The Insecticide Imidacloprid Promotes Algal Growth in Absence of Zooplankton
Abstract
1. Introduction
2. Materials and Methods
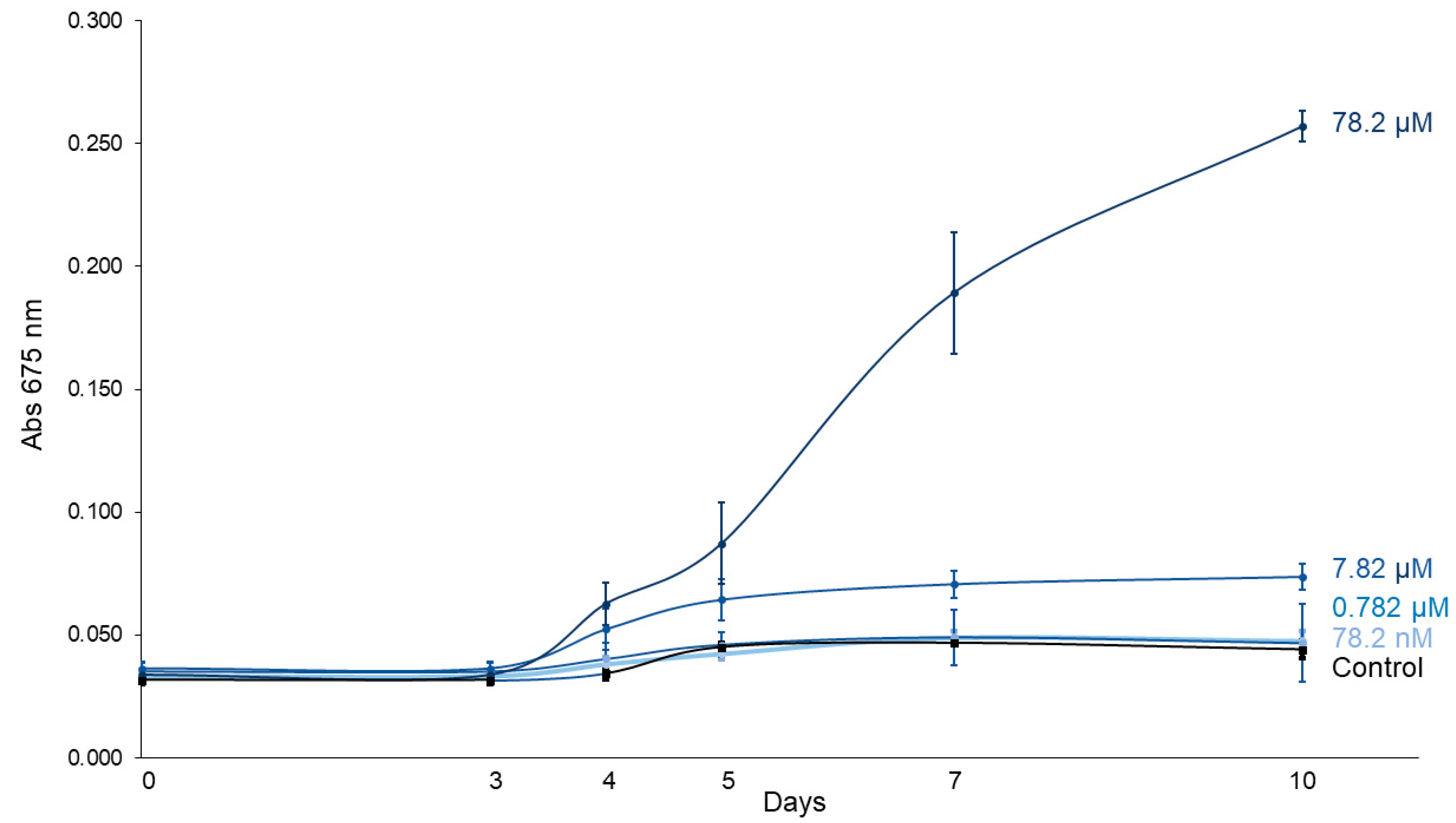
2.1. Assay 1: C. vulgaris Growth Under Different Imidacloprid Concentrations
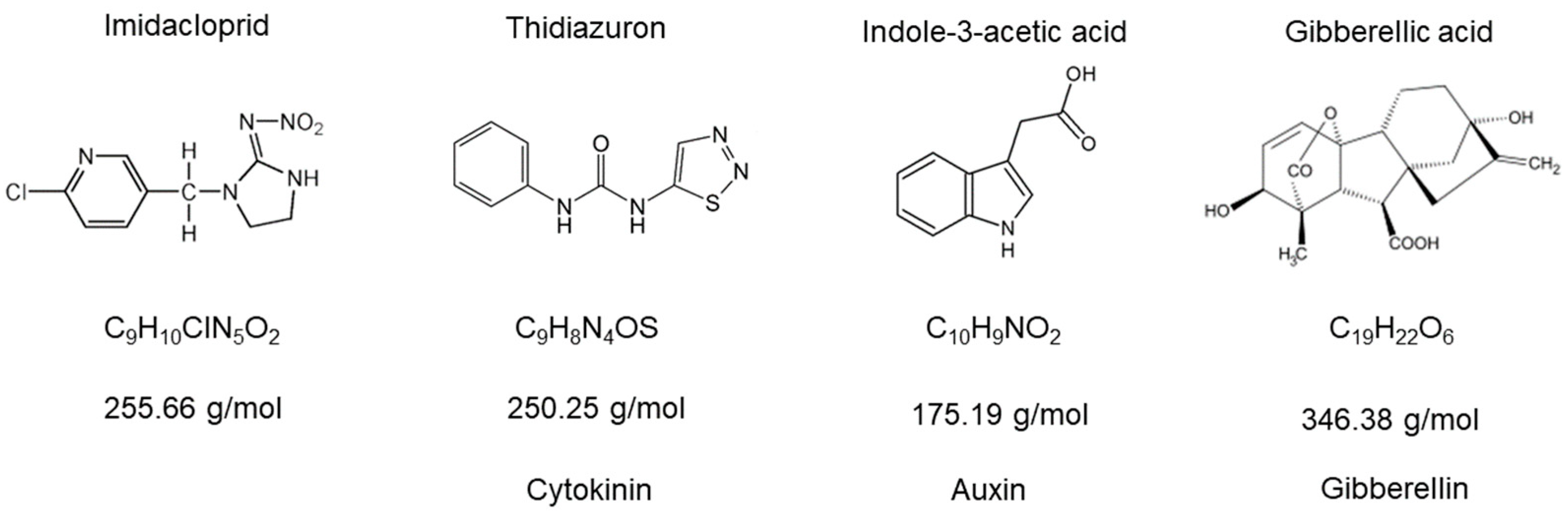
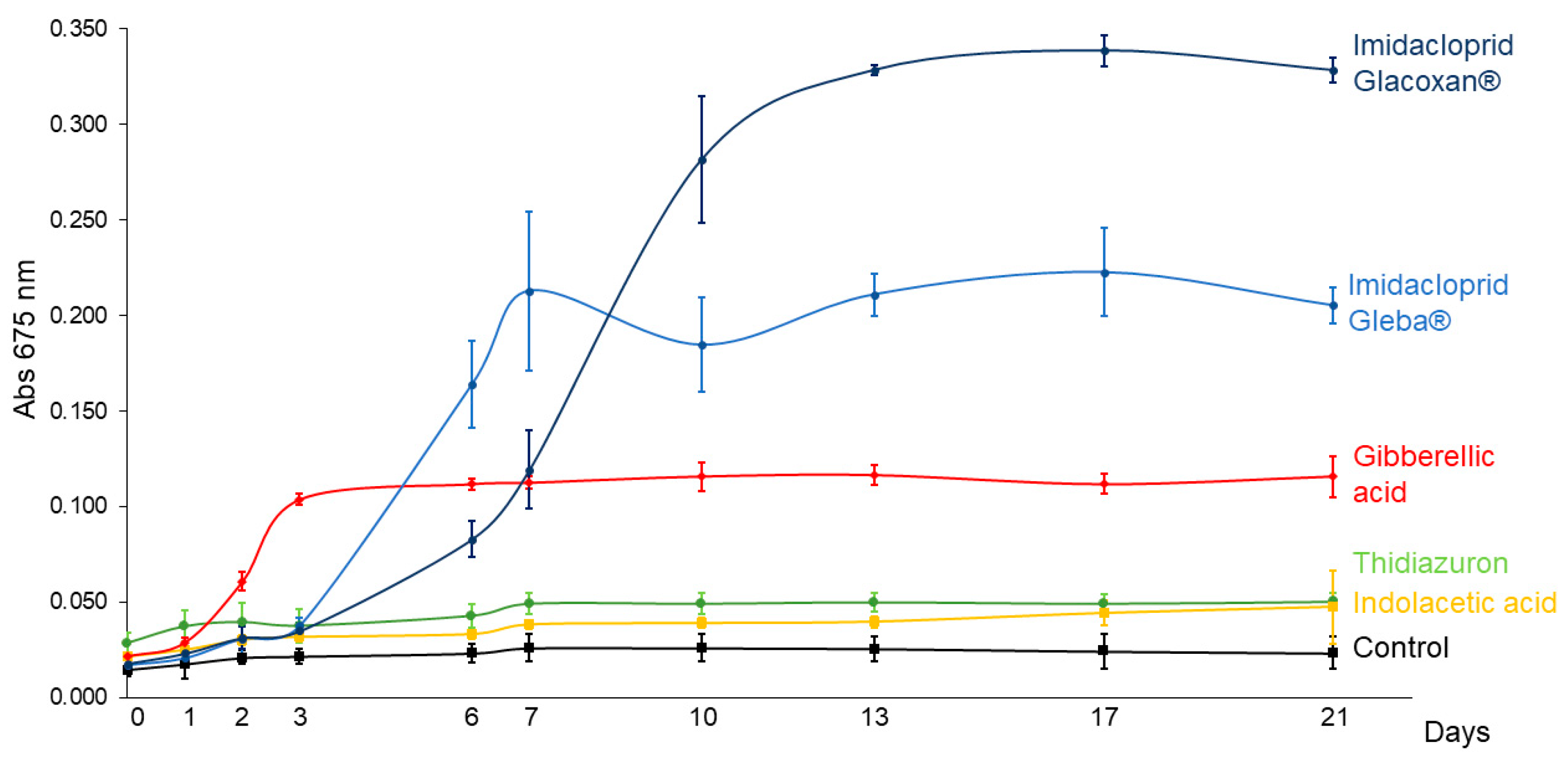
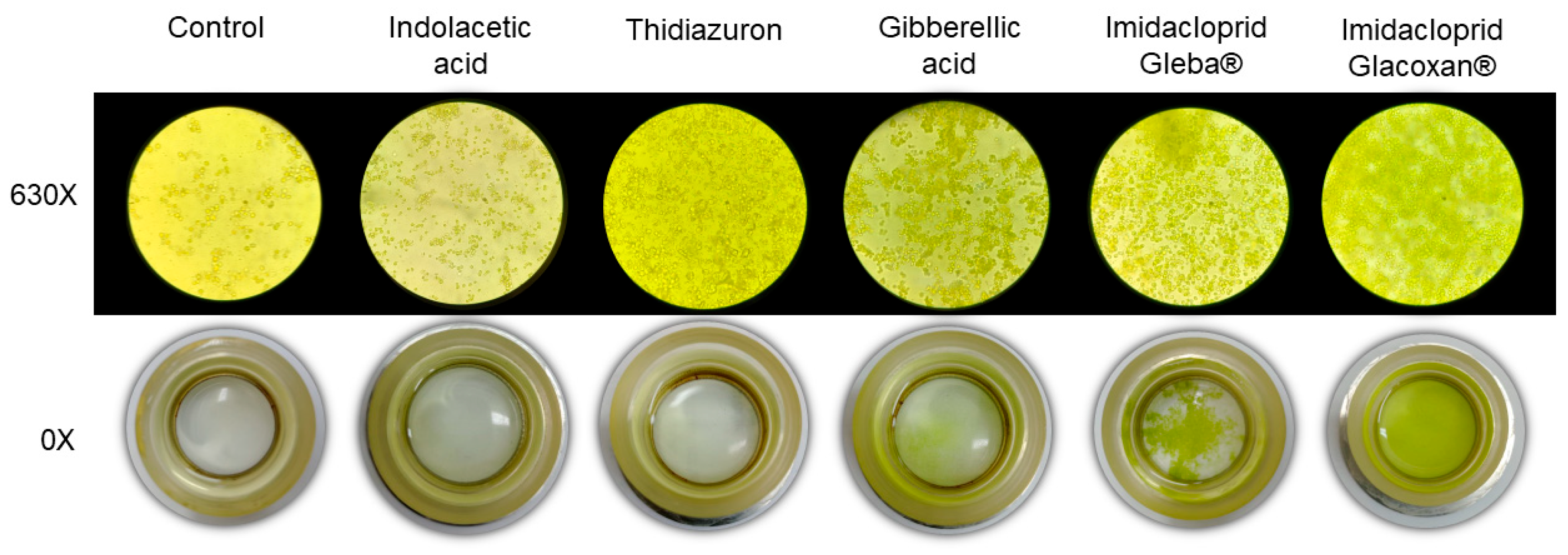
2.2. Assay 2: C. vulgaris Growth Under Imidacloprid and Plant Hormones Treatments
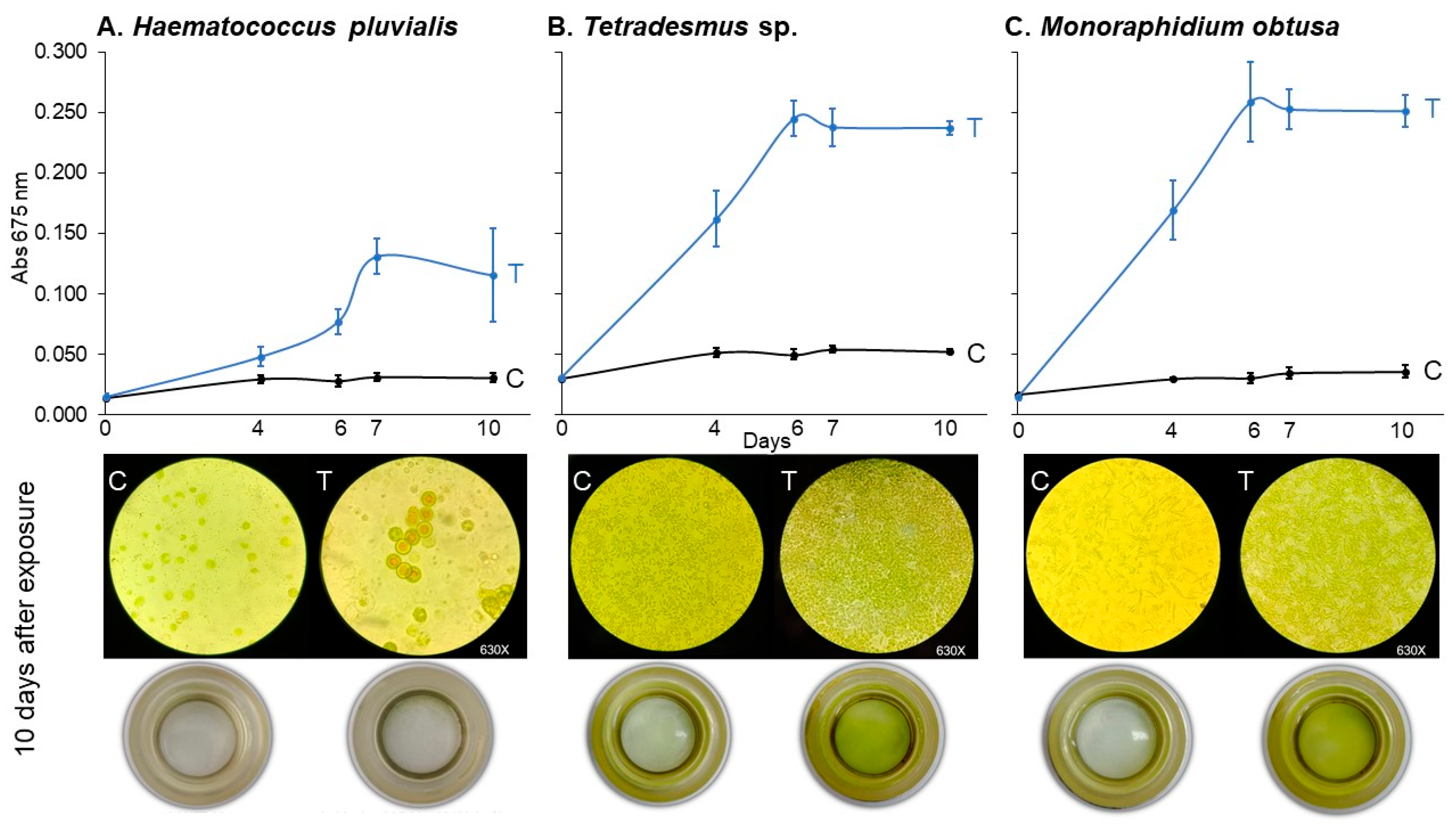
2.3. Assay 3: Growth Curves of Haematococcus pluvialis, Tetrademus sp., and Monoraphidium obtusa Under 7.82 μM of Imidacloprid Gleba Treatment
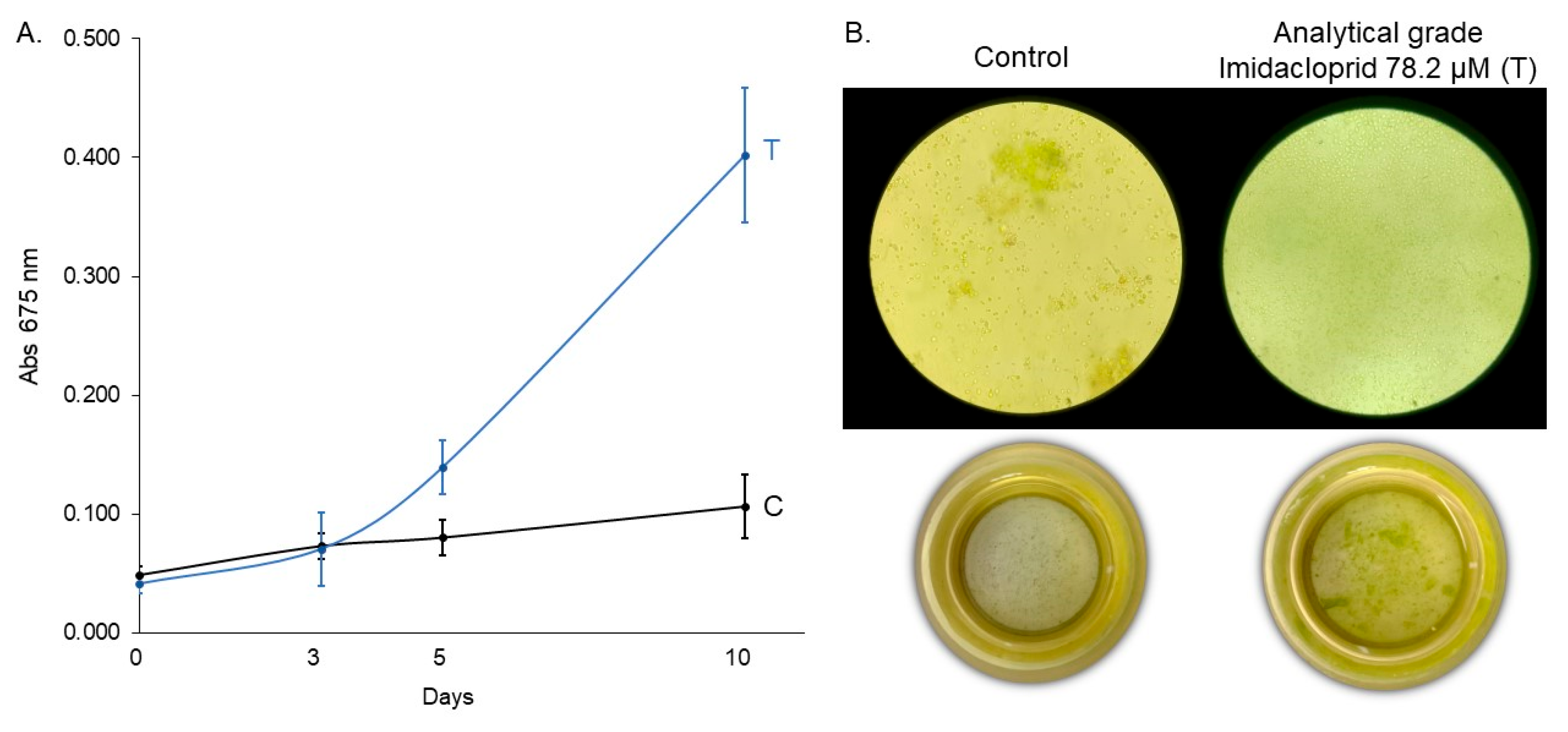
2.4. Assay 4: Growth Response of C. vulgaris to Analytical Grade Imidacloprid
3. Results
3.1. C. vulgaris Growth Under Different Imidacloprid Concentrations (Assay 1)
3.2. C. vulgaris Growth Under Imidacloprid Formulations and Plant Hormones Treatments (Assay 2)
3.3. Growth Curves of Haematococcus pluvialis, Tetradesmus sp., and Monoraphidium obtusa Under 78.2 μM of Imidacloprid Gleba® Treatment (Assay 3)
3.4. Growth Response of C. vulgaris to Analytical-Grade Imidacloprid (Assay 4)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheets, L.P. Imidacloprid: A neonicotinoid insecticide. In Hayes’ Handbook of Pesticide Toxicology; Academic Press: New York, NY, USA, 2010; pp. 2055–2064. [Google Scholar]
- Senn, R.; Hofer, D.; Thieme, T.; Zang, L. Method for Improving Plant Growth. U.S. Patent No. 6,753,296, 22 June 2004. [Google Scholar]
- Gonias, E.D.; Oosterhuis, D.M.; Bibi, A.C. How the insecticide Trimax TM improves the growth and yield of cotton. In Proceedings of the Beltwide Cotton Conference, San Antonio, TX, USA, 3–6 January 2006. [Google Scholar]
- Ford, K.A.; Casida, J.E.; Chandran, D.; Gulevich, A.G.; Okrent, R.A.; Durkin, K.A.; Bunnelle, E.M.; Wildermuth, M.C. Neonicotinoid insecticides induce salicylate-associated plant defense responses. Proc. Natl. Acad. Sci. USA 2010, 107, 17527–17532. [Google Scholar] [CrossRef]
- Awad, N.; Vega-Estévez, S.; Griffiths, G. Salicylic acid and aspirin stimulate growth of Chlamydomonas and inhibit lipoxygenase and chloroplast desaturase pathways. Plant Physiol. Biochem. 2020, 149, 256–265. [Google Scholar] [CrossRef]
- Fu, L.; Li, Q.; Chen, C.; Zhang, Y.; Liu, Y.; Xu, L.; Zhou, Y.; Li, C.; Zhou, D.; Rittmann, B.E. Benzoic and salicylic acid are the signaling molecules of Chlorella cells for improving cell growth. Chemosphere 2021, 265, 129084. [Google Scholar] [CrossRef]
- Lukaszewicz, G.; Iturburu, F.G.; Garanzini, D.S.; Menone, M.L.; Pflugmacher, S. Imidacloprid modifies the mitotic kinetics and causes both aneugenic and clastogenic effects in the macrophyte Bidens laevis L. Heliyon 2019, 5, e02118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, H.; Deng, Z.; Wang, J.; Liu, Z.; Chen, Y.; Ma, Y.; Li, B.; Yang, L.; Zhang, Z.; et al. Efficient removal of Imidacloprid and nutrients by algae-bacteria biofilm reactor (ABBR) in municipal wastewater: Performance, mechanisms and the importance of illumination. Chemosphere 2022, 305, 135418. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhu, J.; Yang, L.; Zhang, Z.; Li, B.; Xia, L.; Wu, L. Microalgae fuel cells enhanced biodegradation of imidacloprid by Chlorella sp. Biochem. Eng. J. 2022, 179, 108327. [Google Scholar]
- Van Dijk, T.C.; Van Staalduinen, M.A.; Van der Sluijs, J.P. Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS ONE 2013, 8, e62374. [Google Scholar] [CrossRef] [PubMed]
- Malev, O.; Klobučar, R.S.; Fabbretti, E.; Trebše, P. Comparative toxicity of imidacloprid and its transformation product 6-chloronicotinic acid to non-target aquatic organisms: Microalgae Desmodesmus subspicatus and amphipod Gammarus fossarum. Pestic. Biochem. Physiol. 2012, 104, 178–186. [Google Scholar] [CrossRef]
- Tišler, T.; Jemec, A.; Mozetič, B.; Trebše, P. Hazard identification of imidacloprid to aquatic environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Encarnação, T.; Santos, D.; Ferreira, S.; Valente, A.J.; Pereira, J.C.; Campos, M.G.; Burrows, H.D.; Pais, A.A. Removal of imidacloprid from water by microalgae Nannochloropsis sp. and its determination by a validated RP-HPLC method. Bull. Environ. Contam. Toxicol. 2021, 107, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, Z.; Wang, J.; Wu, L. Study on Efficient Removal of Imidacloprid and Nutrients from Sewage by Chlorella sp. Environ. Sci. Technol. 2022, 45, 10036504. [Google Scholar]
- Li, D.; Li, J.; Yeerhazi, B.; Cheng, Y. Efficient removal of imidacloprid from sewage by Scenedesmus sp. TXH and the effects of environmental factors on its removal. J. Chem. Technol. Biotechnol. 2023, 98, 1014–1024. [Google Scholar] [CrossRef]
- Reyad, A.G.; Abbassy, M.A.; Marei, G.I.K.; Rabea, E.I.; Badawy, M.E. Removal of fenamiphos, imidacloprid, and oxamyl pesticides from water by microalgal Nannochloropsis oculata biomass and their determination by validated HPLC method. J. Environ. Sci. Health Part B 2023, 58, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Ördög, V.; Van Staden, J.; Jäger, K. Cytokinin-and auxin-like activity in Cyanophyta and microalgae. J. Appl. Phycol. 2002, 14, 215–221. [Google Scholar] [CrossRef]
- Pinto, S.; Tajeshwar, N.; Gordon, K.; Borrero, P.; Novák, O.; Strnad, M.; Foellmer, M.; Heyl, A. Cytokinin Response of the Streptophyte Alga Coleochaete scutata provides a clue to the evolution of cytokinin signaling. Front. Plant Physiol. 2023, 1, 1275205. [Google Scholar] [CrossRef]
- Yao, K.S.; Li, D.; Lei, H.J.; Van den Brink, P.J.; Ying, G.G. Imidacloprid treatments induces cyanobacteria blooms in freshwater communities under sub-tropical conditions. Aquat. Toxicol. 2021, 240, 105992. [Google Scholar]
- Sumon, K.A.; Ritika, A.K.; Peeters, E.T.; Rashid, H.; Bosma, R.H.; Rahman, M.S.; Fatema, M.K.; Van den Brink, P.J. Effects of imidacloprid on the ecology of sub-tropical freshwater microcosms. Environ. Pollut. 2018, 236, 432–441. [Google Scholar] [CrossRef] [PubMed]

| numDF | denDF | F-Value | p-Value | |
|---|---|---|---|---|
| (Intercept) | 1 | 50 | 1882.23 | <0.0001 |
| Treatment | 4 | 10 | 83.56 | <0.0001 |
| Day | 5 | 50 | 137.91 | <0.0001 |
| Treatment–Day | 20 | 50 | 46.19 | <0.0001 |
| Day | 78.2 nM | 0.782 µM | 7.82 μM | 78.2 μM |
|---|---|---|---|---|
| 0 | 0.9960 | 1.0000 | 0.9955 | 1.0000 |
| 3 | 0.9960 | 0.9363 | 0.8387 | 0.9987 |
| 4 | 0.8595 | 0.7276 | 0.0180 | 0.0241 |
| 5 | 0.9493 | 0.9995 | 0.0115 | 0.0016 |
| 7 | 0.9681 | 0.9871 | 0.0026 | <0.0001 |
| 10 | 0.8595 | 0.9790 | 0.0005 | <0.0001 |
| numDF | denDF | F-Value | p-Value | |
|---|---|---|---|---|
| (Intercept) | 1 | 170 | 3489.65 | <0.0001 |
| Treatment | 5 | 17 | 369.92 | <0.0001 |
| Day | 10 | 170 | 176.09 | <0.0001 |
| Treatment–Day | 50 | 170 | 144.20 | <0.0001 |
| Day | Indolacetic Acid | Thidiazuron | Gibberellic Acid | Imidacloprid Gleba® | Imidacloprid Glacoxan® |
|---|---|---|---|---|---|
| 0 | 0.9990 | 0.9734 | 0.9989 | 1.0000 | 1.0000 |
| 1 | 0.9987 | 0.8953 | 0.9898 | 1.0000 | 0.9996 |
| 2 | 0.9960 | 0.9151 | 0.3336 | 0.9985 | 0.9928 |
| 3 | 0.9940 | 0.9536 | 0.0051 | 0.9907 | 0.9790 |
| 6 | 0.9944 | 0.8985 | 0.0026 | 0.0004 | 0.0551 |
| 7 | 0.9855 | 0.8181 | 0.0031 | <0.0001 | 0.0015 |
| 10 | 0.9811 | 0.8181 | 0.0022 | 0.0001 | <0.0001 |
| 13 | 0.9734 | 0.7894 | 0.0020 | <0.0001 | <0.0001 |
| 15 | 0.9108 | 0.7639 | 0.0022 | <0.0001 | <0.0001 |
| 17 | 0.8937 | 0.7733 | 0.0028 | <0.0001 | <0.0001 |
| 21 | 0.7939 | 0.7150 | 0.0017 | <0.0001 | <0.0001 |
| numDF | denDF | F-Value | p-Value | ||
|---|---|---|---|---|---|
| (Intercept) | 1 | 20 | 447.30 | <0.0001 | |
| H. pluvialis | Treatment | 1 | 5 | 83.99 | 0.0003 |
| Day | 4 | 20 | 53.17 | <0.0001 | |
| Treatment–Day | 4 | 20 | 14.51 | <0.0001 | |
| (Intercept) | 1 | 24 | 2860.91 | <0.0001 | |
| Tetradesmus sp. | Treatment | 1 | 6 | 1406.98 | <0.0001 |
| Day | 4 | 24 | 187.59 | <0.0001 | |
| Treatment–Day | 4 | 24 | 138.47 | <0.0001 | |
| (Intercept) | 1 | 24 | 930.68 | <0.0001 | |
| Treatment | 1 | 6 | 1013.19 | 0.0003 | |
| M. obtusa | Day | 4 | 24 | 61.09 | <0.0001 |
| Treatment–Day | 4 | 24 | 88.54 | <0.0001 |
| Day | Haematococcus pluvialis | Tetrademus sp. | Monoraphidium obtusa |
|---|---|---|---|
| 0 | 0.9009 | 0.8678 | 0.8910 |
| 4 | 0.1729 | <0.0001 | <0.0001 |
| 6 | 0.0037 | <0.0001 | <0.0001 |
| 7 | 0.0001 | <0.0001 | <0.0001 |
| 10 | 0.0003 | <0.0001 | <0.0001 |
| numDF | denDF | F-Value | p-Value | |
|---|---|---|---|---|
| (Intercept) | 1 | 42 | 1106.68 | <0.0001 |
| Treatment | 1 | 42 | 142.60 | <0.0001 |
| Day | 3 | 42 | 166.54 | <0.0001 |
| Treatment–Day | 3 | 42 | 97.55 | <0.0001 |
| Day | 78.2 μM |
|---|---|
| 0 | 0.6309 |
| 3 | 0.8752 |
| 5 | 0.0002 |
| 10 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, V.L.; Alvarez Dalinger, F.S.; Moraña, L.B. The Insecticide Imidacloprid Promotes Algal Growth in Absence of Zooplankton. J. Xenobiot. 2025, 15, 90. https://doi.org/10.3390/jox15030090
Lozano VL, Alvarez Dalinger FS, Moraña LB. The Insecticide Imidacloprid Promotes Algal Growth in Absence of Zooplankton. Journal of Xenobiotics. 2025; 15(3):90. https://doi.org/10.3390/jox15030090
Chicago/Turabian StyleLozano, Verónica Laura, Florencia Soledad Alvarez Dalinger, and Liliana Beatriz Moraña. 2025. "The Insecticide Imidacloprid Promotes Algal Growth in Absence of Zooplankton" Journal of Xenobiotics 15, no. 3: 90. https://doi.org/10.3390/jox15030090
APA StyleLozano, V. L., Alvarez Dalinger, F. S., & Moraña, L. B. (2025). The Insecticide Imidacloprid Promotes Algal Growth in Absence of Zooplankton. Journal of Xenobiotics, 15(3), 90. https://doi.org/10.3390/jox15030090






