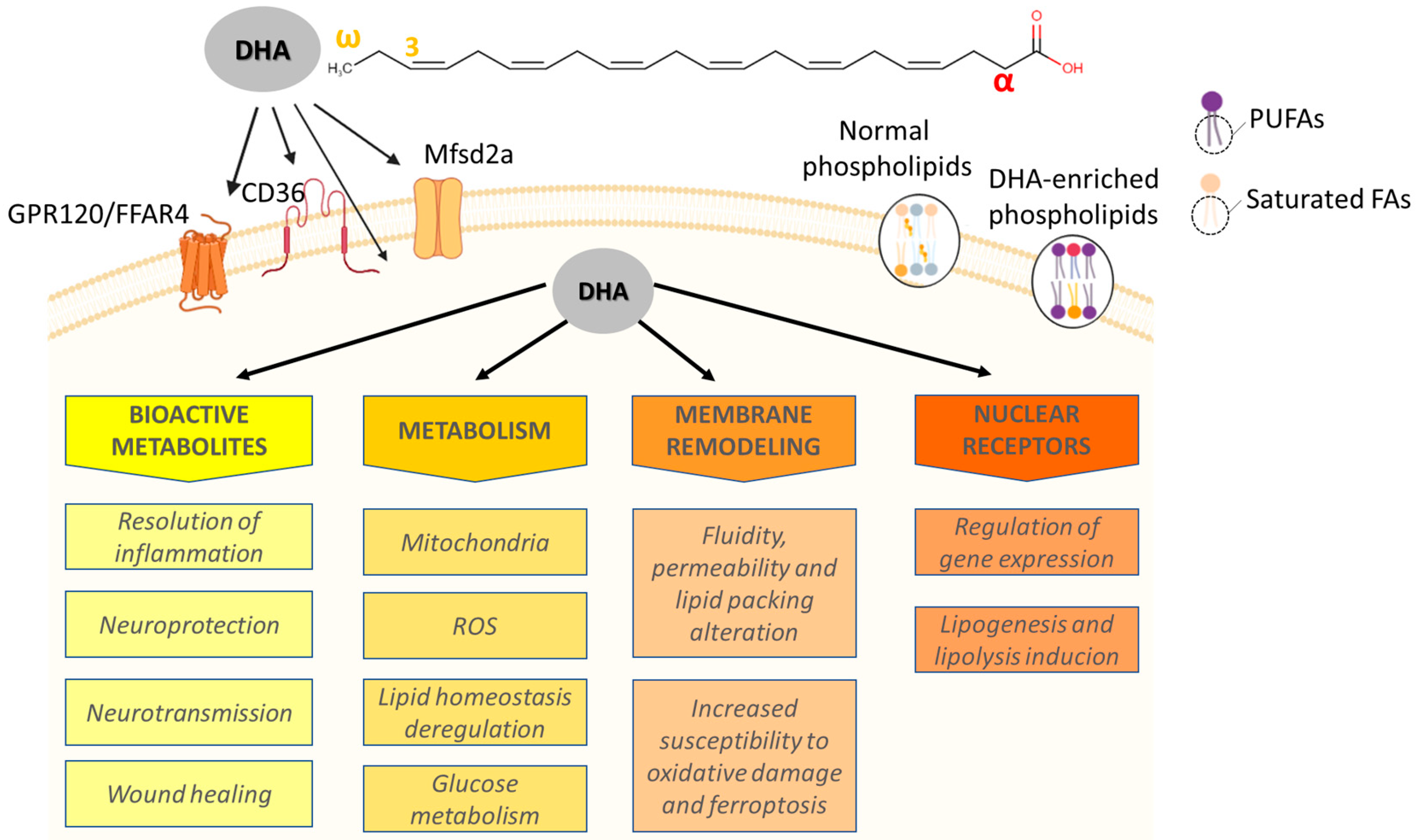
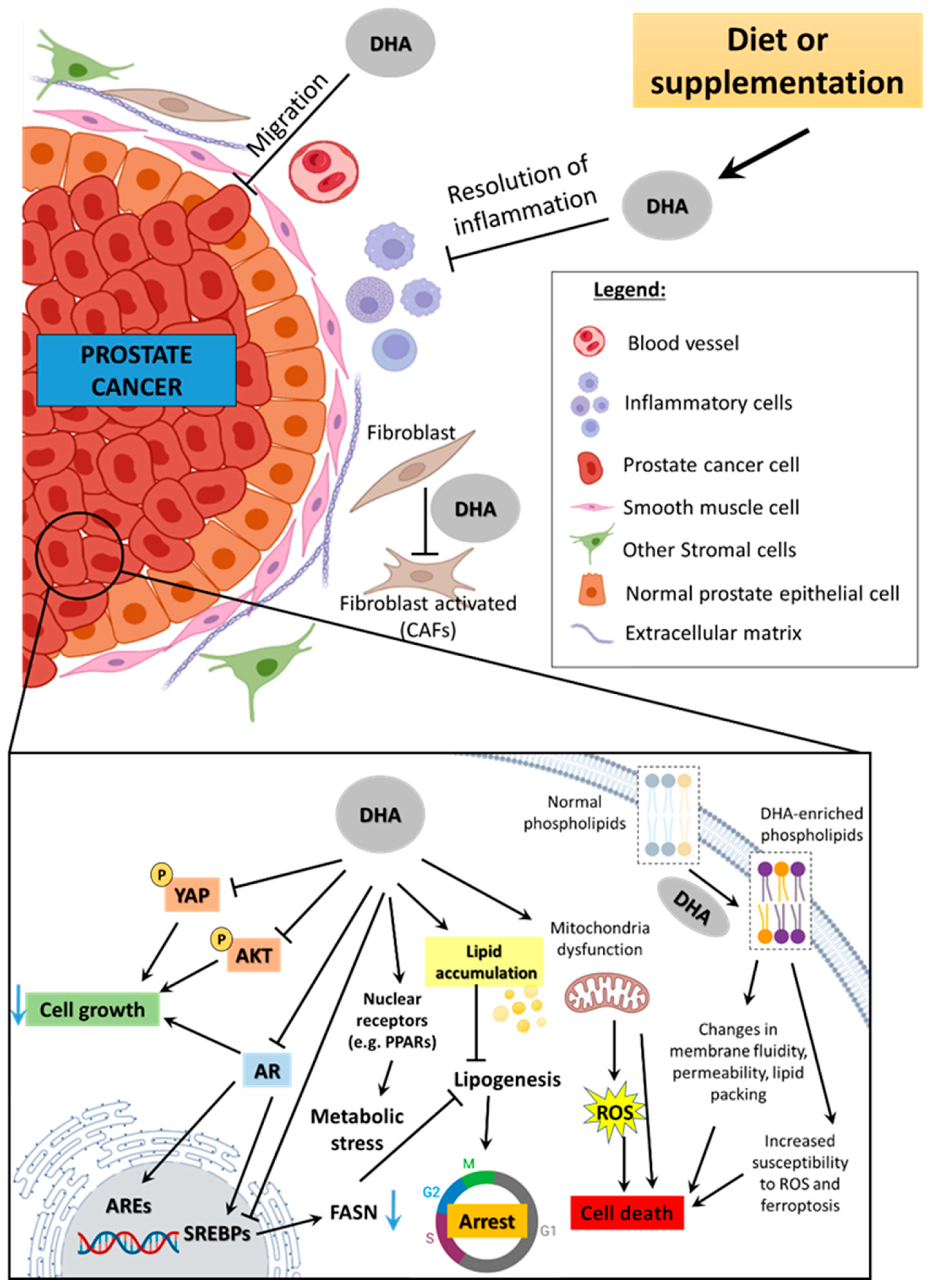
| MECHANISM | MAIN GAPS FOUND IN THE LITERATURE | POTENTIAL TRANSLATIONAL APPROACH |
|---|---|---|
| Androgen signaling | In vivo studies; in vitro combination with ARSIs simultaneously or considering DHA pre-incubation; lack of study using AR genetic editing for validation | Combination of DHA intake with ARSIs currently available |
| Nuclear Receptors | Determination of whether PPARs are protective or promote PCa; in vivo and in vitro studies with DHA combined with PPARs inhibitors; association with androgenic background | Combination of DHA intake with PPAR inhibitors in patients stratified according to PTEN expression; combination of DHA with drugs that regulate GR. |
| Metabolism | Combination with lipogenesis and cholesterol biosynthesis inhibitors in vivo and in vitro; lack of carbon-labeled experiments to evaluate the metabolic flux | Synergistic effect of DHA intake with lipid metabolism inhibitors under clinical trial |
| Cell Death | In vivo assays; lacks assessment of combination either in vitro or in vivo with cell death inhibitors with proper controls | Synergistic effect of DHA intake with cell death inducers under clinical trial, simultaneously or as pretreatment |
| TME | Co-culture assays to validate hypothesis; functional assays ex vivo to validate lymphoid and myeloid function; specific assessment of DHA in COLD and HOT TME | Stratification of patients according to inflammatory infiltrates; combination of DHA intake with the current therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamarindo, G.H.; Amaro, G.M.; da Silva, A.D.T.; Góes, R.M. Effects of Docosahexaenoic Acid on Prostate Cancer. J. Xenobiot. 2025, 15, 111. https://doi.org/10.3390/jox15040111
Tamarindo GH, Amaro GM, da Silva ADT, Góes RM. Effects of Docosahexaenoic Acid on Prostate Cancer. Journal of Xenobiotics. 2025; 15(4):111. https://doi.org/10.3390/jox15040111
Chicago/Turabian StyleTamarindo, Guilherme Henrique, Gustavo Matheus Amaro, Alana Della Torre da Silva, and Rejane Maira Góes. 2025. "Effects of Docosahexaenoic Acid on Prostate Cancer" Journal of Xenobiotics 15, no. 4: 111. https://doi.org/10.3390/jox15040111
APA StyleTamarindo, G. H., Amaro, G. M., da Silva, A. D. T., & Góes, R. M. (2025). Effects of Docosahexaenoic Acid on Prostate Cancer. Journal of Xenobiotics, 15(4), 111. https://doi.org/10.3390/jox15040111





