Abstract
Cowpea, Vigna sp., is an important, low-cost protein source in subtropical and semi-arid regions, where seasonal rainfall makes storage necessary. However, the weevils Callosobruchus maculatus and C. chinensis cause significant grain losses during storage. While synthetic fumigants are commonly used to control these pests, their risks to mammals have prompted the search for safer alternatives. In this context, we tested palo santo, Bursera graveolens, essential oil with limonene, α-phellandrene, o-cymene and β-phellandrene, menthofuran, and germacrene-D as a sustainable approach. This plant is readily accessible, produces high fruit yields, and is used in households for various purposes. We evaluated the fumigant toxicity, repellency, and ovicidal effects of B. graveolens essential oil on both Callosobruchus species. Our results showed that B. graveolens oil was toxic to C. maculatus (LC50 = 80.90 [76.91–85.10] µL) and C. chinensis (LC50 = 63.9 [60.95–66.99] µL), with C. chinensis being more susceptible (SR = 1.27). Molecular docking analyses revealed that all the oil’s compounds bind to both the GABA and octopamine receptors, exhibiting high energy affinities; however, germacrene shows the strongest affinity in these receptors. C. chinensis was strongly repelled at all concentrations, while C. maculatus was repelled only at lethal concentrations. No ovicidal effect was observed in either species. In conclusion, our findings suggest that B. graveolens essential oil is a promising and sustainable protectant for stored cowpeas in small-scale storage units.
Keywords:
Callosobruchus spp.; cowpea; weevils; eco-friendly; essential oils; GABA; octopamine; target site 1. Introduction
Cowpea Vigna genus (L.) is a staple food in semi-arid tropical regions due to its high-quality nutritional content and low cost compared to other protein sources [1,2]. In 2022, global production exceeded 9.77 million metric tons [3]. Like all grains, cowpeas must be stored for later consumption, which can lead to infestations by adapted insects. The primary pests of cowpea across the five continents are the cosmopolitan weevils Callosobruchus maculatus Fabricius and Callosobruchus chinensis L. [4,5]. Infestation typically begins in the field, as females lay eggs in open bean pods, and continues in storage. The rapid reproduction and high fertility of C. maculatus and C. chinensis, with a new generation emerging in less than 30 days and a mean fertility of 100 eggs per female [6,7] make effective management essential in storage units. Conversely, these infestations can result in losses exceeding 30% within a few months [8].
The most common method for controlling stored pests is phosphine-based fumigants. While these fumigants are highly effective, relatively inexpensive, and food-safe, they have some drawbacks, including corrosive effects on metals, environmental pollution [9], and the development of resistance [10,11,12]. As a result, research into alternative pest control methods has increased, with a focus on essential oils and their bioactive compounds. Essential oils are a complex mixture of molecules with potential biological effects, including acute and chronic toxicity [13,14], repellent activity [15,16], and the inhibition of oviposition, growth, feeding, and the development of organisms [17,18,19,20]. This wide range of possible actions of essential oils is due to their diverse chemical composition, in which the effects on organisms can respond to the main compounds or the interaction between various compounds. Additionally, these products minimize harm to non-target organisms such as pollinators, natural enemies, and mammals [21,22].
The complex composition of essential oils makes it difficult for insects to develop resistance to these products. However, it also complicates the understanding of the specific sites of action on target organisms. Recent advances in bioinformatics have identified potential target sites for isolated compounds in essential oils. For example, it is known that the primary physiological targets of essential oils are specific proteins involved in insect metabolism or key neurotransmitters that are inhibited or altered [21,23]. Studies have identified several targets, including gamma-aminobutyric acid (GABA) receptors, transient receptor potential (TRP) channels, octopamine receptor (OctpR), acetylcholine esterase (AChE), enzymatic [superoxide dismutase (SOD), catalase (CAT), peroxidases (POx), glutathione-S-transferase (GST) and glutathione reductase (GR)], and non-enzymatic [glutathione (GSH)] antioxidant defense systems [23,24,25,26].
Thus, Bursera graveolens (Burseraceae), commonly called palo santo, could serve as a valuable source of biomolecules with insecticide potential, as it is rich in monoterpenes and sesquiterpenes [27,28]. Additionally, since the fruit is used to extract essential oil with a yield of over >3%, its use is both profitable and sustainable. The biological properties of B. graveolens include insecticide, acaricide, and antimicrobial effects, as well as medicinal benefits [29,30,31,32,33]. This study evaluates the fumigant effect of B. graveolens essential oil on C. maculatus and C. chinensis, the two primary pests of stored cowpeas. Additionally, 3D molecular modeling and in silico molecular docking were used to investigate the binding patterns of these compounds to target sites in the insects.
2. Materials and Methods
2.1. Rearing Insects Callosobruchus maculatus and C. chinensis
The original populations of C. maculatus and C. chinensis, the main pests of cowpea bean (genus Vigna), were field-collected from small farms in the Viçosa region (Minas Gerais State, Brazil—20°45′20.23″ S, 42°51′50.95″ W) during the years 2018–2019. Both species were reared on their primary host, free from insecticide residues and other infestations. Cowpea (V. unguiculate) was obtained from the local market, and the grains were stored at −10 °C for two weeks before being provided to the weevils, ensuring the grains were free from other insect contamination. The adults were placed in ventilated glass jars (4 L) with the beans and maintained under laboratory conditions (27 ± 2 °C, 75 ± 5% relative humidity, 12 h of scotophase) [19].
2.2. Bursera graveolens Essential Extraction Oil
The essential oil (EO) of B. graveolens was extracted from fresh fruits using the hydrodistillation method with a Clevenger-type apparatus at approximately 100 °C for three hours. The EO was then dried over anhydrous sodium sulfate and stored in sealed vials protected from light at 4 °C until further analysis and use [29]. Chromatographic analysis using gas-chromatography mass spectrometry (GC-MS) with a gas chromatograph SHIMADZU GCMS-QP500, (Shimadzu Corporation, Kyoto, Japan) equipped with a mass detector by electron impact ionization (70 eV) and a GC-2014 (SHIMADZU) gas chromatograph equipped with a flame ionization detector (GC-FID), revealed that the B. graveolens essential oil used here has aliphatic monoterpenes limonene (43.6%), α-phellandrene (20.4%), o-cymene (12.7%), and β-phellandrene (3.7%) as major compounds; the oxygenated monoterpene menthofuran (10.7%) and the aliphatic sesquiterpene germacrene D (2.1%) [16].
2.3. Bioassays Toxicity Against Callosobruchus maculatus and Callosobruchus chinensis
The fumigant effect of the essential oil was tested on two species of weevils in cowpea beans and their primary host, following standardized methods described by Ndomo et al. [34] and Zapata and Smagghe [35]. Briefly, the experiment involved a 250 cm3 glass jar containing 75 g of cowpea grains and 20 newly emerged adult insects (<24 h) and sexed (1:1 male-to-female ratio). Several concentrations [40, 60, 80, 140 µL/L (C. chinensis) and 40, 60, 80, 100, 180, 220 µL/L (C. maculatus)] of B. graveolens essential oil were used. Each concentration was applied in a small 4 cm2 piece of Whatman No. 1 filter paper that was attached to the metal lid with cotton thread and suspended inside the jar without touching the beans, acting as a diffuser. The jars were then sealed with parafilm (PM996, Parafilm, Detroit, MI, USA) and placed in a BOD incubator with constant conditions of temperature (27 ± 2 °C), relative humidity (70 ± 5%), and 12 h scotophase. After 24 h, the dead insects in each jar were counted and considered dead if they did not respond to a contact stimulus applied with a fine brush. Five replicates were conducted for each treatment. The control group followed the same experimental setup, but without the application of essential oil.
2.4. Molecular Docking Between Essential Oil Biomolecules of Bursera graveolens and Gamma-Aminobutyric Acid (GABA) and Octopamine Receptors of Callosobruchus maculatus
The proteome of C. maculatus (cowpea weevil) is available from the National Center for Biotechnology Information (NCBI) database. We performed a BLASTp of C. maculatus proteome search against the (NCBI) databases and identified the protein gamma-aminobutyric acid receptor (GABA) subunit beta XP_050512277.1 of Diabrotica virgifera virgifera (Coleoptera; Chrysomelidae) with 90% identity and an e-value of 0.0, matching with the VEN59736.1 unnamed protein (C. maculatus). We downloaded the VEN59736.1 amino acid sequences of C. maculatus and used the method with Swiss Model Workspace https://swissmodel.expasy.org/interactive (15 January 2025) in order to build the protein by inspecting protein structure crashes, the amino acid positions at the active site [36], Ramachandran plots [37], and the Global Model Quality Estimate (GMQE). The prediction was based on the structure generated using AlphaFold DB [38] for the protein identified as A0A653DHQ0 (Uniprot ID: A0A653DHQ0_CALMS). The resulting model exhibited a typical conformational architecture of GABA receptors, with well-defined transmembrane domains. The model quality assessment using GMQE of (0.77) and the values of Ramachandran, favored with 90%, indicated a reliable confidence level in the prediction. In the BLASTp search for an octopamine receptor of the C. maculatus proteome, using the (NCBI) databases, we identified the protein octopamine receptor beta-3R-like XP_023024593.1 of Leptinotarsa decemlineata (Coleoptera; Chrysomelidae), with 83.4% of identity and e-value 3 × 10−163 matching with the VEN45923.1 unnamed protein (C. maculatus). The prediction was based on the structure generated using AlphaFold DB for the protein identified as A0A6P7F675 (Uniprot ID: A0A6P7F675_DIAVI). The resulting model exhibits the typical architecture of G-protein-coupled receptors (GPCRs), with well-defined transmembrane domains, consistent with its role in neurotransmission. The model quality assessment using a GMQE of 0.83 and values of Ramachandran of 95.5% indicated a reliable confidence level in the prediction. For both modeled proteins, GABA and octopamine, the energy minimization was performed using the Yasara forced field [39].
We prepared the limonene, α-phellandrene, o-cymene, menthofuran, β-phellandrene, and germacrene D molecules of B. graveolens using PubChem [40] and NCBI and stored them in a Structure Data Format (SDF) for molecular docking predictions. These molecules and proteins were prepared with Autodock Tools 1.5.7. [41]. The best ligand–receptor complex, which returned affinity energy values (kcal/mol) using the AutoDock Vina [42] was used to generate 2D interaction maps with Discovery Studio Visualizer.
2.5. Repellency Bioassay of Essential Oil Against Callosobruchus spp.
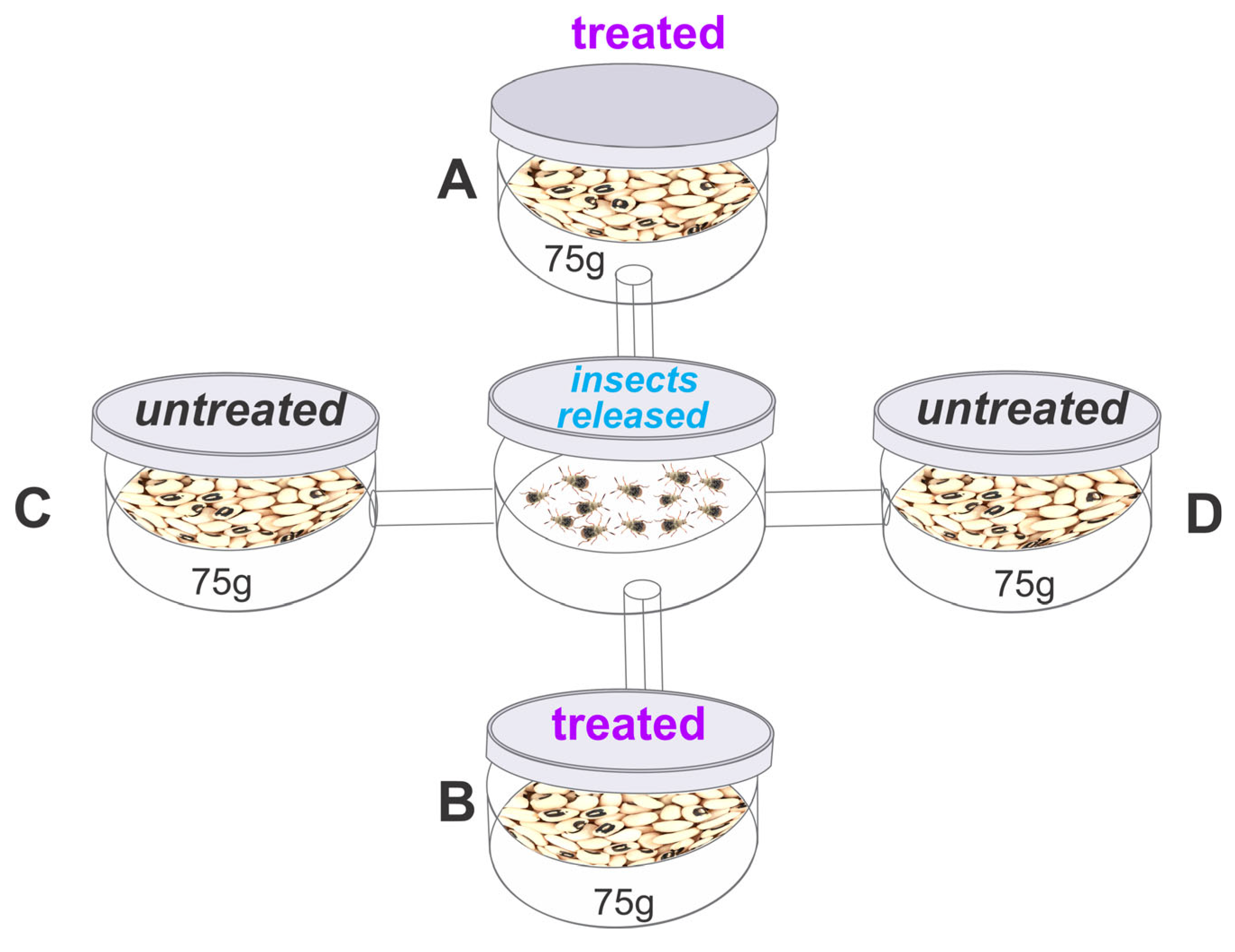
The bioassay to assess the repellent activity of the essential oil was conducted using an apparatus consisting of five circular plastic containers (12 cm diameter, 8 cm height). A central container (E) was connected to four surrounding containers (A, B, C, and D) using plastic cylinders (12 cm long, 1 cm in diameter) (Figure 1), as described by Jumbo, Corrêa, Gomes, Armijos, Valarezo, Mantilla-Afanador, Machado, Rocha, Aguiar, and Oliveira [16], Freitas et al. [43]. Containers A and B were arranged diagonally and filled with 75 g of cowpea beans treated with an estimated sublethal or lethal concentration of B. graveolens essential oil [(in μL/L): LC15 or LC50 or LC95)] for each species. The essential oil was applied to filter paper hanging from the lid of the container, as described in the toxicity bioassay. Containers C and D were filled with 75 g of untreated beans (control). In the central container, 40 unsexed adults (<24 h old) were released, and after 24 h, the total number of insects in each container was recorded. The repellency index (RI) was calculated as proposed by Mazzonetto and Vendramim [44] RI = (2T)/(T + C), where RI is the repellency index, C is the percentage of insects in the untreated containers, and T is the percentage of insects in the treated containers. The RI values ranged from 0 to 2, with the following interpretation: RI = 1 (neutral activity), RI > 1 (attraction), and RI < 1 (repellence). A safety margin for classification was established by adding or subtracting the standard deviation (SD) of each treatment from a value of 1 (indicating neutrality).
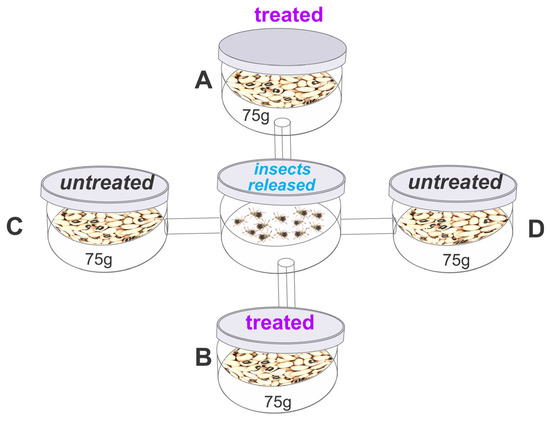
Figure 1.
Layout of the arena used in the repellency bioassay; The arenas A, B with Bursera graveolens essential oil and arenas C, D without treatment.
2.6. Tests Effect Ovicidal and Emergency Adults Callosobruchus spp.
To determine the ovicidal activity of the essential oil, the methodology of Nattudurai et al. [45] was adapted. Glass jars, as described in the toxicity assay process, were used for each species. Twenty insects (10 males and 10 females) of C. maculatus or C. chinensis were placed in 20 g of cowpea bean for egg laying. After 48 h, the beetles were removed, and the beans containing eggs were exposed to four estimated concentrations (LC25, LC50, LC75, LC95) of B. graveolens essential oil for 24 h, as performed in the toxicity assay. After five days, the number of larvae that hatched from the eggs was counted. The beans from each exposure were transferred to new containers, and the progeny of the adult of each species and treatment were assessed after 35 days [19]. Four replicates were set up for each concentration of essential oil and the control.
2.7. Statistical Analysis
Concentration–mortality curves were estimated using probit analyses with the PROC PROBIT procedure in SAS software, version 9.1 [46]. The 95% confidence intervals for susceptibility rate (SR) were calculated as described by Robertson et al. [47], with values considered significant if the range did not include 1. The number of hatched larvae in each treatment was compared to control emergence using the Bonferroni test (p < 0.05) and GraphPad Prisma 8.1.
3. Results
3.1. Essential Oil Toxicity Bioassays to Callosobruchus maculatus and Callosobruchus chinensis
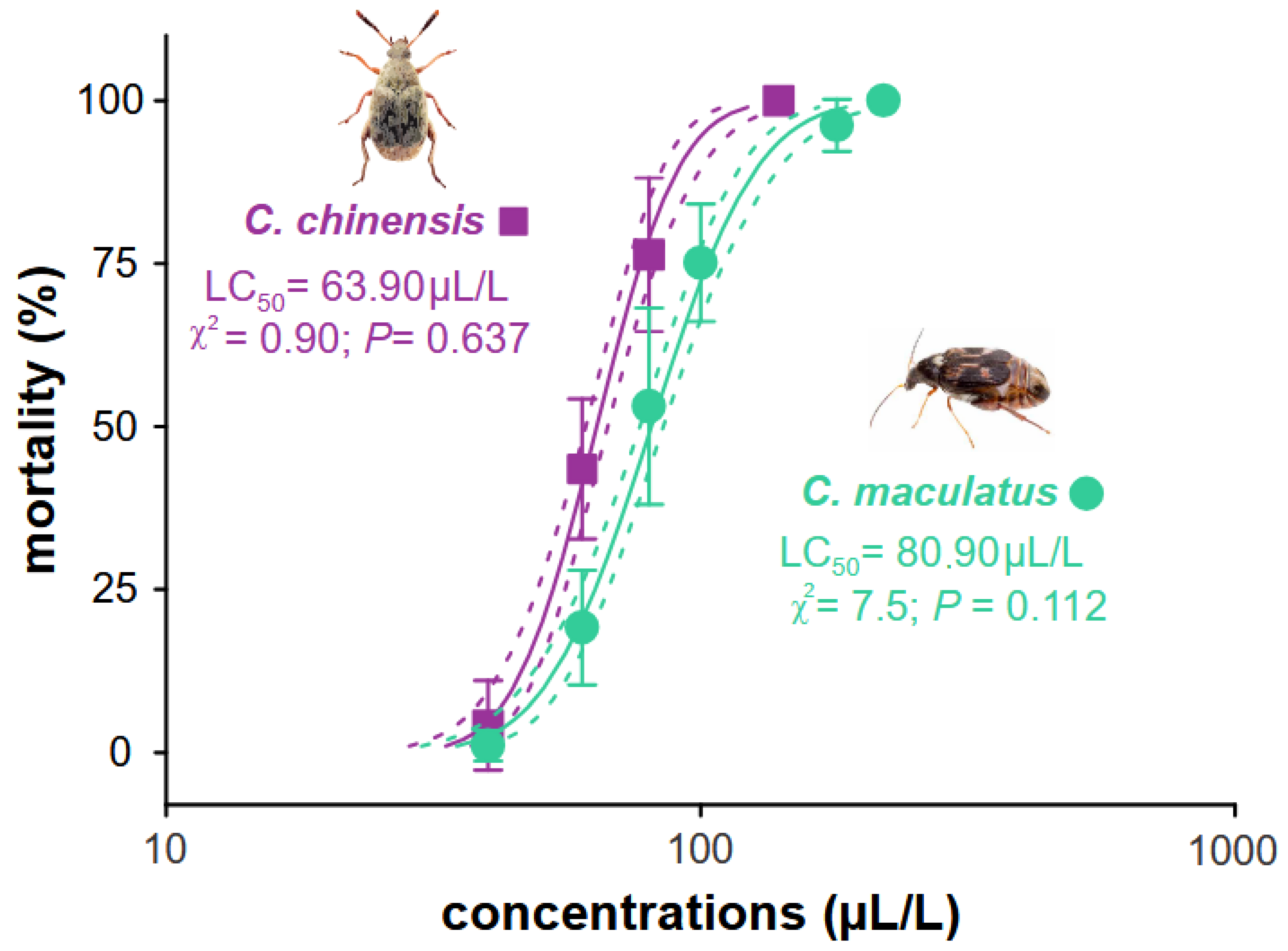
Our results showed that B. graveolens essential oil is toxic to the two main pests of stored cowpea beans when used as a fumigant. The lethal concentration (LC50) was estimated to be C. maculatus LC50 80.90 (76.91–85.10) µL, which was greater than for C. chinensis 63.90 (60.95–66.99) µL, with a susceptibility ratio (SR) of 1.27 for C. maculatus (Table 1). These values for each species were obtained from the concentration–mortality curves from the probit model (goodness-of-fit tests exhibited low χ2 values and high p-values [>0.05] when all the concentrations for each species were obtained (Figure 2)).

Table 1.
Toxicity of Bursera graveolens essential oil against the primary pests of stored cowpea, Callosobruchus chinensis and Callosobruchus maculatus, when exposed as a fumigant for 24 h.
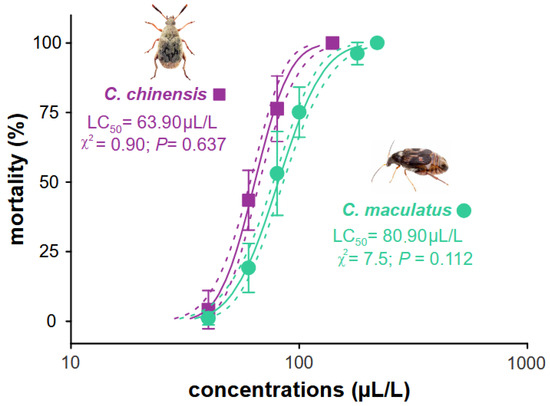
Figure 2.
Concentration–mortality curve estimated for Bursera graveolens essential oil for Callosobruchus maculatus and C. chinensis. The full lines represent the estimated lethal concentration (LC) of each species, and the dotted lines represent the confidence intervals (95%). Symbols represent the average (±SE) mortality recorded for each essential oil concentration of four replicates.
3.2. Interactions Between Bursera graveolens Essential Oil and the Site’s Target of Callosobruchus spp.
3.2.1. Bursera graveolens Essential Oil Interaction with Gamma-Aminobutyric Acid (GABA)
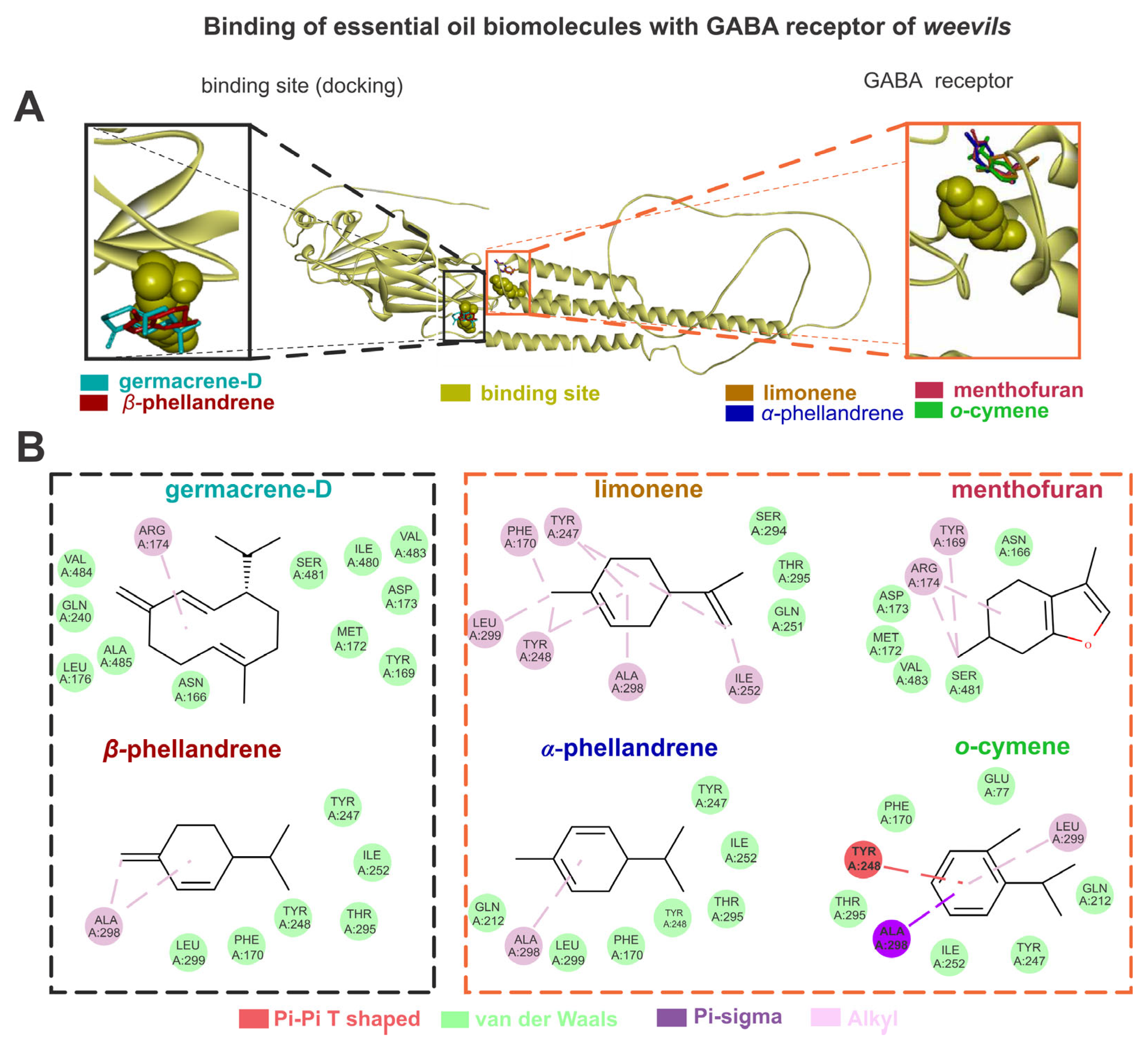
The GABA exhibited higher energy affinities (AutoDockVina affinity energy kcal mol–1) when complexed with germacrene D (–6.3), followed by limonene (–5.1), α-phellandrene (–5.0), o-cymene (–5.0), menthofuran (–5.0), and β-phellandrene (–4.9). The germacrene D showed van der Waals interactions with LEU176, ALA485, GLN240, GLU233, VAL484, ASN166, TYR169, MET172, ASP173, SER481, ILE480, and VAL483, as well as alkyl interactions with ARG174. The limonene showed van der Waals interactions with THR295, SER294, and GLN251, as well as alkyl interactions with TYR247, LEU299, TYR248, PHE170, ALA298, and ILE252. The α-phellandrene showed van der Waals interactions with TYR247, ILE252, THR295, TYR248, PHE170, LEU299, and GLN212, as well as alkyl interactions with ALA298. The o-cymene showed van der Waals interactions with ILE252, TYR247, GLN212, GLU77, PHE170, and THR295, pi-sigma interactions with ALA298, pi-pi T-shaped interactions with TYR248, and alkyl interactions with LEU299. The menthofuran showed van der Waals interactions with ASN166, SER481.VAL483, MET172, and ASP173, as well as alkyl interactions with TYR169 and ARG174. The β-phellandrene showed van der Waals interactions with TYR247, ILE252, THR295, TYR248, PHE170, and LEU299, as well as alkyl interactions with ALA298 (Figure 3).
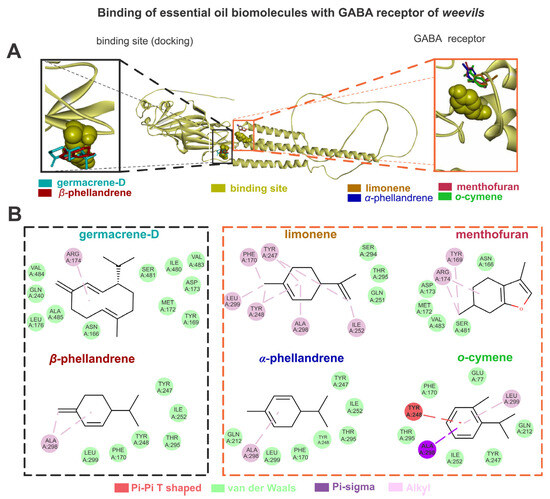
Figure 3.
The limonene, o-cymene, α-phellandrene, β-phellandrene, germacrene D, and menthofuran bind with gamma-aminobutyric acid (GABA) sites of weevils (A); the molecular interactions of the same constituents with the amino acids from GABA active sites are also shown (B).
3.2.2. Bursera graveolens Essential Oil Interaction with Octopamine Receptors
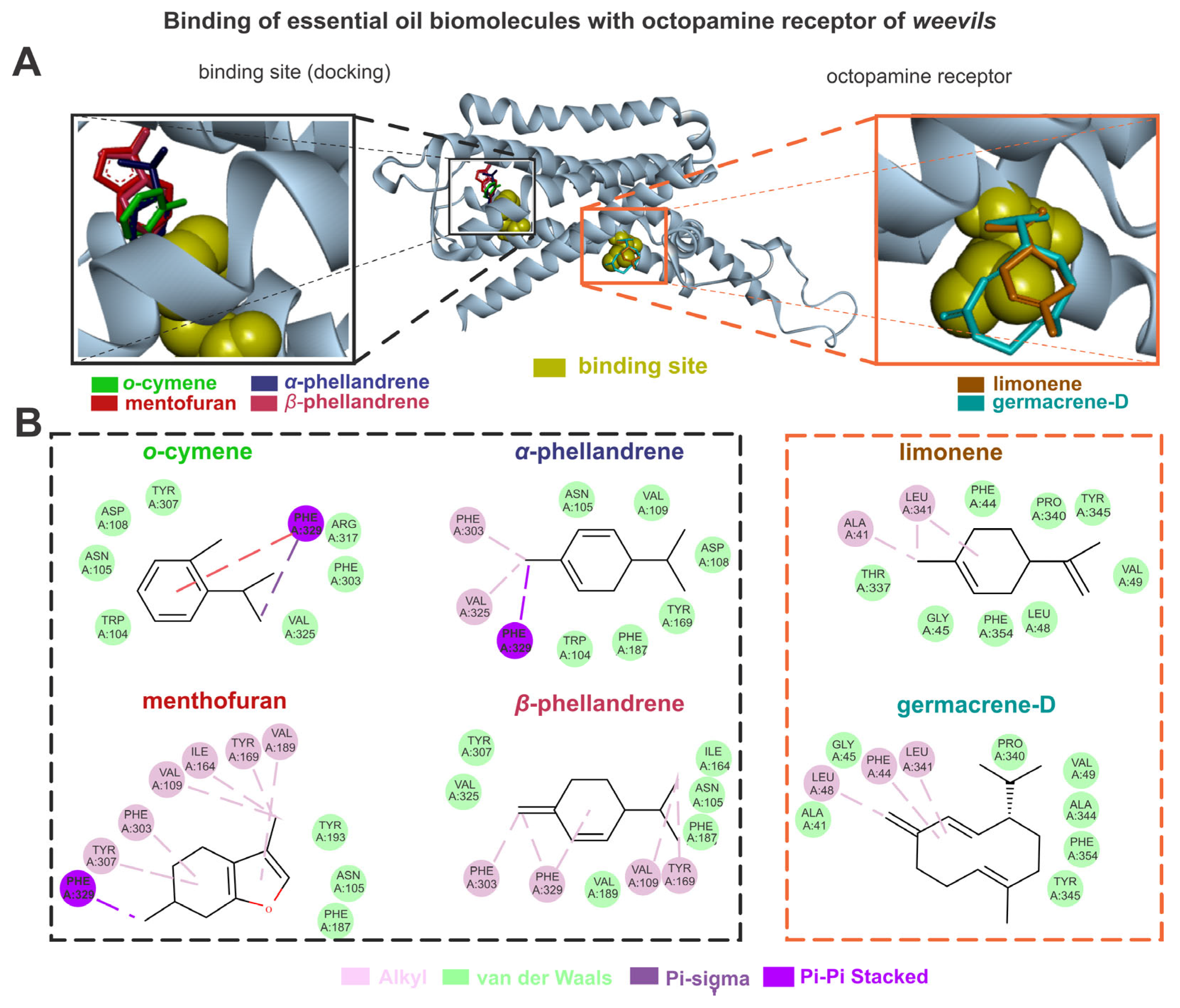
The biomolecules of the B. graveolens and octopamine receptors exhibited higher energy affinities when complexed with germacrene D (–6.7), menthofuran (–6.1), α-phellandrene and β-phellandrene (5.8), o-cymene (–5.7), and limonene (–5.4). The germacrene D showed van der Waals interactions with TYR345, PHE354, ALA344, VAL49, PRO340, GLY45, and ALA41, and alkyl interactions with LEU341, LEU48, and PHE44. The menthofuran showed van der Waals interactions with TYR193, ASN105, and PHE187, alkyl interactions with TYR307, PHE303, VAL109, ILE164, TYR169, and VAL189, and pi-sigma interactions with PHE329. The α-phellandrene showed van der Waals interactions with PHE187, TRP104, TYR169, ASP108, VAL109, and ASN105, as well as alkyl interactions with VAL325 and PHE303, and pi-sigma interactions with PHE329. The β-phellandrene showed van der Waals interactions with ILE164, ASN105, PHE187, VAL189, TYR307, VAL325, and VAL189, as well as alkyl interactions with TYR169, VAL109, PHE329, and PHE303. The o-cymene showed van der Waals interactions with VAL325, PHE303, ARG317, TYR307, ASP108, ASN105, and TRP104, pi-sigma interactions with PHE329, and pi-pi stacked interactions with PHE329. The limonene showed van der Waals interactions with PHE44, PRO340, TYR345, VAL49, LEU48, PHE354, GLY45, and THR337, as well as alkyl interactions with LEU341 and ALA41 (Figure 4).
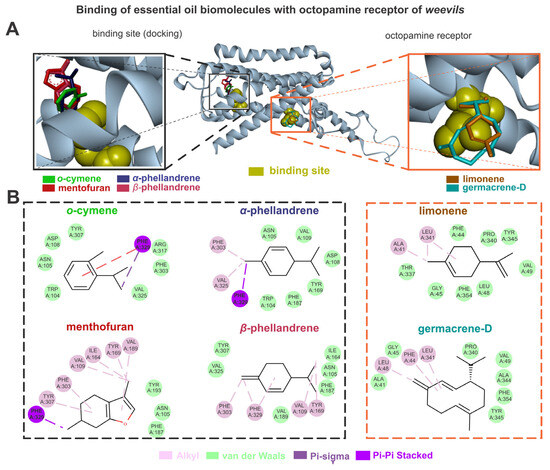
Figure 4.
The limonene, o-cymene, α-phellandrene, β-phellandrene, germacrene D, and menthofuran bind with octopamine sites of weevils (A); the molecular interactions of the same constituents with the amino acids from octopamine active sites are also shown (B).
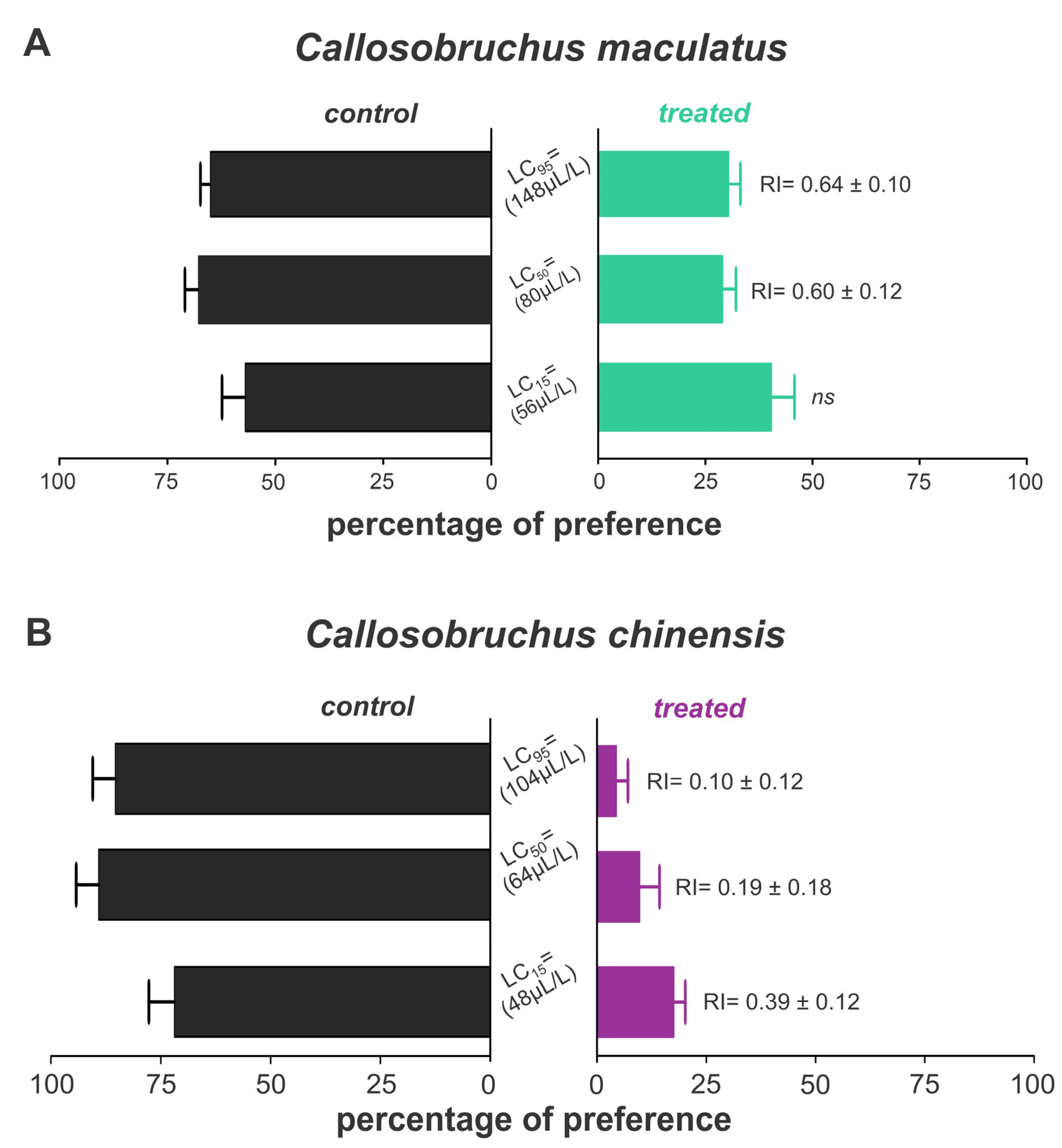
3.3. Repellency of Bursera graveolens Essential Oil to Adult Callosobruchus maculatus and Callosobruchus chinensis
The essential oils of B. graveolens exhibited repellent effects on both weevil species, with variations depending on species and concentration. Thus, C. maculatus was repelled when exposed only to lethal concentrations, with approximately 30% of insects in the treated grains (Figure 5A). On the other hand, we found 4.47, 9.78, and 17.61% of C. chinensis on the grains treated with LC15, LC25, and LC50, respectively, indicating a strong repellent activity against this species (Figure 5B).
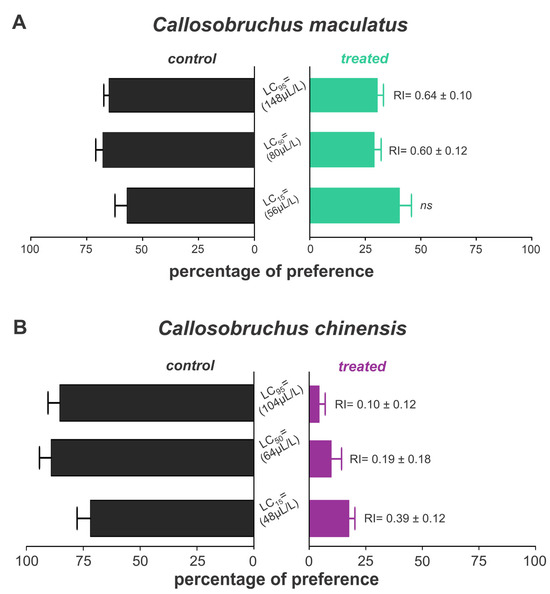
Figure 5.
Repellency of Bursera graveolens essential oil to adult Callosobruchus maculatus (A) and Callosobruchus chinensis (B) in cowpea beans. Bars represent the average (±SE) percentage of insects found after 24 h of exposure.
3.4. Ovicidal Effect of Bursera graveolens Essential Oil
The early-age eggs (24 h) from C. chinensis and C. maculatus were not susceptible to B. graveolens essential oil when applied as a fumigant. In both species, the emergence rate was between 50 and 70%, approximately, and no difference was found from the control in C. chinensis (F4,19 = 2.28; p = 0.108) and C. maculatus (F4,19 = 2.25; p = 0.111) (Table 2).

Table 2.
Percentage of egg hatching in Callosobruchus maculatus and Callosobruchus chinensis exposed at an early age (24 h) to estimated concentrations of Bursera graveolens essential oil.
4. Discussion
Our results suggest that B. graveolens essential oil may be effective in controlling two major cowpea storage pests, C. maculatus and C. chinensis. In silico analysis indicates that all compounds in B. graveolens essential oil (germacrene, limonene, α-phellandrene, menthofuran, β-phellandrene, and o-cymene) interact with both γ-aminobutyric acid (GABA) and octopamine receptors found in the weevils’ nervous system, with the sesquiterpene germacrene showing the strongest affinity. While no ovicidal effects were observed, the oil shows promise as both a curative treatment due to its toxicity and a preventive measure because of its repellent properties.
The B. graveolens essential oil was toxic to both Callosobruchus species, although the susceptibility rates differed. C. chinensis was more susceptible than C. maculatus. Similar susceptibility of C. chinensis to other biocontrol methods has been reported, including ozone [5], essential oils [48], microorganisms [49] and synthetic insecticides [50]. The differences in insect susceptibility to a particular compound can mainly be attributed to species-specific detoxification strategies when exposed to xenobiotics. Gupta et al. [51] demonstrated variation in detoxification activity (acetylcholine esterase and glutathione-S-transferase) in these same species when exposed to the same essential oil. Additionally, the toxicity of B. graveolens to weevils was linked to its major compounds, though the synergic effect of minor components should not be overlooked. Previous studies have highlighted the effectiveness of B. graveolens essential oil against stored-grain pests and other arthropods [16,29,33,52], as well as the toxicity of major compounds like limonene [53] or phellandrene [54,55] via contact or fumigant methods. However, due to the complexity of essential oils, which contain compounds with additive, antagonistic, or synergistic effects, the exact mode of action on target organisms remains uncertain. Therefore, individual analyses of the major compounds are necessary.
In silico molecular docking analysis revealed that the main compounds of B. graveolens, such as limonene, α-phellandrene, o-cymene, menthofuran, β-phellandrene, and germacrene, showed the highest binding affinity for both γ-aminobutyric acid (GABA) and octopamine receptors. However, the germacrene compound demonstrated greater structural stability in both receptors, enhancing hydrophobic interactions. The germacrene-GABA receptor complex exhibited stronger van der Waals interactions, indicating more contact points and increased stability with the GABA protein. This high affinity was also evident with the octopamine receptor, contributing to a strong hydrophobic complementarity with the protein [56]. Given that y-aminobutyric acid (GABA) receptors are critical for inhibitory neurotransmission in insects, and octopamine receptors serve as neurotransmitters, neuromodulators, or neurohormones in both central and peripheral nervous systems, even small changes in these receptors can trigger significant physiological events [57,58]. GABA receptors in insects are known targets of synthetic insecticides, classified as Group 2 molecules by IRAC [59]. This group is represented by phenylpyrazoles, cyclodienes, organochlorines, meta-diamides, and isoxazolines [60,61], while octopamine receptors are targeted by antagonists like amitraz (IRAC Group 19) and new molecules with potential insecticide such as Phenyl imidazolinium-2-one [62,63,64]. Synergism effects in insecticides targeting GABA or octopamine receptors have been documented [65,66], suggesting that similar synergistic interactions may occur with the organic compounds in essential oil. Essential oils have been reported to act on octopaminergic receptors in insects as agonists and/or antagonists, inducing intracellular changes in cAMP production [67,68]. Additionally, B. graveolens essential oils have been shown to disrupt the functions of acetylcholinesterase (AChE) enzymes and transient receptor potential (TRP) channels in insects [16]. Our in silico analyses support GABA and octopamine receptors as potential targets, mainly for germacrene.
Studies have shown that essential oils containing the sesquiterpene germacrene as a major compound are toxic and deterrent to insects, including stored-grain pests [69,70,71]. Although limonene and phellandrene have a lower affinity for GABA and octopamine receptors compared to germacrene, these compounds are predominant in the essential oil and likely contribute significantly to its toxicity. The toxicity of essential oils containing limonene or phellandrene, as well as pure compounds, has been demonstrated [72,73]. Recent studies have shown that essential oils rich in limonene and phellandrene inhibit enzymes such as glutathione-S-transferase, acetylcholinesterase, and Na+/K+-ATPase in C. chinensis and C. maculatus, among other insects [73,74,75]. Additionally, germacrene has been shown to increase Reactive Oxygen Species (ROS) and disrupt antioxidant defense systems in these species [76], as well as cause damage to other insects’ organs [77,78].
As a preventive measure against C. maculatus and C. chinensis colonization in stored cowpea beans, B. graveolens essential oil proved effective due to its repellent effect. While higher concentrations were more repellent to C. maculatus, C. chinensis was strongly repelled even at lower concentrations. Similar findings were reported by Jayaram et al. [79] with Tagetes minuta essential oil, which showed stronger deterrence for C. chinensis. However, it is already known that the repellent effect varies by organism type and/or concentration applied [14,80]. The repellent properties of B. graveolens essential oil against stored-grain weevils, including the common bean weevil, have been documented [16,31,33] with monoterpenes like limonene and phellandrene, primarily responsible for these effects. The repellent activity of these compounds was also observed by Cao, Pang, Guo, Wang, Geng, Sang, Guo, and Du [54], supporting our hypothesis, which was further confirmed through electroantennography studies showing insect olfactory responses to limonene [81,82]. Additionally, the germacrene compound, which exhibited a high affinity for GABA and octopamine receptors, also demonstrated strong repellent effects against other insects [83,84].
The B. graveolens essential oil was toxic and repellent to C. maculatus and C. chinensis; however, no ovicidal effects were observed at any of the applied concentrations. Previous studies on the fumigant effect of essential oils on Callosobruchus species eggs have yielded mixed results, with some reporting no effects, while others found 100% ovicidal activity on 24-hour-old eggs [45,85,86,87]. The effectiveness against eggs depends on the essential oil composition, concentration, and egg age, as demonstrated by Acanthoscelides obtectus (Say 1831) [88,89]. Based on our results, B. graveolens may be less effective at controlling these beetles when the grains are infested with early-stage eggs or may require prolonged exposure to affect embryo development.
5. Conclusions
Our results demonstrate the preventive and curative potential of Bursera graveolens essential oil against the two primary pests of stored cowpeas, Callosobruchus maculatus and C. chinensis. Given that the oil is extracted from the tree’s fruit with a high yield, it could provide a promising and sustainable solution for controlling these pests in tropical regions, especially in small-scale farming, where cowpea beans are widely cultivated. However, further research is needed to stabilize the product and conduct selectivity tests on mammals.
Author Contributions
Conceptualization, L.O.V., E.E.O., G.R.S. and R.W.S.A.; Methodology, L.O.V., J.M.G., E.V., M.J.G., P.B.S. and T.S.; Software, L.T.M.C., J.G.M.-A., O.M.H., J.M.G. and P.B.S.; Validation, L.O.V., E.E.O., G.R.S. and R.W.S.A.; Formal analysis, L.O.V., E.E.O., J.G.M.-A. and E.V.; Investigation, L.O.V., M.J.G., J.M.G., P.B.S., O.M.H., T.S. and L.T.M.C.; Resources, L.O.V., E.E.O., G.R.S. and R.W.S.A.; Data curation, J.M.G., M.J.G., P.B.S., O.M.H., T.S. and L.T.M.C.; Writing—original draft preparation, L.O.V., J.G.M.-A., M.J.G. and T.S.; Writing—review and editing, L.O.V., E.E.O. and E.V.; Visualization, L.O.V. and E.E.O.; Supervision, E.E.O., G.R.S. and R.W.S.A.; Project administration, E.E.O., G.R.S. and R.W.S.A.; Funding acquisition, E.E.O., G.R.S. and E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Council of Scientific and Technological Development (CNPq, 308576/2018-7, and 427304/2018-0 for EEO), the Coordination for the Improvement of Higher Education Personnel (CAPES; Finance code 001), and the Minas Gerais State Foundation for Research Aid (FAPEMIG, APQ-03771-18 for EEO).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest, and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Martins, S.P.; Nelson Silva, G.; de Sousa, A.H.; e Silva Barbosa, D.R.; da Silva, R.A.; Ducatti, K.R.; Donegá, M.A.; dos Santos, E.F.; Carvalho, M.S.; de Queiroz, M.V.B.M. Cowpea storage: Can small farmers use polyethylene terephthalate bottles and wood ash as an alternative to avoid damage caused by Callosobruchus maculatus? J. Stored Prod. Res. 2024, 106, 102301. [Google Scholar] [CrossRef]
- Nounagnon, M.; Roko, G.; Agbodjato, N.A.; Dah-Nouvlessounon, D.; Babalola, O.O.; Baba-Moussa, L. Cowpea (Vigna unguiculata). In Potential Pulses: Genetic and Genomic Resources; CABI: Wallingford, UK, 2024; pp. 58–77. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products; FAOSTAT: Rome, Italy, 2022. [Google Scholar]
- Kébé, K.; Alvarez, N.; Tuda, M.; Arnqvist, G.; Fox, C.W.; Sembène, M.; Espíndola, A. Global phylogeography of the insect pest Callosobruchus maculatus (Coleoptera: Bruchinae) relates to the history of its main host, Vigna unguiculata. J. Biogeogr. 2017, 44, 2515–2526. [Google Scholar] [CrossRef]
- Gad, H.A.; Abo Laban, G.F.; Metwaly, K.H.; Al-Anany, F.S.; Abdelgaleil, S.A.M. Efficacy of ozone for Callosobruchus maculatus and Callosobruchus chinensis control in cowpea seeds and its impact on seed quality. J. Stored Prod. Res. 2021, 92, 101786. [Google Scholar] [CrossRef]
- Beck, C.W.; Blumer, L.S. A Handbook on Bean Beetles, Callosobruchus maculatus; National Science Foundation: Alexandria, VA, USA, 2014; pp. 1–17. [Google Scholar]
- Hosamani, G.B.; Jagginavar, S.; Karabhantanal, S. Biology of pulse beetle Callosobruchus chinensis on different pulses. J. Entomol. Zool. Stud. 2018, 6, 1898–1900. [Google Scholar]
- Kang, J.K.; Pittendrigh, B.R.; Onstad, D.W. Insect resistance management for stored product pests: A case study of cowpea weevil (Coleoptera: Bruchidae). J. Econ. Entomol. 2013, 106, 2473–2490. [Google Scholar] [CrossRef]
- Hasan, M.M.; Reichmuth, C. Relative toxicity of phosphine against the bean bruchid Acanthoscelides obtectus (Say) (Col., Bruchidae). J. Appl. Entomol. 2004, 128, 332–336. [Google Scholar] [CrossRef]
- Aidbhavi, R.; Muralimohan, K.; Bandi, S.M. The status of resistance to phosphine in common bruchid species infesting edible stored pulses in India. J. Stored Prod. Res. 2023, 103, 102164. [Google Scholar] [CrossRef]
- Zongo, S.; Coulibaly, A.Y.; Drabo, S.F.; Gnankiné, O.; Kiendrebeogo, M.; Doumma, A.; Sembène, M.; Sanon, A. Metabolic resistance to pyrethroids (Py) and organophosphates (Op) in Callosobruchus maculatus (fab.) (Coleoptera: Chrysomelidae: Bruchinae) a major pest of stored cowpeas in West Africa. Int. J. Pest Manag. 2020, 67, 338–345. [Google Scholar] [CrossRef]
- Machuca-Mesa, L.M.; Turchen, L.M.; Guedes, R.N.C. Phosphine resistance among stored product insect pests: A global meta-analysis-based perspective. J. Pest Sci. 2023, 97, 1485–1498. [Google Scholar] [CrossRef]
- Krzyżowski, M.; Baran, B.; Łozowski, B.; Francikowski, J. The effect of Rosmarinus officinalis essential oil fumigation on biochemical, behavioral, and physiological parameters of Callosobruchus maculatus. Insects 2020, 11, 344. [Google Scholar] [CrossRef]
- Jumbo, L.O.V.; Faroni, L.R.; Oliveira, E.E.; Pimentel, M.A.; Silva, G.N. Potential use of clove and cinnamon essential oils to control the bean weevil, Acanthoscelides obtectus Say, in small storage units. Ind. Crops Prod. 2014, 56, 27–34. [Google Scholar] [CrossRef]
- Demeter, S.; Lebbe, O.; Hecq, F.; Nicolis, S.C.; Kenne Kemene, T.; Martin, H.; Fauconnier, M.-L.; Hance, T. Insecticidal activity of 25 essential oils on the stored product pest, Sitophilus granarius. Foods 2021, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Jumbo, L.O.V.; Corrêa, M.J.M.; Gomes, J.M.; Armijos, M.J.G.; Valarezo, E.; Mantilla-Afanador, J.G.; Machado, F.P.; Rocha, L.; Aguiar, R.W.S.; Oliveira, E.E. Potential of Bursera graveolens essential oil for controlling bean weevil infestations: Toxicity, repellence, and action targets. Ind. Crops Prod. 2022, 178, 114611. [Google Scholar] [CrossRef]
- Hategekimana, A.; Erler, F. Fecundity and fertility inhibition effects of some plant essential oils and their major components against Acanthoscelides obtectus Say (Coleoptera: Bruchidae). J. Plant Dis. Prot. 2020, 127, 615–623. [Google Scholar] [CrossRef]
- Kanda, D.; Kaur, S.; Koul, O. A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: Acute toxins or feeding deterrents. J. Pest Sci. 2017, 90, 531–545. [Google Scholar] [CrossRef]
- Viteri Jumbo, L.O.; Haddi, K.; Faroni, L.R.D.; Heleno, F.F.; Pinto, F.G.; Oliveira, E.E. Toxicity to, oviposition and population growth impairments of Callosobruchus maculatus exposed to clove and cinnamon essential oils. PLoS ONE 2018, 13, e0207618. [Google Scholar] [CrossRef]
- Massango, H.G.L.L.; Faroni, L.R.A.; Haddi, K.; Heleno, F.F.; Viteri Jumbo, L.O.; Oliveira, E.E. Toxicity and metabolic mechanisms underlying the insecticidal activity of parsley essential oil on bean weevil, Callosobruchus maculatus. J. Pest Sci. 2017, 90, 723–733. [Google Scholar] [CrossRef]
- Costa, L.T.M.; Smagghe, G.; Viteri Jumbo, L.O.; Santos, G.R.; Aguiar, R.W.S.; Oliveira, E.E. Selective Actions of Plant-Based Biorational Insecticides: Molecular Mechanisms and Reduced Risks to Non-Target Organisms. Curr. Opin. Environ. Sci. Health. 2025, 44, 100601. [Google Scholar] [CrossRef]
- Haddi, K.; Turchen, L.M.; Viteri Jumbo, L.O.; Guedes, R.N.; Pereira, E.J.; Aguiar, R.W.; Oliveira, E.E. Rethinking biorational insecticides for pest management: Unintended effects and consequences. Pest Manag. Sci. 2020, 76, 2286–2293. [Google Scholar] [CrossRef]
- Corrêa, E.J.A.; Carvalho, F.C.; de Castro Oliveira, J.A.; Bertolucci, S.K.V.; Scotti, M.T.; Silveira, C.H.; Guedes, F.C.; Melo, J.O.F.; de Melo-Minardi, R.C.; de Lima, L.H.F. Elucidating the molecular mechanisms of essential oils’ insecticidal action using a novel cheminformatics protocol. Sci. Rep. 2023, 13, 4598. [Google Scholar] [CrossRef]
- Toledo, P.F.S.; Viteri Jumbo, L.O.; Rezende, S.M.; Haddi, K.; Silva, B.A.; Mello, T.S.; Della Lucia, T.M.C.; Aguiar, R.W.S.; Smagghe, G.; Oliveira, E.E. Disentangling the ecotoxicological selectivity of clove essential oil against aphids and non-target ladybeetles. Sci. Total Environ. 2020, 718, 137328. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Araujo, S.H.; Mantilla-Afanador, J.G.; Svacina, T.; Nascimento, T.F.; Da Silva Lima, A.; Camara, M.B.P.; Viteri Jumbo, L.O.; Dos Santos, G.R.; Da Rocha, C.Q.; De Oliveira, E.E. Contributions of γ-Aminobutyric Acid (GABA) Receptors for the activities of Pectis brevipedunculata essential oil against Drosophila suzukii and pollinator bees. Plants 2024, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Toledo, P.F.S.; Ferreira, T.P.; Bastos, I.M.A.S.; Rezende, S.M.; Viteri Jumbo, L.O.; Didonet, J.; Andrade, B.S.; Melo, T.S.; Smagghe, G.; Oliveira, E.E.; et al. Essential oil from Negramina (Siparuna guianensis) plants controls aphids without impairing survival and predatory abilities of non-target ladybeetles. Environ. Pollut. 2019, 255, 113153. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, C.; Imayoshi, Y.; Iwabuchi, H.; Komemushi, S.; Sawabe, A. Chemical composition of three extracts of Bursera graveolens. Flavour Fragr. J. 2006, 21, 234–238. [Google Scholar] [CrossRef]
- Young, D.G.; Chao, S.; Casablanca, H.; Bertrand, M.-C.; Minga, D. Essential oil of Bursera graveolens (Kunth) triana et planch from Ecuador. J. Essent. Oil Res. 2007, 19, 525–526. [Google Scholar] [CrossRef]
- Rey-Valeirón, C.; Guzmán, L.; Saa, L.R.; López-Vargas, J.; Valarezo, E. Acaricidal activity of essential oils of Bursera graveolens (Kunth) Triana & Planch and Schinus molle L. on unengorged larvae of cattle tick Rhipicephalus (Boophilus) microplus (Acari:Ixodidae). J. Essent. Oil Res. 2017, 29, 344–350. [Google Scholar] [CrossRef]
- Monzote, L.; Hill, G.M.; Cuellar, A.; Scull, R.; Setzer, W.N. Chemical composition and anti-proliferative properties of Bursera graveolens essential oil. Nat. Prod. Commun. 2012, 7, 1934578X1200701130. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Yepes-Fuentes, L.; Tirado-Ballestas, I.; Orozco, M. Actividad Repelente del aceite esencial de Bursera graveolens Jacq. ex L., frente Tribolium castaneum Herbst, 1797 (Coleoptera: Tenebrionidae). An. Biol. 2018, 40, 87–93. [Google Scholar] [CrossRef]
- Leyva, M.; del Carmen Marquetti, M.; Montada, D.; Payroll, J.; Scull, R.; Morejón, G.; Pino, O. Aceites esenciales de Eucalyptus globulus (Labill) y Bursera graveolens (Kunth) Triana & Planch para el control de mosquitos de importancia médica. Biologist 2020, 18, 239–250. [Google Scholar] [CrossRef]
- Jaramillo-Colorado, B.E.; Suarez-López, S.; Marrugo-Santander, V. Volatile chemical composition of essential oil from Bursera graveolens (Kunth) Triana & Planch and their fumigant and repellent activities. Acta Sci. Biol. Sci. 2019, 41, 46822. [Google Scholar] [CrossRef]
- Ndomo, A.; Tapondjou, L.; Ngamo, L.; Hance, T. Insecticidal activities of essential oil of Callistemon viminalis applied as fumigant and powder against two bruchids. J. Appl. Entomol. 2010, 134, 333–341. [Google Scholar] [CrossRef]
- Zapata, N.; Smagghe, G. Repellency and toxicity of essential oils from the leaves and bark of Laurelia sempervirens and Drimys winteri against Tribolium castaneum. Ind. Crops Prod. 2010, 32, 405–410. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Haas, J.; Barbato, A.; Behringer, D.; Studer, G.; Roth, S.; Bertoni, M.; Mostaguir, K.; Gumienny, R.; Schwede, T. Continuous Automated Model EvaluatiOn (CAMEO) complementing the critical assessment of structure prediction in CASP12. Proteins Struct. Funct. Bioinf. 2018, 86, 387–398. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinf. 2009, 77, 114–122. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Freitas, R.C.P.; Faroni, L.R.D.A.; Haddi, K.; Jumbo, L.O.V.; Oliveira, E.E. Allyl isothiocyanate actions on populations of Sitophilus zeamais resistant to phosphine: Toxicity, emergence inhibition and repellency. J. Stored Prod. Res. 2016, 69, 257–264. [Google Scholar] [CrossRef]
- Mazzonetto, F.; Vendramim, J.D. Efeito de pós de origem vegetal sobre Acanthoscelides obtectus (Say)(Coleoptera: Bruchidae) em feijão armazenado. Neotrop. Entomol. 2003, 32, 145–149. [Google Scholar] [CrossRef]
- Nattudurai, G.; Baskar, K.; Paulraj, M.G.; Islam, V.I.H.; Ignacimuthu, S.; Duraipandiyan, V. Toxic effect of Atalantia monophylla essential oil on Callosobruchus maculatus and Sitophilus oryzae. Environ. Sci. Pollut. Res. 2017, 24, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- SAS; Version 9.1; SAS Institute Inc.: Cary, NC, USA, 2003.
- Robertson, J.L.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- El-Sawaha, M.H.; Raddyb, H.M.; Gadb, H.A.; Fahmya, H.H. Chemical composition and insecticidal activities of Origanum majorana L. essential oil nanoemulsion against Callosobruchus maculatus and Callosobruchus chinensis. Egypt. J. Chem. 2024, 67, 371–381. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Gad, H.A.; Hamza, A.F.; Al-Anany, M.S. Insecticidal efficacy of two inert dusts and Trichoderma harzianum, applied alone or in combination, against Callosobruchus maculatus and Callosobruchus chinensis on stored cowpea seeds. Crop Prot. 2021, 146, 105656. [Google Scholar] [CrossRef]
- Hasan, M.; Reichmuth, C. Phosphine tolerance in two bruchid beetles, Callosobruchus chinensis (L.) and C. maculatus (F.) (Coleoptera: Bruchidae). In Advances in Stored Product Protection, Proceedings of the 8th International Working Conference on Stored Product Protection, York, UK, 22–26 July 2002; CABI: Wallingford, UK, 2003; pp. 656–661. [Google Scholar]
- Gupta, H.; Deeksha; Urvashi; Reddy, S.G.E. Insecticidal and detoxification enzyme inhibition activities of essential oils for the control of pulse beetle, Callosobruchus maculatus (F.) and Callosobruchus chinensis (L.) (Coleoptera: Bruchidae). Molecules 2023, 28, 492. [Google Scholar] [CrossRef]
- Parichanon, P.; Ascrizzi, R.; Tani, C.; Echeverria, M.C.; Andrade, S.O.; Paredes, H.; Taglieri, I.; Flamini, G.; Venturi, F.; Conti, B. Chemical Profiling, Sensory Qualities, and Bioactivities of Essential Oils Obtained from Aloysia citrodora and Bursera graveolens Ecuadorian Plants Against the Mosquito Aedes albopictus (Skuse) (Diptera: Culicidae). Insects 2025, 16, 202. [Google Scholar] [CrossRef]
- Liang, J.-Y.; An, Y.; Hou, Z.-B.; Wang, X.-D.; Zhou, F.; Zhang, J.; Wang, J.-L. Acute toxicity of Zanthoxylum bungeanum against two stored product insects and synergistic interactions between two major compounds limonene and linalool. J. Environ. Sci. Health B 2022, 57, 739–744. [Google Scholar] [CrossRef]
- Cao, J.-Q.; Pang, X.; Guo, S.-S.; Wang, Y.; Geng, Z.-F.; Sang, Y.-L.; Guo, P.-J.; Du, S.-S. Pinene-rich essential oils from Haplophyllum dauricum (L.) G. Don display anti-insect activity on two stored-product insects. Int. Biodeterior. Biodegrad. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Cheng, Z.; Jiang, J.; Yang, X.; Chu, H.; Jin, M.; Li, Y.; Tao, X.; Wang, S.; Huang, Y.; Shang, L.; et al. The research of genetic toxicity of β-phellandrene. Environ. Toxicol. Pharmacol. 2017, 54, 28–33. [Google Scholar] [CrossRef]
- Podlewska, S.; Bojarski, A.J. Chapter 3—Post-processing of Docking Results: Tools and Strategies. In Molecular Docking for Computer-Aided Drug Design, Coumar, M.S., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 57–74. [Google Scholar]
- Farooqui, T. Review of octopamine in insect nervous systems. Open Access Insect Physiol. 2012, 4, 1–17. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Z.; Ma, H.; Zheng, W.; Liu, J.; Zhou, Y.; Man, Y.; Zhou, X.; Zeng, A. Pharmacological Properties and Function of the PxOctβ3 Octopamine Receptor in Plutella xylostella (L.). Insects 2022, 13, 735. [Google Scholar] [CrossRef] [PubMed]
- Salgado, V. BASF Insecticide Mode of Action Technical Training Manual; BASF Corporation: Ludwigshafen, Germany, 2013. [Google Scholar]
- Zhou, T.; Wu, W.; Ma, S.; Chen, J.; Huang, J.; Qiao, X. Effects of RDL GABA Receptor Point Mutants on Susceptibility to Meta-Diamide and Isoxazoline Insecticides in Drosophila melanogaster. Insects 2024, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Zhou, T.; Zhang, J.; Zhang, L.; Lu, Y.; Huang, J. Functional validation of A2′N mutation of the RDL GABA receptor against fipronil via molecular modeling and genome engineering in Drosophila. Pest Manag. Sci. 2024, 80, 1924–1929. [Google Scholar] [CrossRef]
- Kita, T.; Hayashi, T.; Ohtani, T.; Takao, H.; Takasu, H.; Liu, G.; Ohta, H.; Ozoe, F.; Ozoe, Y. Amitraz and its metabolite differentially activate α- and β-adrenergic-like octopamine receptors. Pest Manag. Sci. 2017, 73, 984–990. [Google Scholar] [CrossRef]
- Guo, L.; Fan, X.-y.; Qiao, X.; Montell, C.; Huang, J. An octopamine receptor confers selective toxicity of amitraz on honeybees and Varroa mites. eLife 2021, 10, e68268. [Google Scholar] [CrossRef]
- Deng, X.-L.; Guo, L.; Ma, H.-H.; Hu, X.-P.; Zhou, X.-M. Phenyl imidazolidin-2-ones antagonize a β-adrenergic-like octopamine receptor in diamondback moth (Plutella xylostella). Pest Manag. Sci. 2021, 77, 3224–3232. [Google Scholar] [CrossRef]
- Ahmed, M.A.I.; Vogel, C.F.A. The role of octopamine receptor agonists in the synergistic toxicity of certain insect growth regulators (IGRs) in controlling dengue vector Aedes aegypti (Diptera: Culicidae) mosquito. Acta Trop. 2016, 155, 1–5. [Google Scholar] [CrossRef]
- Xie, N.; Gross, A.D. Muscarinic acetylcholine receptor activation synergizes the knockdown and toxicity of GABA-gated chloride channel insecticides. Pest Manag. Sci. 2022, 78, 4599–4607. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Ohta, H.; Ozoe, Y. Chapter Two—Molecular Signalling, Pharmacology, and Physiology of Octopamine and Tyramine Receptors as Potential Insect Pest Control Targets. In Advances in Insect Physiology; Cohen, E., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 46, pp. 73–166. [Google Scholar]
- Pavela, R.; Govindarajan, M. The essential oil from Zanthoxylum monophyllum a potential mosquito larvicide with low toxicity to the non-target fish Gambusia affinis. J. Pest Sci. 2017, 90, 369–378. [Google Scholar] [CrossRef]
- Vaglica, A.; Peri, E.; Badalamenti, N.; Ilardi, V.; Bruno, M.; Guarino, S. Chemical composition and evaluation of insecticidal activity of Seseli bocconei essential oils against stored products pests. Plants 2022, 11, 3047. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.N.d.L.; Da Silva, M.F.R.; Da Silva, P.C.B.; De Lira Pimentel, C.S.; Lino Da Rocha, S.K.; Farias De Aguiar, J.C.R.d.O.; Neto, A.C.A.; Paiva, P.M.G.; Gomes, M.G.M.; Da Silva-Júnior, E.F.; et al. Oviposition deterrence, larvicidal activity and docking of β-germacrene-D-4-ol obtained from leaves of Piper corcovadensis (Piperaceae) against Aedes aegypti. Ind. Crops Prod. 2022, 182, 114830. [Google Scholar] [CrossRef]
- Durán Aguirre, C.E.; Pratissoli, D.; Damascena, A.P.; Romário de Carvalho, J.; de Araujo Junior, L.M. Lethal and sublethal effects of Citrus aurantium and Citrus sinensis essential oils and their major component limonene on Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Essent. Oil-Bear. Plants 2024, 27, 838–848. [Google Scholar] [CrossRef]
- Gupta, H.; Singh, P.P.; Reddy, S.G.E. Exploring the chemical profiling and insecticidal properties of essential oils from fresh and discarded lemon peels, Citrus limon against pulse beetle. Int. Biodeter. Biodegr. 2025, 196, 105924. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Badawy, M.E.I.; Mahmoud, N.F.; Marei, A.E.-S.M. Acaricidal activity, biochemical effects and molecular docking of some monoterpenes against two-spotted spider mite (Tetranychus urticae Koch). Pestic. Biochem. Physiol. 2019, 156, 105–115. [Google Scholar] [CrossRef]
- Nenaah, G.E.; Almadiy, A.A.; Al-Assiuty, B.A.; Mahnashi, M.H. The essential oil of and its nanoemulsion and isolated monoterpenes: Investigation of their activity against with insights into the adverse effects on non-target organisms. Pest Manag. Sci. 2022, 78, 1035–1047. [Google Scholar] [CrossRef]
- Kiran, S.; Kujur, A.; Patel, L.; Ramalakshmi, K.; Prakash, B. Assessment of toxicity and biochemical mechanisms underlying the insecticidal activity of chemically characterized Boswellia carterii essential oil against insect pest of legume seeds. Pestic. Biochem. Physiol. 2017, 139, 17–23. [Google Scholar] [CrossRef]
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Silva, M.A.N.; Deschamps, C.; Molento, M.B. Insecticide activity of Curcuma longa (leaves) essential oil and its major compound α-phellandrene against Lucilia cuprina larvae (Diptera: Calliphoridae): Histological and ultrastructural biomarkers assessment. Pestic. Biochem. Physiol. 2019, 153, 17–27. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.X.; Song, B. Pesticidal Activity and Mode of Action of Monoterpenes. J. Agric. Food Chem. 2022, 70, 4556–4571. [Google Scholar] [CrossRef]
- Jayaram, C.S.; Chauhan, N.; Dolma, S.K.; Reddy, S.G.E. Chemical composition and insecticidal activities of essential oils against the pulse beetle. Molecules 2022, 27, 568. [Google Scholar] [CrossRef]
- González Armijos, M.J.; Viteri Jumbo, L.; D’Antonino Faroni, L.R.; Oliveira, E.E.; Flores, A.F.; Haddi, K. Fumigant toxicity of eugenol and its negative effects on biological development of Callosobruchus maculatus L. Rev. Cienc. Agric. 2019, 36, 5–15. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schütz, S.; de Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Peng, Y.; Zhao, X.; Yue, S.; Huang, Y.; Cao, H. Identification of odorant receptors of Tribolium confusum in response to limonene repellent activity. Pestic. Biochem. Physiol. 2023, 195, 105555. [Google Scholar] [CrossRef] [PubMed]
- Ashitani, T.; Garboui, S.S.; Schubert, F.; Vongsombath, C.; Liblikas, I.; Pålsson, K.; Borg-Karlson, A.K. Activity studies of sesquiterpene oxides and sulfides from the plant Hyptis suaveolens (Lamiaceae) and its repellency on Ixodes ricinus (Acari: Ixodidae). Exp. Appl. Acarol. 2015, 67, 595–606. [Google Scholar] [CrossRef]
- Liakakou, A.; Angelis, A.; Papachristos, D.P.; Fokialakis, N.; Michaelakis, A.; Skaltsounis, L.A. Isolation of Volatile Compounds with Repellent Properties against Aedes albopictus (Diptera: Culicidae) Using CPC Technology. Molecules 2021, 26, 3072. [Google Scholar] [CrossRef]
- Vojoudi, S.; Esmaili, M.; Farrokhi, M.; Saber, M. Acute toxicity of kaolin and essential oils from Mentha pulegium and Zingiber officinale against different stages of Callosobruchus maculatus under laboratory conditions. Arch. Phytopathol. Plant Prot. 2014, 47, 285–291. [Google Scholar] [CrossRef]
- Kéı̈ta, S.M.; Vincent, C.; Schmit, J.-P.; Ramaswamy, S.; Bélanger, A. Effect of various essential oils on Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2000, 36, 355–364. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdelbagi, O.; Ishag, A.; Hammad, A. Effects of garlic oils on the fecundity and hatchability of Callosobruchus maculatus L. (Coleoptera: Bruchidae). Univers. J. Agric. Res. 2019, 7, 63–68. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Stamopoulos, D.C. Fumigant toxicity of three essential oils on the eggs of Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2004, 40, 517–525. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, P.; Prakash, B.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of essential oils of Lippia alba (Mill.) N.E. Brown and Callistemon lanceolatus (Sm.) Sweet and their major constituents on mortality, oviposition and feeding behaviour of pulse beetle, Callosobruchus chinensis L. J. Sci. Food Agric. 2011, 91, 2277–2283. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).