Micro- and Nano-Plastics in Drinking Water: Threat or Hype? Critical State-of-the-Art Analysis of Risks and Approaches
Abstract
1. Introduction
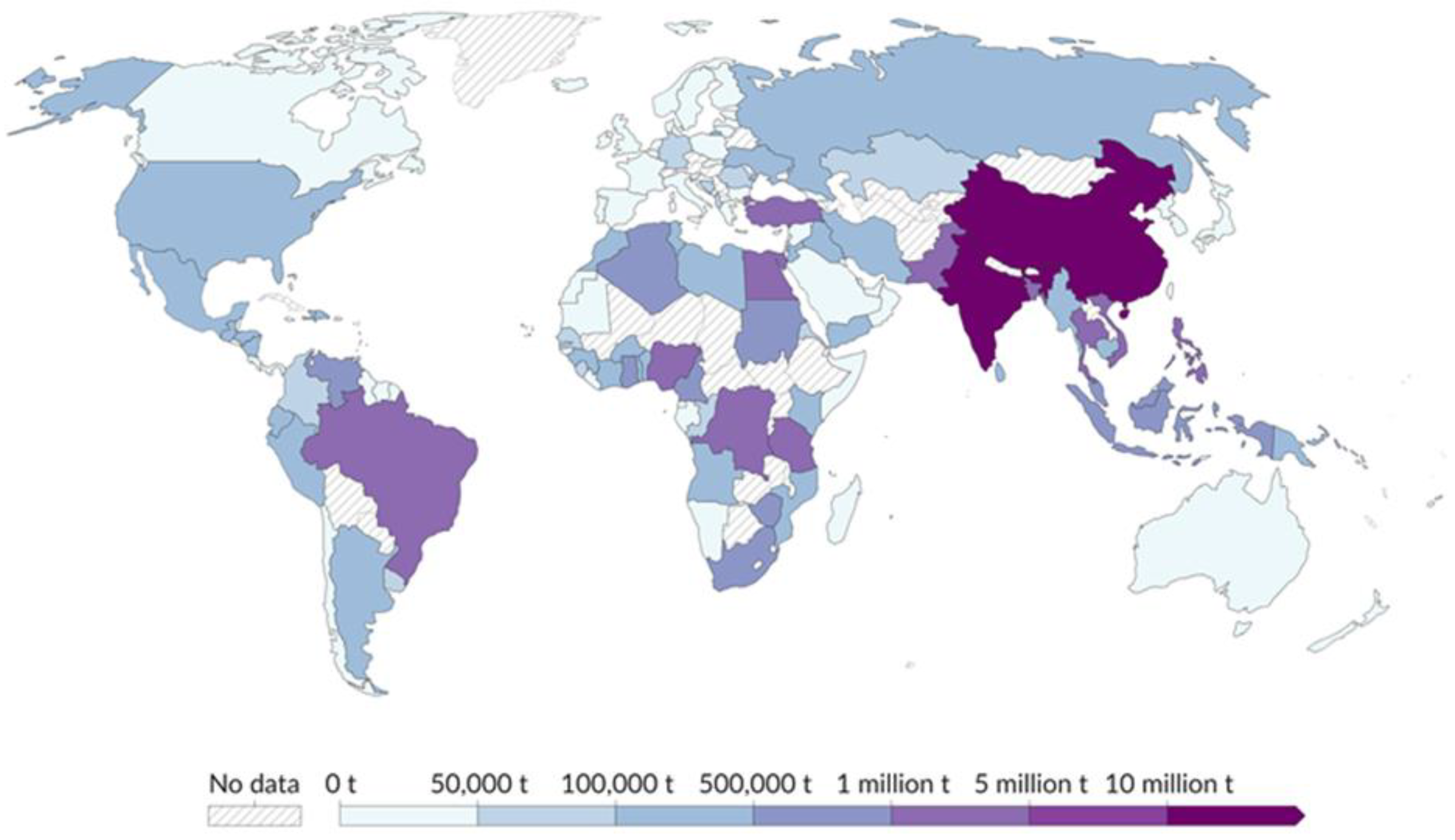
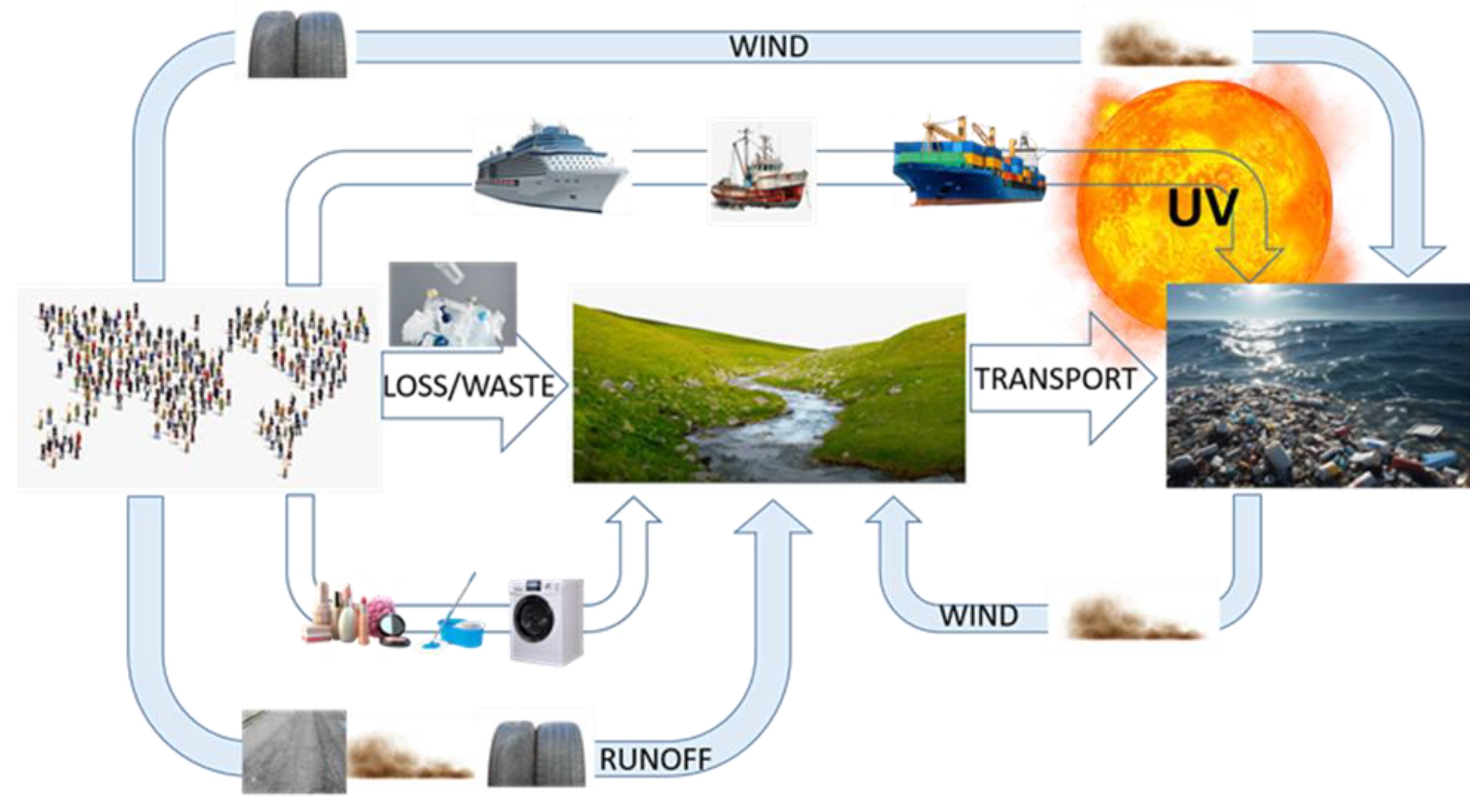
2. Methodology
3. MPs in Aquatic Environments
3.1. MP Detection Issues
3.2. Nanoplatics: An Entirely Separate Issue?
3.3. MP/NP Ingestion and Human Health Risk
4. Drinking Water Treatment Technologies and MP/NP Removal
4.1. AOPs and MPs
4.2. Membranes and MPs
4.3. NP Removal
5. Possible Approches
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ENTECH. The Value of Plastics. Available online: https://www.entecpolymers.com/resources/product-guides/the-value-of-plastics#:~:text=Plastics%20require%202%25%20to%204,generate%2061%25%20more%20greenhouse%20gases! (accessed on 15 November 2024).
- Meng, F.; Brandão, M.; Cullen, J.M. Replacing Plastics with Alternatives Is Worse for Greenhouse Gas Emissions in Most Cases. Environ. Sci. Technol. 2024, 58, 2716–2727. [Google Scholar] [CrossRef]
- McKinsey & Company. Climate Impact of Plastics. Available online: https://www.mckinsey.com/industries/chemicals/our-insights/climate-impact-of-plastics (accessed on 6 October 2024).
- Sustainable Packaging Coalition. 2021–2022 Centralized Study on the Availability of Recycling. Available online: https://sustainablepackaging.org/wp-content/uploads/2022/03/UPDATED-2020-21-Centralized-Study-on-Availability-of-Recycling-SPC-3-2022.pdf (accessed on 2 October 2024).
- d’Ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. Field Actions Sci. Rep. 2019, 19, 12–21. [Google Scholar]
- Capodaglio, A.G. Developments and Issues in Renewable Ecofuels and Feedstocks. Energies 2024, 17, 3560. [Google Scholar] [CrossRef]
- Geyer, R. Chapter 2—Production. In Production, Use and Fate of Synthetic Polymers in Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2017. [CrossRef]
- UNEP. Drowning in Plastics—Marine Litter and Plastic Waste Vital Graphics; United Nations Environment Programme (UNEP), Secretariats of the Basel, Rotterdam and Stockholm Conventions (BRS) and GRID-Arendal: Basel, Switzerland, 2021. [Google Scholar]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process Imp. 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. Compt. Rend. Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Burns, E.E.; Boxall, A.B.A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796. [Google Scholar] [CrossRef]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; LaRue, M.; McDonald, A.J. First evidence of microplastics in Antarctic snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- Bank, M.S.; Hansson, S.V. Chapter 1—The Microplastic Cycle: An Introduction to a Complex Issue. In Microplastic in the Environment: Pattern and Process; Bank, M.S., Ed.; Springer Nature AG: Cham, Switzerland, 2022. [Google Scholar]
- PLASTICS FOR CHANGE. The 7 Different Types of Plastic. Available online: https://www.plasticsforchange.org/blog/different-types-of-plastic (accessed on 30 September 2024).
- Our World in Data. Mismanaged Plastic Waste. 2019. Available online: https://ourworldindata.org/grapher/plastic-waste-mismanaged (accessed on 28 September 2024).
- Capodaglio, A.G. Microplastics in the urban water cycle: A critical analysis of issues and of possible (needed?) solutions. Sci. Total Environ. 2024, 954, 176580. [Google Scholar] [CrossRef]
- Choudhury, T.R.; Riad, S.; Uddin, F.J.; Maksud, M.A.; Alam, M.A.; Chowdhury, A.M.S.; Mubin, A.L.; Towfiqul Islam, A.R.M.; Malafaia, G. Microplastics in multi-environmental compartments: Research advances, media, and global management scenarios. J. Contam. Hydrol. 2024, 265, 104379. [Google Scholar] [CrossRef]
- Travis, C.C.; Hester, S.T. Global chemical pollution. Environ. Sci. Technol. 1991, 25, 814–819. [Google Scholar] [CrossRef]
- Copetti, D.; Marziali, L.; Viviano, G.; Valsecchi, L.; Guzzella, L.; Capodaglio, A.G.; Tartari, G.; Polesello, S.; Valsecchi, S.; Mezzanotte, V.; et al. Intensive monitoring of conventional and surrogate quality parameters in a highly urbanized river affected by multiple combined sewer overflows. Water Sci. Technol. Water Supply 2019, 19, 953–966. [Google Scholar] [CrossRef]
- Xia, W.; Rao, Q.; Deng, X.; Chen, J.; Xie, P. Rainfall is a significant environmental factor of microplastic pollution in inland waters. Sci. Total Environ. 2020, 732, 139065. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Moss, K.; Le Roux, G.; Phoenix, V.R.; Sonke, J.E. Examination of the ocean as a source for atmospheric microplastics. PLoS ONE 2020, 15, e0232746. [Google Scholar] [CrossRef]
- The New York Times. The ‘Great Pacific Garbage Patch’ Is Ballooning, 87,000,000,000 Tons of Plastic and Counting. Available online: https://www.nytimes.com/2018/03/22/climate/great-pacific-garbage-patch.html#:~:text=In%20the%20Pacific%20Ocean%20between,’%20worth%2C%20researchers%20said%20Thursday (accessed on 30 September 2024).
- Agostini, L.; Fornazier Moreira, J.C.; Gonçalves Bendia, A.; Pezzo Kmit, M.C.; Waters, L.G.; Ferreira Mourão Santana, M.; Sumida, M.Y.G.; Turra, A.; Pellizari, V.H. Deep-sea plastisphere: Long-term colonization by plastic-associated bacterial and archaeal communities in the Southwest Atlantic Ocean. Sci. Total Environ. 2021, 793, 148335. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Vielfaure, H.; Polerecky, L.; Kienhuis, M.V.M.; van der Meer, M.T.J.; Pflüger, T.; Egger, M.; Niemann, H. Biodegradation of polyethylene by the marine fungus Parengyodontium album. Sci. Total Environ. 2024, 934, 172819. [Google Scholar] [CrossRef]
- Jebashalomi, V.; Partheeban, E.C.; Rajaram, R. Microbial degradation of low-density polyethylene (LDPE) and polystyrene using Bacillus cereus (OR268710) isolated from plastic-polluted tropical coastal environment. Sci. Total Environ. 2024, 924, 171580. [Google Scholar] [CrossRef]
- Mishra, S.; Dash, D.; Das, A.P. Aquatic Microbial Diversity on Plastisphere: Colonization and Potential Role in Microplastic Biodegradation. Geomicrobiol. J. 2024, 41, 312–323. [Google Scholar] [CrossRef]
- Philp, R.B. Ecosystems and Human Health: Toxicology and Environmental Hazards, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze estuary system, China: First observations on occurrence, distribution. Marine Poll. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Tulcan, R.X.S.; Lu, X. Microplastics in ports worldwide: Environmental concerns or overestimated pollution levels? Crit. Rev. Environ. Sci. Technol. 2024, 54, 1803–1826. [Google Scholar] [CrossRef]
- Schell, T.; Rico, A.; Vighi, M. Occurrence, fate and fluxes of plastics and microplastics in terrestrial and freshwater ecosystems. Rev. Environ. Contam. Toxicol. 2020, 238, 22–28. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Hofman-Caris, R.C.H.M.; Pieke, E.N.; ter Laak, T.L. Fate of microplastics in the drinking water production. Water Res. 2022, 221, 118790. [Google Scholar] [CrossRef]
- Sangkham, S.; Islam, M.A.; Adhikari, S.; Kumar, R.; Sharma, P.; Sakunkoo, P.; Bhattacharya, P.; Tiwari, A. Evidence of microplastics in groundwater: A growing risk for human health. Groundwater Sustain. Dev. 2023, 23, 100981. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Qian, N.; Gao, X.; Lang, X.; Deng, H.; Bratu, T.M.; Chen, Q.; Stapleton, P.; Yan, B.; Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2300582121. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Jones, T.; Santosh, M.; Liu, P.; Zhang, M.; Xu, L.; Li, W.; Lu, J.; Yang, C.X.; et al. Airborne microplastics: A review of current perspectives and environmental implications. J. Clean. Prod. 2022, 347, 131048. [Google Scholar] [CrossRef]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of drinking water: A systematic review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhu, Y.; Zeng, G.; Zhang, Y.; Yang, Y.; Wen, X.; Chen, M.; Yi, H. Removal of microplastics via drinking water treatment: Current knowledge and future directions. Chemosphere 2020, 251, 126612. [Google Scholar] [CrossRef]
- Bujnicki, J.; Dykstra, P.; Fortunato, E.; Grobert, N.; Heuer, R.; Keskitalo, C.; Nurse, P. Environmental and Health Risks of Microplastic Pollution, Scientific Opinion 6/2019; Directorate-General for Research and Innovation, European Commission, Group of Chief Scientific Advisors. Brussels 2019. Available online: https://op.europa.eu/en/publication-detail/-/publication/f235d1e3-7c4d-11e9-9f05-01aa75ed71a1/language-en (accessed on 28 February 2025).
- ISO 24187:2023; Principles for the Analysis of Microplastics Present in the Environment. International Organization for Standardization: Geneva, Switzerland, 2023.
- Chen, C.Y.; Olshammar, M.; Thorsén, G.; Strömberg, E. Identification and Quantification Techniques for Microplastics: Strengths, Weaknesses, and Recommendations for Harmonization; IVL, Swedish Environmental Research Institute REPORT C867: Stockholm, Sweden, 2024. [Google Scholar]
- EU. Directive 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Off. J. Eur. Union L 2020, 435, 1–62. [Google Scholar]
- EU. Commission Delegated Decision 2024/1441 of 11 March 2024 supplementing Directive (EU) 2020/2184 of the European Parliament and of the Council by laying down a methodology to measure microplastics in water intended for human consumption (notified under document C(2024) 1459). Off. J. Eur. Union L Ser. 2024. [Google Scholar]
- Belz, S.; Cella, C.; Geiss, O.; Gilliland, D.; La Spina, R.; Mėhn, D.; Sokull-Kluettgen, B. Analytical Methods to Measure Microplastics in Drinking Water; JRC136859; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar] [CrossRef]
- European Commission. Proposal for a Directive of the European Parliament and of the Council Concerning Urban Wastewater Treatment; COM/2022/541 Final; Council of the European Union: Brussels, Belgium, 2022.
- ASTM D8332-20; Standard Practice for Collection of Water Samples with High, Medium, or Low Suspended Solids for Identification and Quantification of Microplastic Particles and Fibers. ANSI: Washington, DC, USA, 2020.
- Galgani, F.; Ruiz Orejon Sanchez Pastor, L.; Ronchi, F.; Tallec, K.; Fischer, E.; Matiddi, M.; Anastasopoulou, A.; Andresmaa, E.; Angiolillo, M.; Bakker Paiva, M.; et al. Guidance on the Monitoring of Marine Litter in European Seas; JRC133594; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Sobhani, Z.; Al Amin, M.; Naidu, R.; Megharaj, M.; Fang, C. Identification and visualisation of microplastics by Raman mapping. Anal. Chim. Acta 2019, 1077, 191–199. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, Q.; Xing, X.; Chen, W.; She, Z.; Luo, Z. Raman spectra and surface changes of microplastics weathered under natural environments. Sci. Total Environ. 2020, 739, 139990. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- RIVM. Factsheet over Microplastics in Nederlandse Wateren. 2019. Available online: https://www.rivm.nl/sites/default/files/2019-06/Factsheet%20Microplastics%20in%20Nederlandse%20wateren.pdf (accessed on 28 February 2025).
- WHO. Dietary and Inhalation Exposure to Nano- and Microplastic Particles and Potential Implications for Human Health. Nutrition and Food Safety (NFS), Standards & Scientific Advice on Food Nutrition (SSA); World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Mortensen, N.P.; Johnson, L.H.; Grieger, K.D.; Ambroso, J.L.; Fennell, T.R. Biological interactions between nanomaterials and placental development and function following oral exposure. Reprod. Toxicol. 2019, 90, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F.; Clapper, H.M.; Burgess, R.T.; Ho, K.T. Human and ecological health effects of nanoplastics: May not be a tiny problem. Curr. Opin. Toxicol. 2021, 28, 43–48. [Google Scholar] [CrossRef]
- Gigault, L.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- N.N.I. Nanotechnology—Big Things from a Tiny World; National Nanotechnology Coordination Office, National Nanotechnology Initiative: Alexandria, VA, USA, 2018.
- EC. COMMISSION RECOMMENDATION on the Definition of Nanomaterial. Available online: http://eur-lex.europa.eu/LexUreServ/LexUriServ.do?uri=OJ:L:2011:275:0038:0040:EN:PDF (accessed on 28 February 2025).
- EC. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (recast). Off. J. Eur. Union L 2009, 342, 59–209. [Google Scholar]
- EU. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union L 2015, 327, 1–22. [Google Scholar]
- ISO 80004-1:2023; Nanotechnologies—Vocabulary—Part 1: Core Vocabulary. International Organization for Standardization: Vernier, Switzerland, 2023.
- Enyoh, C.E.; Wang, Q.; Chowdhury, T.; Wang, W.; Lu, S.; Xiao, K.; Chowdhury, M.A.H. New Analytical Approaches for Effective Quantification and Identification of Nanoplastics in Environmental Samples. Processes 2021, 9, 2086. [Google Scholar] [CrossRef]
- Xie, D.; Fang, H.; Zhao, X.; Lin, X.; Su, Z. Identification of microplastics and nanoplastics in environmental water by AFM-IR. Anal. Chim. Acta 2025, 1354, 343992. [Google Scholar] [CrossRef] [PubMed]
- Morgana, S.; Casentini, B.; Tirelli, V.; Grasso, F.; Amalfitano, S. Fluorescence-based detection: A review of current and emerging techniques to unveil micro/ nanoplastics in environmental samples. TrAC Trends Analyt Chem. 2024, 172, 117559. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Vladitsi, M.; Nikolaou, C.; Kalogiouri, N.P.; Samanidou, V.F. Analytical Methods for Nanomaterial Determination in Biological Matrices. Methods Protoc. 2022, 5, 61. [Google Scholar] [CrossRef]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and environmental impacts of nanoparticles: A scoping review of the current literature. BMC Publ. Health 2023, 23, 1059. [Google Scholar] [CrossRef]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassi, B.; Rowenczyk, L.; Tufenkji, N.; Fent, S.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, X.; Zhang, X.; Wu, L.; Wei, W.; Ni, B.J. Nanoplastics are significantly different from microplastics in urban waters. Water Res. X 2023, 19, 100169. [Google Scholar] [CrossRef]
- Burton, G.A., Jr. Stressor Exposures Determine Risk: So, Why Do Fellow Scientists Continue To Focus on Superficial Microplastics Risk? Environ. Sci. Technol. 2017, 51, 13515–13516. [Google Scholar] [CrossRef]
- European Food Safety Authority. Presence of microplastics and nanoplastics in food, with particular focus on seafood. Panel on Contaminants in the Food Chain. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Zhu, L.; Kang, Y.L.; Ma, M.D.; Wu, Z.X.; Zhang, L.; Hu, R.X.; Xu, Q.; Zhu, J.; Gu, X.; An, L. Tissue accumulation of microplastics and potential health risks in human. Sci. Total Environ. 2024, 915, 170004. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Zhou, N.; Chen, Y.; Ling, Z.; Xiang, P. Microplastics in the human body: A comprehensive review of exposure, distribution, migration mechanisms, and toxicity. Sci. Total Environ. 2024, 946, 174215. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool. A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Bahar, M.M.; Megharaj, M.; Fang, C.; Rahman, M.M. The Unseen Threat of the Synergistic Effects of Microplastics and Heavy Metals in Aquatic Environments: A Critical Review. Curr. Poll. Rep. 2024, 10, 478–497. [Google Scholar] [CrossRef]
- Barhoumi, B.; Sander, S.G.; Tolosa, I. A review on per- and polyfluorinated alkyl substances (PFASs) in microplastic and food-contact materials. Environ. Res. 2022, 206, 112595. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Chen, Q.; Kalogerakis, N.; Ji, R.; Ma, Y. Interactions between microplastics and organic pollutants: Effects on toxicity, bioaccumulation, degradation, and transport. Sci. Total Environ. 2020, 748, 142427. [Google Scholar] [CrossRef]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liquids 2022, 350, 118580. [Google Scholar] [CrossRef]
- Puckowski, A.; Cwięk, W.; Mioduszewska, K.; Stepnowski, P.; Białk-Bielińska, A. Sorption of pharmaceuticals on the surface of microplastics. Chemosphere 2021, 263, 127976. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; De-la-Torre, G.E. Sorption of chemical contaminants on degradable and non-degradable microplastics: Recent progress and research trends. Sci. Total Environ. 2021, 757, 143875. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Rakib, M.R.J.; Lin, C.; Hung, N.T.Q.; Le, V.G.; Nguyen, H.L.; Malafaia, G.; Idris, A.M. A comprehensive review on ecological effects of microplastic pollution: An interaction with pollutants in the ecosystems and future perspectives. Trends Anal. Chem. 2023, 168, 117294. [Google Scholar] [CrossRef]
- Danopoulos, E.; Twiddy, M.; West, R.; Rotchell, J.M. A rapid review and meta-regression analyses of the toxicological impacts of microplastic exposure in human cells. J. Hazard. Mater. 2022, 427, 127861. [Google Scholar] [CrossRef]
- Backhaus, T.; Wagner, M. Microplastics in the Environment: Much Ado about Nothing? A Debate. Glob. Chall. 2020, 4, 1900022. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Poirier, L.; Kamari, A.; Châtel, A.; Mouneyrac, C.; Lagarde, F. Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ. Pollut. 2016, 211, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; Hernandez, A.; Rubio, L.; Marcos, R.; Cortes, C. Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier. Arch. Toxicol. 2020, 94, 2997–3012. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Ferguson, L.; Awe, A.; Sparks, C. Microplastic concentrations and risk assessment in water, sediment and invertebrates from Simon’s Town, South Africa. Heliyon 2024, 10, e28514. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Tang, J.; Wang, L.; Liu, R.; Giesy, J.P. Combined cytotoxicity of polystyrene nanoplastics and phthalate esters on human lung epithelial A549 cells and its mechanism. Ecotoxicol. Environ. Saf. 2021, 213, 112041. [Google Scholar] [CrossRef]
- ECHA. Plastic Additives Initiative. Available online: https://echa.europa.eu/mapping-exercise-plastic-additives-initiative (accessed on 27 February 2025).
- Yu, Y.; Kumar, M.; Bolan, S.; Padhye, L.P.; Bolan, N.; Li, S.; Wang, L.; Hou, D.; Li, Y. Various additive release from microplastics and their toxicity in aquatic environments. Environ. Pollut. 2024, 343, 123219. [Google Scholar] [CrossRef]
- Barceló, D.; Picó, Y.; Alfarhan, A.H. Microplastics: Detection in human samples, cell line studies, and health impacts. Environ. Toxicol. Pharmacol. 2023, 101, 104204. [Google Scholar] [CrossRef]
- Paul, M.B.; Stock, V.; Cara-Carmona, J.; Lisicki, E.; Shopova, S.; Fessard, V.; Braeuning, A.; Sieg, H.; Bohmert, L. Micro- and nanoplastics—Current state of knowledge with the focus on oral uptake and toxicity. Nanoscale Adv. 2020, 2, 435. [Google Scholar] [CrossRef]
- Vega-Herrera, A.; Garcia-Tornè, M.; Borrell-Diaz, X.; Abad, E.; Llorca, M.; Villanueva, C.M.; Farré, M. Exposure to micro(nano)plastics polymers in water stored in single-use plastic bottles. Chemosphere 2023, 343, 140106. [Google Scholar] [CrossRef]
- Becerra-Herrera, M.; Arismendi, D.; Molina-Balmaceda, A.; Uslar, J.; Manzo, V.; Richter, P.; Caraballo, M.A. Initial phthalates fingerprint and hydrochemical signature as key factors controlling phthalates concentration trends in PET-bottled waters during long storage times. Food Chem. 2022, 372, 131248. [Google Scholar] [CrossRef] [PubMed]
- UNU. Bottled Water Masks World’s Failure to Supply Safe Water for All. United Nations University. Available online: https://unu.edu/press-release/bottled-water-masks-worlds-failure-supply-safe-water-all (accessed on 10 December 2024).
- Akhbarizadeh, R.; Dobaradaran, S.; Schmidt, T.C.; Nabipour, I.; Spitz, J. Worldwide bottled water occurrence of emerging contaminants: A review of the recent scientific literature. J. Hazard. Mater. 2020, 392, 122271. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.; Dauchy, X.; Chagnon, M.C.; Etienne, S. Chemical compounds and toxicological assessments of drinking water stored in polyethylene terephthalate (PET) bottles: A source of controversy reviewed. Water Res. 2012, 46, 571–583. [Google Scholar] [CrossRef]
- Dolcini, J.; Chiavarini, M.; Firmani, G.; Ponzio, E.; D’Errico, M.M.; Barbadoro, P. Consumption of Bottled Water and Chronic Diseases: A Nationwide Cross-Sectional Study. Int. J. Environ. Res. Public Health 2024, 21, 1074. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef]
- Romphophak, P.; Faikhaw, O.; Sairiam, S.; Thuptimdang, P.; Coufort-Saudejaud, C. Removal of microplastics and nanoplastics in water treatment processes: A systematic literature review. J. Water Process Eng. 2024, 64, 105669. [Google Scholar] [CrossRef]
- Na, S.H.; Kim, M.J.; Kim, J.T.Y.; Jeong, S.; Lee, S.; Chung, J.; Kim, E.U. Microplastic removal in conventional drinking water treatment processes: Performance, mechanism, and potential risk. Water Res. 2021, 202, 117417. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Islam, Z.; Jamal, A.H.M.S.I.M.; Momtaz, N.; Beauty, S.A. Removal efficiencies of microplastics of the three largest drinking water treatment plants in Bangladesh. Sci. Total Environ. 2023, 895, 165155. [Google Scholar] [CrossRef]
- Callegari, A.; Boguniewicz-Zablocka, J.; Capodaglio, A.G. Experimental application of an advanced separation process for NOM removal from surface drinking water supply. Separations 2017, 4, 32. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martinez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of microbeads from wastewater using electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.T.; Horn, H.; Schuhen, K. Removal of microplastics from waters through agglomeration-fixation using organosilanes—Effects of polymer types, water composition and temperature. Water 2021, 13, 675. [Google Scholar] [CrossRef]
- Herbort, A.F.; Sturm, M.T.; Fiedler, S.; Abkai, G.; Schuhen, K. Alkoxy-silyl induced agglomeration: A new approach for the sustainable removal of microplastic from aquatic systems. J. Polym. Environ. 2018, 26, 4258–4270. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, J.J.; Liu, L.Y.; Li, Z.; Zeng, E.Y. Drinking Boiled Tap Water Reduces Human Intake of Nanoplastics and Microplastics. Environ. Sci. Technol. Lett. 2024, 11, 273–279. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Yao, Y.; Artigas, F.; Huang, Q.; Zhang, W. Organotin Release from Polyvinyl Chloride Microplastics and Concurrent Photodegradation in Water: Impacts from Salinity, Dissolved Organic Matter, and Light Exposure. Environ. Sci. Technol. 2019, 53, 10741–10752. [Google Scholar] [CrossRef]
- Velasco, A.N.; Ramseier Gentile, S.; Zimmermann, S.; Le Coustumer, P.; Stoll, S. Contamination and removal efficiency of microplastics and synthetic fibres in a conventional drinking water treatment plant in Geneva, Switzerland. Sci. Total Environ. 2023, 880, 163270. [Google Scholar] [CrossRef]
- Han, Z.; Jiang, J.; Xia, J.; Yan, C.; Cui, C. Occurrence and fate of microplastics from a water source to two different drinking water treatment plants in a megacity in eastern China. Environ. Pollut. 2024, 346, 123546. [Google Scholar] [CrossRef]
- Capodaglio, A.G. High-energy oxidation process: An efficient alternative for wastewater organic contaminants removal. Clean. Technol. Environ. Policy 2017, 19, 1995–2006. [Google Scholar] [CrossRef]
- de Oliveira Dos Santos, N.; Busquets, R.; Campos, L.C. Insights into the removal of microplastics and microfibres by Advanced Oxidation Processes. Sci. Total Environ. 2023, 861, 160665. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Zhao, Y.; Xiang, Y.; Li, Y.; Pan, X. Effects of advanced oxidation processes on leachates and properties of microplastics. J. Hazard. Mater. 2021, 413, 125342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, Y.; Li, Y.; Yin, L. Application of advanced oxidation processes for the removal of micro/nanoplastics from water: A review. Chemosphere 2024, 346, 140636. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1619–1666. [Google Scholar] [CrossRef]
- Acarer, S. A review of microplastic removal from water and wastewater by membrane technologies. Water Sci. Technol. 2023, 88, 199. [Google Scholar] [CrossRef]
- Marsono, B.D.; Yuniarto, A.; Purnomo, A.; Soedjono, E.S. Comparison performances of microfiltration and rapid sand filter operated in water treatment plant. OIP Conf. Ser. Earth Environ. Sci. 2022, 1111, 012048. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; He, H.; Zhu, Y.; Dionysiou, D.D.; Liu, Z.; Zhao, C. Do membrane filtration systems in drinking water treatment plants release nano/microplastics? Sci. Total Environ. 2021, 755, 142658. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Tchobanoglous, G.; Capodaglio, A.G.; Tzanakakis, V.A. The Importance of Nonconventional Water Resources under Water Scarcity. Water 2024, 16, 1015. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Pulido-Reyes, G.; Magherini, L.; Bianco, C.; Sethi, R.; von Gunten, U.; Kaegi, R.; Mitrano, D.M. Nanoplastics removal during drinking water treatment: Laboratory- and pilot-scale experiments and modelling. J. Hazard. Mater. 2022, 436, 129011–129024. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Tian, Z.; Cai, X.; Guan, B. Identification and quantification of nanoplastics (20–1000 nm) in a drinking water treatment plant using AFM-IR and Pyr-GC/MS. J. Hazard. Mater. 2024, 463, 132933. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Gao, B.; Wu, L.; Yao, Y.; Zhang, M.; Liu, L. Filtration of engineered nanoparticles in carbon-based fixed bed columns. Chem. Eng. J. 2013, 220, 221–227. [Google Scholar] [CrossRef]
- Piplai, T.; Kumar, A.; Alappat, B.J. Removal of mixture of ZnO and CuO nanoparticles (NPs) from water using activated carbon in batch kinetic studies. Water Sci. Technol. 2017, 75, 928–943. [Google Scholar] [CrossRef]
- Tong, H.; He, L.; Rong, H.; Li, M.; Kim, H. Transport behaviors of plastic particles in saturated quartz sand without and with biochar/Fe3O4-biochar amendment. Water Res. 2020, 169, 115284. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef]
- Ramirez Arenas, L.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Fate and removal efficiency of polystyrene nanoplastics in a pilot drinking water treatment plant. Sci. Total Environ. 2022, 813, 152623. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Örmeci, B. Removal Effectiveness of Nanoplastics (<400 nm) with Separation Processes Used for Water and Wastewater Treatment. Water 2020, 12, 635. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Keerthana Devi, M.; Karmegam, N.; Manikandan, S.; Subbaiya, R.; Song, H.; Kwon, E.E.; Sarkar, B.; Bolan, N.; Kim, W.; Rinklebe, J.; et al. Removal of nanoplastics in water treatment processes: A review. Sci. Total Environ. 2022, 845, 157168–157180. [Google Scholar] [CrossRef]
- Li, J.; Mubashar, M.; Zulekha, R.; Xu, C.; Zhang, X. Applications of coagulation-sedimentation and ultrafiltration for the removal of nanoparticles from water. Sep. Purif. Technol. 2025, 357 Pt A, 129920. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Chen, Z.; Ma, B.; Chen, J.P. Ultrafiltration membrane fouling by microplastics with raw water: Behaviors and alleviation methods. Chem. Eng. J. 2021, 410, 128174. [Google Scholar] [CrossRef]
- Tiwari, E.; Singh, N.; Khandelwal, N.; Monikh, F.A.; Darbha, G.K. Application of Zn/Al layered double hydroxides for the removal of nano-scale plastic debris from aqueous systems. J. Hazard. Mater. 2020, 397, 122769. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Miao, C.; Wang, Y.; Gao, G.; Yang, R.; Zhu, H.; Wang, J.; Li, S.; Lan, Y. Metal–organic framework-based foams for efficient microplastics removal. J. Mater. Chem. A 2020, 8, 14644–14652. [Google Scholar] [CrossRef]
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Castillo, J.F.; Mitchell, S.G.; Guettel, R.; Streb, C. Water Purification and Microplastics Removal using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (magPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1605. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of microplastics from water by magnetic nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Li, J.; Li, Q.; Xu, H.; Ye, Q.; Wang, Y.; Shu, S.; Zhang, J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Sci. Total Environ. 2021, 752, 141837. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Pivokonska, L.; Novotna, K.; Cermakova, M.; Klimtova, M. Occurrence and fate of microplastics at two different drinking water treatment plants within a river catchment. Sci. Total Environ. 2020, 741, 140236. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Twiss, M.R. Standardized methods are required to assess and manage microplastic contamination of the Great Lakes system. J. Great Lakes Res. 2016, 42, 921–925. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, S.; Zhang, T.; Liu, Q.; Alvarez, P.J.J.; Chen, W. Current Methods and Prospects for Analysis and Characterization of Nanomaterials in the Environment. Environ. Sci. Technol. 2022, 56, 7426–7447. [Google Scholar] [CrossRef]
- Ribeiro, F.; O’Brien, J.W.; Galloway, T.; Thomas, K.V. Accumulation and fate of nano- and micro-plastics and associated contaminants in organisms. TrAC Trends Anal. Chem. 2019, 111, 139–147. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, P.; Pathak, S.; Manimekalai, R.; Garg, D.; Dashora, K. Strategies for the Remediation of Micro- and Nanoplastics from Contaminated Food and Water: Advancements and Challenges. J. Xenobiot. 2025, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, J.; Zhu, Z.; Li, L.; Yu, F. Effect of microplastic size on the adsorption behavior and mechanism of triclosan on polyvinyl chloride. Environ. Pollut. 2019, 254, 113104. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lian, X.-Y.; Wang, Y.; Chen, S.; Sun, Y.-R.; Tao, G.-L.; Tan, Q.-W.; Feng, J.-C. Impacts of hydraulic conditions on microplastics biofilm development, shear stresses distribution, and microbial community structures in drinking water distribution pipes. J. Environ. Manag. 2023, 325, 116510. [Google Scholar] [CrossRef]
- Dalmau-Soler, J.; Ballesteros-Cano, R.; Boleda, M.R.; Paraira, M.; Ferrer, N.; Lacorte, S. Microplastics from headwaters to tap water: Occurrence and removal in a drinking water treatment plant in Barcelona Metropolitan area (Catalonia, NE Spain). Environ. Sci. Pollut. Res. 2021, 28, 59462–59472. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, Q.; Hu, X.; Zhong, X. Occurrence and identification of microplastics in tap water from China. Chemosphere 2020, 252, 126493. [Google Scholar] [CrossRef]
- Cherian, A.G.; Liu, Z.; McKie, M.J.; Almuhtaram, H.; Andrews, R.C. Microplastic Removal from Drinking Water Using Point-of-Use Devices. Polymers 2023, 15, 1331. [Google Scholar] [CrossRef]
- Gopakumar, A.N.; Ccanccapa-Cartagena, A.; Bell, K.; Salehi, M. Development of crosslinked polyvinyl alcohol nanofibrous membrane for microplastic removal from water. J. Appl. Polym. Sci. 2024, 141, e55428. [Google Scholar] [CrossRef]
- Li, Q.; Lai, Y.; Yu, S.; Li, P.; Zhou, X.; Dong, L.; Liu, X.; Yao, Z.; Liu, J. Sequential Isolation of Microplastics and Nanoplastics in Environmental Waters by Membrane Filtration, Followed by Cloud-Point Extraction. Anal. Chem. 2021, 93, 4559–4566. [Google Scholar] [CrossRef]
- Bai, Y.; Shan, F.; Zhu, Y.Y.; Xu, J.Y.; Wu, Y.S.; Luo, X.G.; Wu, Y.U.; Hu, H.Y.; Zhang, B.L. Long-term performance and economic evaluation of full-scale MF and RO process—A case study of the Changi NEWater Project Phase 2 in Singapore. Water Cycle 2020, 1, 128–135. [Google Scholar] [CrossRef]
- Payment, P.; Franco, R.; Richardson, L.; Siemiatyck, J. Gastrointestinal health effects associated with the consumption of drinking water produced by point-of-use domestic reverse-osmosis filtration units. Appl. Environ. Microbiol. 1991, 57, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. Urban Water Supply Sustainability and Resilience under Climate Variability: Innovative Paradigms, Approaches and Technologies. ACS ES&T Water 2024, 4, 5185–5206. [Google Scholar]
| Method | Strength | Weakness | Notes |
|---|---|---|---|
| Visual analysis | Straightforward. Allows the exam of large filter surfaces, leading to quick analysis. | No polymer identification. Serious risk of particle misidentification. | Requires skilled and experienced analysts. Useful for sample pre-screening prior to other analyses. It can be improved with training and experience. |
| FTIR | High resolution. Polymer type identification. Less instrument settings than Raman. μ-FTIR: resolution below 20 μm, with automatic sample scan μ-FTIR provides information on MP aging (through carbonyl index). | Measures smaller filter surface area than visual analysis. Commonly used after visual analysis on selected particles, selection bias can occur. Possible fragment counts are overestimated compared to a stereomicroscope. Accuracy affected by MP morphology. It may not identify particles <10 μm. μ-FTIR operation is time-consuming as it measures individual particles (unless using focal plane array-based detection requiring liquid N for cooling. | Advanced instruments require trained personnel and routine maintenance/calibration to operate. Requires cleaner samples: chemical treatment can affect results. Overlapping particles may induce refractive error. Additional costs for special filters (i.e., anodisc, PTFE, gold coated). Spectral libraries affect identification accuracy. Different laboratories use different hit quality indices and spectral matching libraries, resulting in varying matching success. Harmonization of spectral libraries is needed. Expertise in interpreting spectra of weathered particles is essential. |
| Raman spectroscopy | Higher resolution than visual analysis. Polymer identification. A good complement to visual analysis. Less affected by polymer degradation than FTIR, not affected by thickness. Can identify particle <1 μm. It can be automated to reduce spectral interpretation operating time. μ-Raman in combination with an optical microscope to analyze particles ˂1 μm. | Risk of contamination by adhesive polymer fragments on instrument surface. Spectra interfered by particle color, addictive, fluorescence, and pigment content. Risk of sample damage by laser beam | Advanced instruments require trained personnel and routine maintenance/calibration to operate. Requires clean sample to reduce spectral interference. Similar to FTIR, different spectral libraries influence final results. |
| Py-GC/MS | Identifies the total mass of each polymer type in a sample. Characterization of both polymers and additives | No size class of particles is given unless prior particles are manual sorting. | Advanced instruments require trained personnel and routine maintenance/calibration to operate. Requires a clean sample to achieve a cleaner program. Requires dedicated libraries for polymers and additives. |
| Approach | Advantages | Disadvantages | Principle | MPs Type | MPs Size | Removal Efficiency | Refs. |
|---|---|---|---|---|---|---|---|
| Membrane Filtration | High removal efficiency | Membrane fouling, High TMP required | UF, RO RO | All All | 1–5000 μm 20–1000 nm | ≈100% up to 99% | [124,125,136] |
| Sand filtration | Effective for larger-size MPs | Low removal efficiency | Rapid sand filtration | All | <10 μm | 29.0–44.4% | [100] |
| Effective for small size particles | Removal efficiency can be improved by adsorbents addition | Low rate filtration | All | 20–1000 nm | Up to 99% | [125] | |
| Adsorption | High efficiency, simple operation | Adsorbent regeneration | GAC | All | 20–1000 nm | Up to 99.9% | [130] |
| Zn/Al layered hydroxides | PS | 55 nm | 96% | [137] | |||
| Metal–organic framework- foams | PS, PMMA, PVDF | 325 nm, 183 nm, 260 nm | 88.2% 85.7% 90.1% | [138] | |||
| Magnetic removal | Simple, economical, and fast | Addition of magnetic materials to treated solution | MagPOM–SILP | PS | 1 or 10 μm | 100% | [139] |
| Nano-Fe3O4 | PE, PP, PS, PET | 200–900 μm | 62.83–86.87% | [140] | |||
| M−CNTs | PA, PET, PE | 48 μm | 100% | [141] | |||
| Coagulation | Simple operation, low cost | Consumption of flocculants | Coagulation | PS, PE | <5000 μm | 77.83%, 29.70% | [142] |
| Coagulation | PE | <5000 μm | 8.3–61.2% | [124] | |||
| Coagulation | All MP | <5000 μm | 40.5–54.5% | [100] | |||
| Coagulation + GAC | All MP | <5000 μm | 62% | [143] | |||
| Co-precipitation | Simple requires water boiling | Only effective in hard water | CaCO3 precipitation | PS, PE, PP | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capodaglio, A.G. Micro- and Nano-Plastics in Drinking Water: Threat or Hype? Critical State-of-the-Art Analysis of Risks and Approaches. J. Xenobiot. 2025, 15, 85. https://doi.org/10.3390/jox15030085
Capodaglio AG. Micro- and Nano-Plastics in Drinking Water: Threat or Hype? Critical State-of-the-Art Analysis of Risks and Approaches. Journal of Xenobiotics. 2025; 15(3):85. https://doi.org/10.3390/jox15030085
Chicago/Turabian StyleCapodaglio, Andrea G. 2025. "Micro- and Nano-Plastics in Drinking Water: Threat or Hype? Critical State-of-the-Art Analysis of Risks and Approaches" Journal of Xenobiotics 15, no. 3: 85. https://doi.org/10.3390/jox15030085
APA StyleCapodaglio, A. G. (2025). Micro- and Nano-Plastics in Drinking Water: Threat or Hype? Critical State-of-the-Art Analysis of Risks and Approaches. Journal of Xenobiotics, 15(3), 85. https://doi.org/10.3390/jox15030085







