Ecotoxicity of Fire Retardants to Zebrafish (Danio rerio) in Early Life Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Chemicals
2.2. Cultivation System and Obtaining of Zebrafish Embryos
2.3. Toxicity Test with Zebrafish Embryos (FET)
2.4. Chemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Parameters
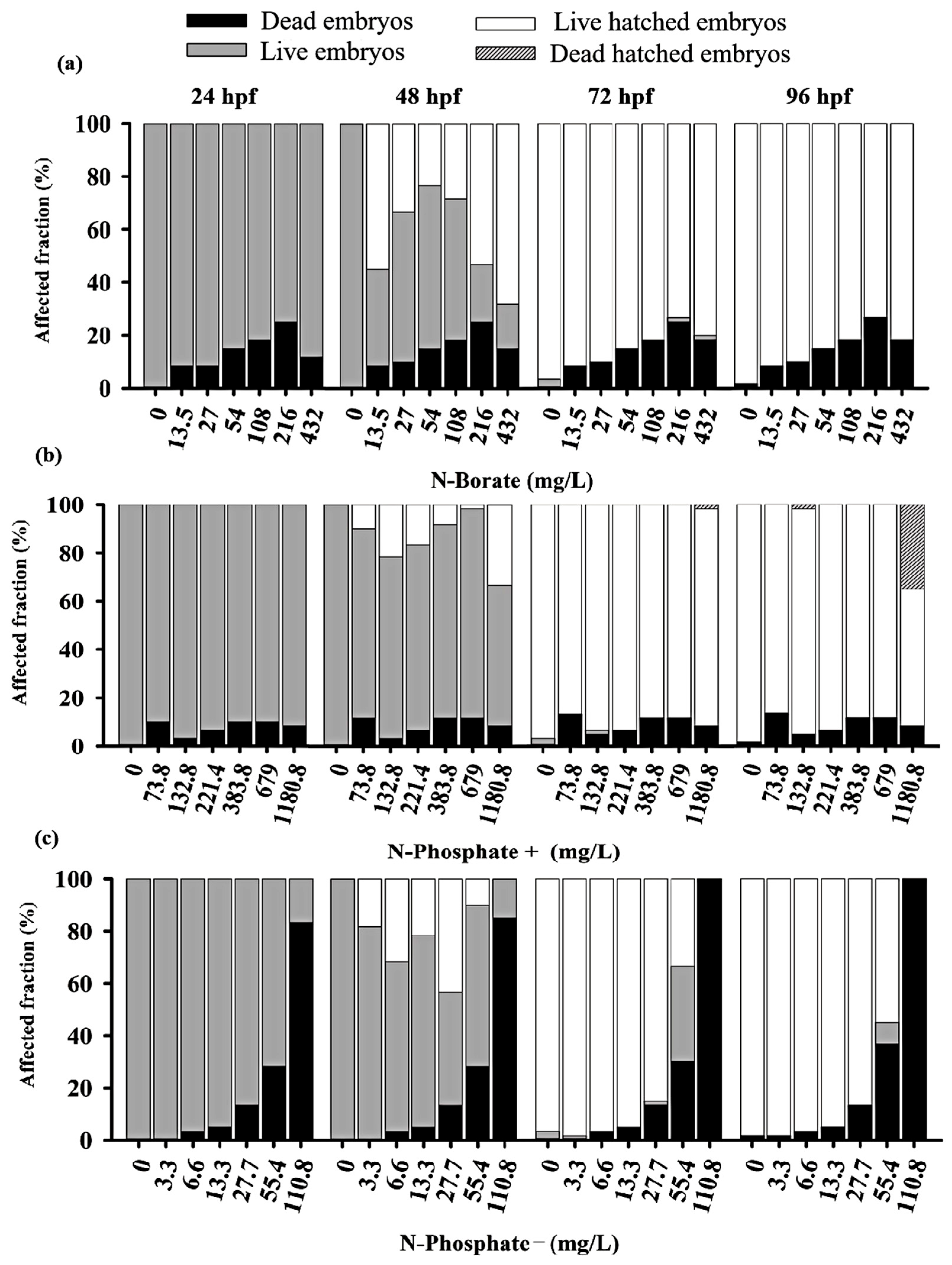
3.2. Mortality and Hatching Rates of Zebrafish Embryos
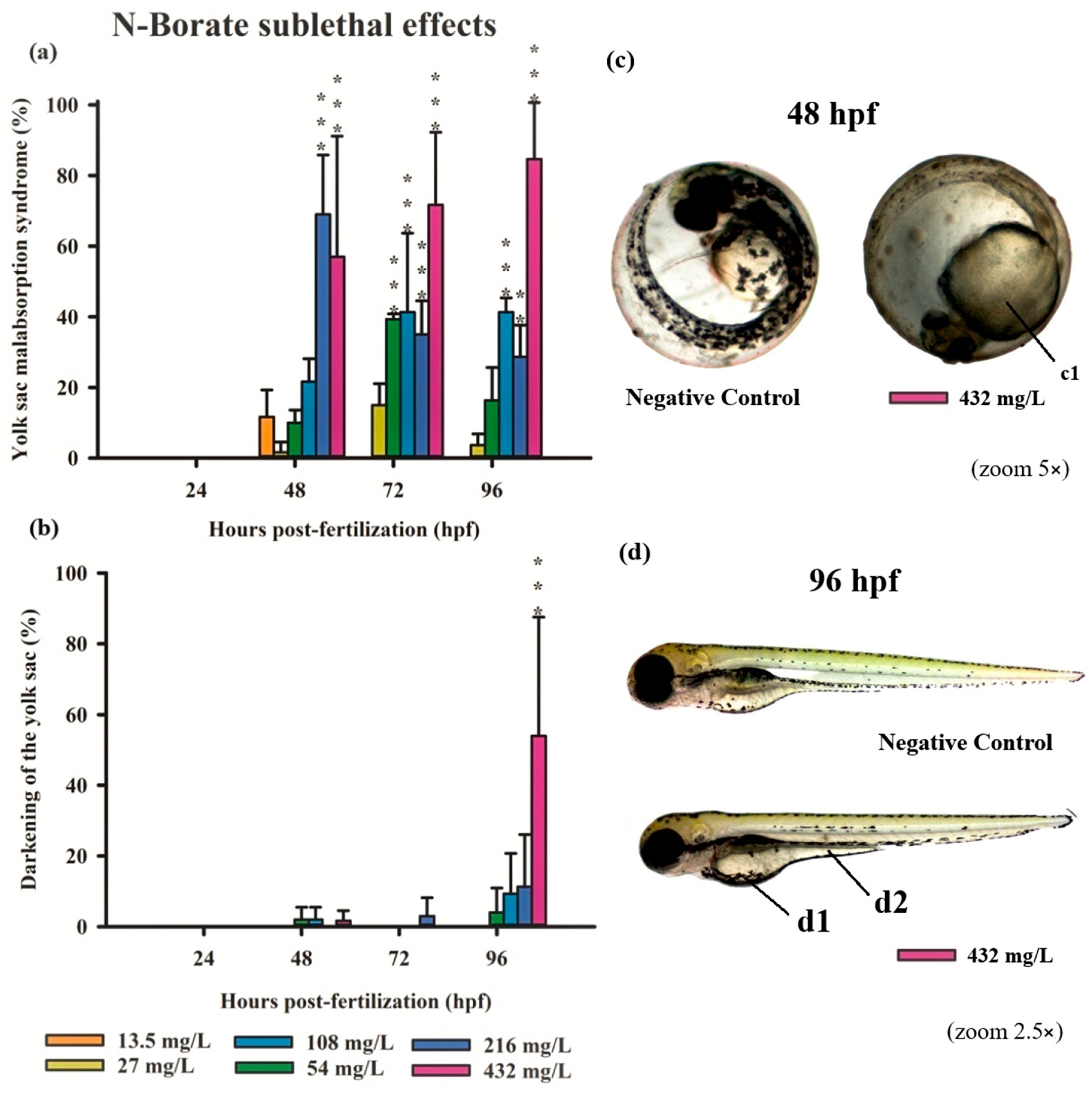
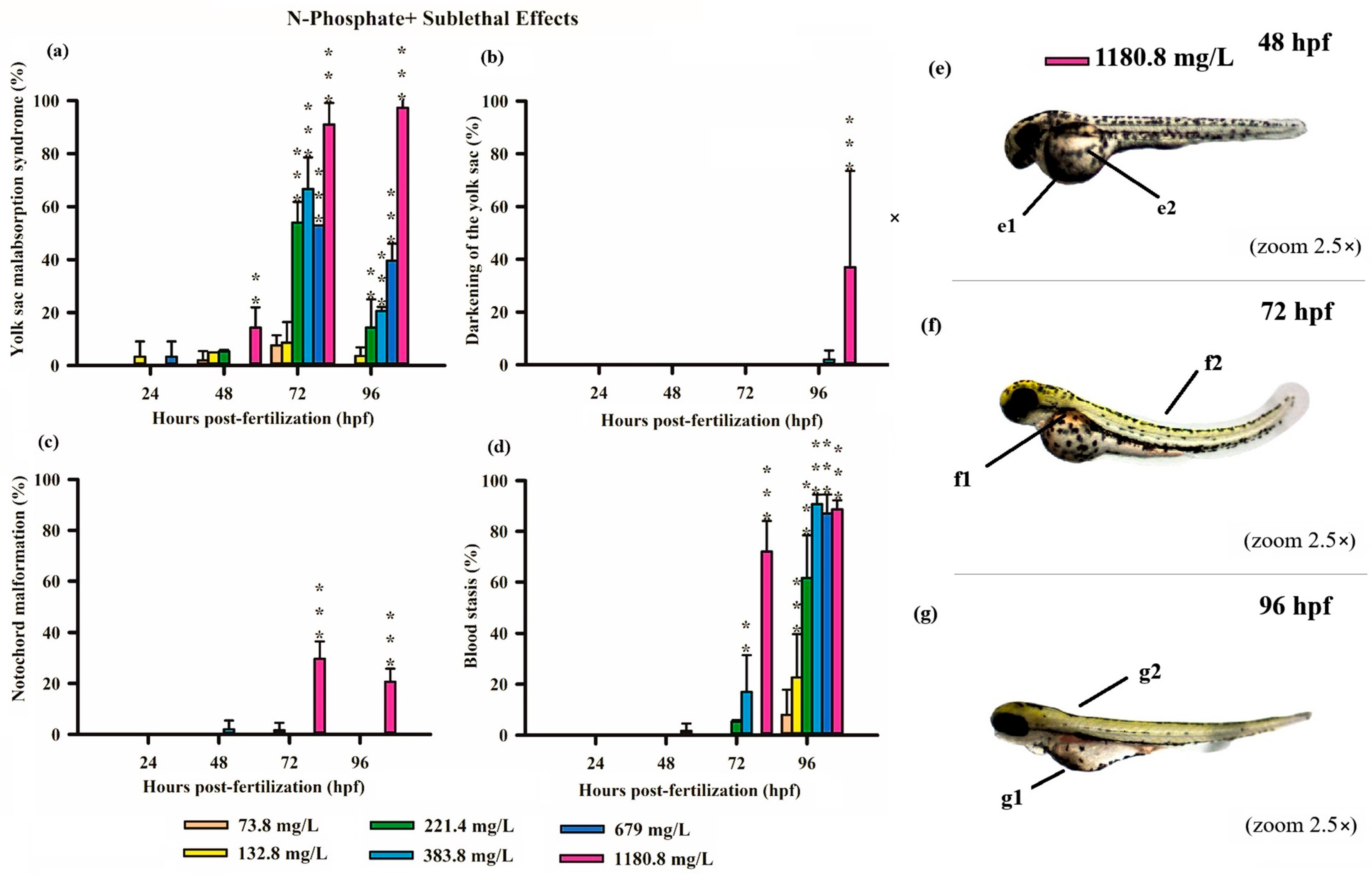
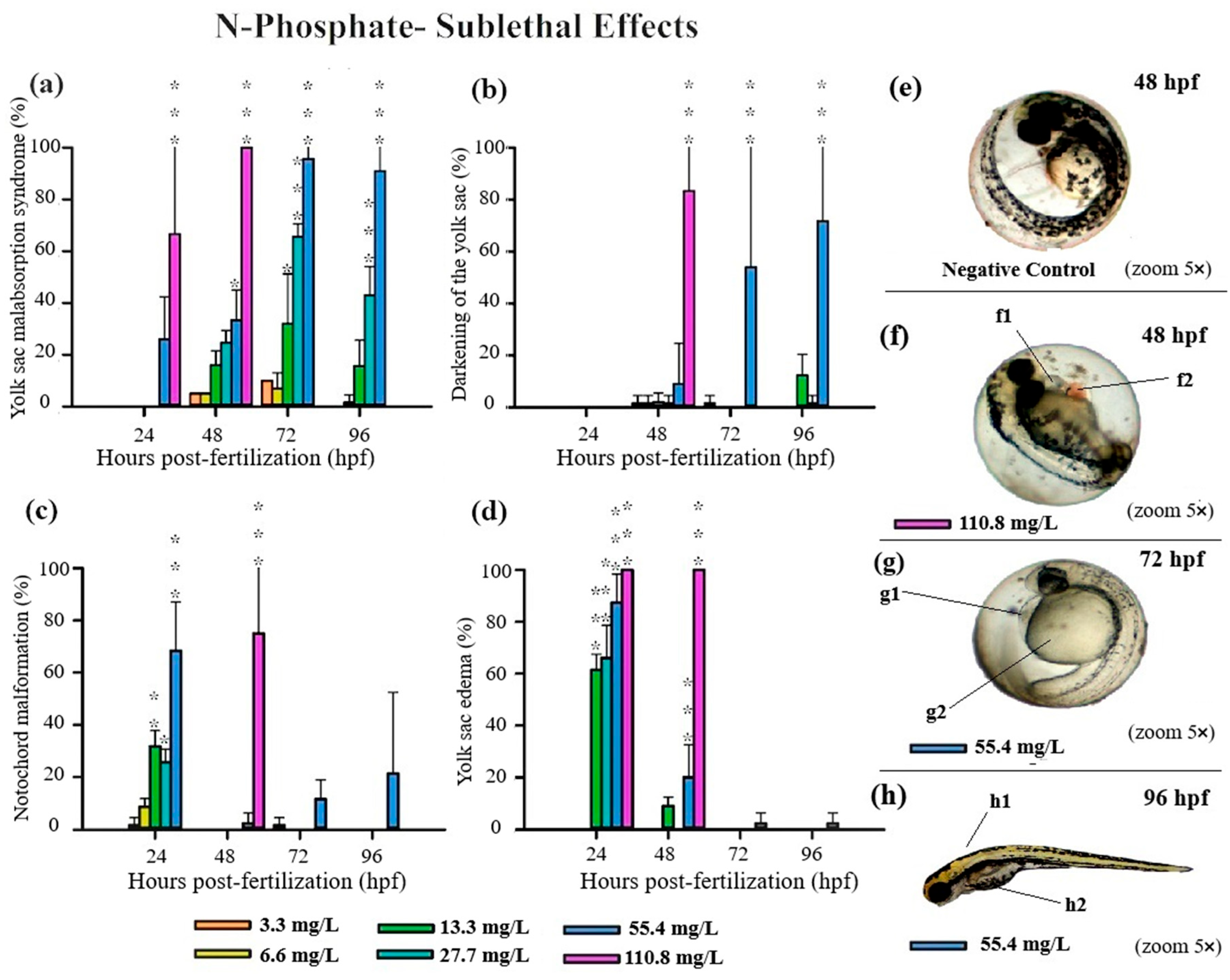
3.3. Teratogenicity of Zebrafish Embryos
4. Discussion
4.1. Acute and Sublethal Toxicity of FRs
4.2. Causes of FR Toxicity
4.3. Ecological Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Faria, B.L.; Brando, P.M.; Macedo, M.N.; Panday, P.K.; Soares-Filho, B.S.; Coe, M.T. Current and Future Patterns of Fire-Induced Forest Degradation in Amazonia. Environ. Res. Lett. 2017, 12, 095005. [Google Scholar] [CrossRef]
- Hope, P.; Black, M.T.; Lim, E.P.; Dowdy, A.; Wang, G.; Pepler, A.S.; Fawcett, R.J.B. On determining the impact of increasing atmospheric CO2 on the record fire weather in eastern Australia in February 2017. Bull. Am. Meteorol. Soc. 2019, 100, S111–S117. [Google Scholar] [CrossRef]
- Lizundia-Loiola, J.; Otón, G.; Ramo, R.; Chuvieco, E. A spatio-temporal active-fire clustering approach for global burned area mapping at 250 m from MODIS data. Remote Sens. Environ. 2020, 236, 111493. [Google Scholar] [CrossRef]
- San-Miguel-Ayanz, J.; Durrant, T.; Boca, R.; Libertà, G.; Branco, A.; De Rigo, D.; Ferrari, D.; Maianti, P.; Artes Vivancos, T.; Pfeiffer, H.; et al. Forest Fires in Europe, Middle East and North Africa (2018). EUR 29856 EN; Publications Office of the European Union: Luxembourg, 2019; ISBN 978-92-76-12591-4. [Google Scholar]
- Karma, S.; Schismenos, S.; Emmanouloudis, D.; Chalaris, M. Challenges and Lessons Learned from Past Major Environmental Disasters Due to Technological or Wildland-Urban Interface Fire Incidents. Contributing Paper to GAR. In United Nations Global Assessment Report on Disaster Risk Reduction (GAR) 2019; United Nations Office for Disaster Risk Reduction: Geneva, Switzerland, 2019. [Google Scholar]
- Godfree, R.C.; Knerr, N.; Encinas-Viso, F.; Albrecht, D.; Bush, D.; Cargill, D.C.; Clements, M.; Gueidan, C.; Guja, L.K.; Harwood, T.; et al. Implications of the 2019–2020 megafires for the biogeography and conservation of Australian vegetation. Nat. Commun. 2021, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.M.; Christianson, A.C.; Gray, R.W.; Daniels, L. Western Canada’s new wildfire reality needs a new approach to fire management. Environ. Res. Lett. 2022, 17, 061001. [Google Scholar] [CrossRef]
- De la Barrera, F.; Barraza, F.; Favier, P.; Ruiz, V.; Quense, J. Megafires in Chile 2017: Monitoring Multiscale Environmental Impacts of Burned Ecosystems. Sci. Total Environ. 2018, 637, 1526–1536. [Google Scholar] [CrossRef]
- Silveira, M.V.F.; Silva, C.H.L., Jr.; Anderson, L.O.; Aragao, L. Amazon Fires in the 21st Century: The Year of 2020 in Evidence. Glob. Ecol. Biogeogr. 2022, 31, 2026–2040. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2021—The Physical Science Basis: Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Jones, M.W.; Abatzoglou, J.T.; Veraverbeke, S.; Andela, N.; Lasslop, G.; Forkel, M.; Smith, A.J.P.; Burton, C.; Betts, R.A.; van der Werf, G.R.; et al. Global and regional trends and drivers of fire under climate change. Rev. Geophys. 2022, 60, e2020RG000726. [Google Scholar] [CrossRef]
- Jolly, W.M.; Cochrane, M.A.; Freeborn, P.H.; Holden, Z.A.; Brown, T.J.; Williamson, G.J.; Bowman, D.M. Climate-induced variations in global wildfire danger from 1979 to 2013. Nat. Commun. 2015, 6, 7537. [Google Scholar] [CrossRef]
- Schmidt, I.B.; Eloy, L. Fire Regime in the Brazilian Savanna: Recent Changes, Policy and Management. Flora 2020, 268, 151613. [Google Scholar] [CrossRef]
- Courtens, E.N.P.; Meerburg, F.; Mausen, V.; Vlaeminck, S.E. When the Smoke Disappears: Dealing with Extinguishing Chemicals in Firefighting Wastewater. Water Sci. Technol. 2014, 69, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Doerr, S.H.; Santín, C. Global Trends in Wildfire and Its Impacts: Perceptions versus Realities in a Changing World. Philos. Trans. R. Soc. B. 2016, 371, 20150345. [Google Scholar] [CrossRef] [PubMed]
- Graetz, S.; Ji, M.; Hunter, S.; Sibley, P.K.; Prosser, R.S. Deterministic risk assessment of firefighting water additives to aquatic organisms. Ecotoxicology 2020, 29, 1377–1389. [Google Scholar] [CrossRef]
- Instituto Brasileiro do Meio Ambiente e Recursos Naturais (IBAMA). Technical Report No. 514/2018-COASP/CGASQ/DIQUA. Brasília. 2018. Available online: https://www.gov.br/ibama/pt-br/assuntos/quimicos-e-biologicos/petroleo-combustiveis-e-produtos-perigosos/parecer-tecnico-sobre-produtos-retardantes-de-chamas-para-uso-em-incendios-florestais (accessed on 20 May 2025).
- Gaikowski, M.P.; Hamilton, S.J.; Buhl, K.J.; McDonald, S.F.; Summers, C.H. Acute toxicity of three fire-retardant and two fire-suppressant foam formulations to the early life stages of rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 1996, 15, 1365–1374. [Google Scholar] [CrossRef]
- González-Prieto, S.J. Firefighting Chemicals. In Handbook of Fire and the Environment; The Society of Fire Protection Engineers Series; Meacham, B.J., McNamee, M., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Montagnolli, R.N.; Matos Lopes, P.R.; Matos Cruz, J.; Marina Turini Claro, E.; Quiterio, G.M.; Bidoia, E.D. The Effects of Fluoride-Based Fire-Fighting Foams on Soil Microbiota Activity and Plant Growth during Natural Attenuation of Perfluorinated Compounds. Environ. Toxicol. Pharmacol. 2017, 50, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Houtz, E.; Wang, M.; Park, J.S. Identification and fate of aqueous film forming foam derived per- and polyfluoroalkyl substances in a wastewater treatment plant. Environ. Sci. Technol. 2018, 52, 13212–13221. [Google Scholar] [CrossRef]
- Tunstill, K.; Grogan, L.F.; Morrison, C.; McCallum, H.; Lanctôt, C. Effects of Two Firefighting Chemical Formulations, Phos–Chek LC95W and BlazeTamer380, on Striped Marsh Frog (Limodynastes peronii) Tadpole Survival, Growth, Development and Behaviour. Aquat. Toxicol. 2022, 252, 106326. [Google Scholar] [CrossRef]
- Anderson, J.; Prosser, R.S. Potential Risk to Aquatic Biota from Aerial Application of Firefighting Water Additives. Environ. Pollut. 2023, 316, 120651. [Google Scholar] [CrossRef] [PubMed]
- AFAC. Use of Chemicals in Bushfire Control and Prescribed Burning; Australasian Fire and Emergency Service Authorities Council: Melbourne, Australia. 2016; Available online: https://www.afac.com.au/insight/doctrine/article/current/use-of-chemicals-in-bushfire-control-and-prescribed-burning (accessed on 1 May 2025).
- McDonald, J.; McCormack, P.C. Responsibility and Risk-Sharing in Climate Adaptation: A Case Study of Bushfire Risk in Australia. Clim. Law 2022, 12, 128–161. [Google Scholar] [CrossRef]
- USA Forest Service. Interagency Wildland Fire Chemicals Policy and Guidance. Forest Service, USA Department of Agriculture. 2022. Available online: https://www.fs.usda.gov/managing-land/fire/chemicals (accessed on 5 February 2024).
- Morrison, C.; Grogan, L.F.; Clemann, N.; Lanctôt, C. Impacts of Fire-Fighting Chemicals on Native Fauna and Ecosystems in Australia: Identification of Key Knowledge Gaps and Research Priorities. Environ. Manag. 2025, 75, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Moss, G.L.; Smithyman, D. Short-Term Effects of Aerially-Applied Fire-Suppressant Foams on Water Chemistry and Macroinvertebrates in Streams after Natural Wild-Fire on Kangaroo Island, S. Aust. Hydrobiologia 2003, 498, 177–189. [Google Scholar] [CrossRef]
- Rakowska, J.; Prochaska, K.; Twardochleb, B.; Rojewska, M.; Porycka, B.; Jaszkiewicz, A. Selection of Surfactants as Main Components of Ecological Wetting Agent for Effective Extinguishing of Forest and Peat-Bog Fires. Chem. Pap. 2014, 68, 823–833. [Google Scholar] [CrossRef]
- Kalabokidis, K.D. Effects of wildfire suppression chemicals on people and the environment—A review. Glob. Nest J. 2000, 2, 129–137. [Google Scholar]
- Buhl, K.J.; Hamilton, S.J. Acute Toxicity of Fire-Control Chemicals, Nitrogenous Chemicals, and Surfactants to Rainbow Trout. Trans. Am. Fish. Soc. 2000, 129, 408–418. [Google Scholar]
- Martín, S.; Rodríguez, M.; Moreno, J.M.; Angeler, D.G. Complex ecological responses to drought and fire-retardant contamination impacts in ephemeral waters. Water Air Soil Pollut. 2014, 225, 2078. [Google Scholar] [CrossRef]
- Tobin, B.W.; Schwartz, B.F.; Kelly, M.; Despain, J.D. Fire retardant and post-fire nutrient mobility in a mountain surface water–karst groundwater system: The hidden fire, Sequoia National Park, California, USA. Environ. Earth Sci. 2015, 73, 951–960. [Google Scholar] [CrossRef]
- Buhl, K.J.; Hamilton, S.J. Acute toxicity of fire-retardant and foam-suppressant chemicals to early life stages of chinook salmon (Oncorhynchus tshawytscha). Environ. Toxicol. Chem. 1998, 17, 1589–1599. [Google Scholar] [CrossRef]
- Brito, D.Q.; Henke-Oliveira, C.; Oliveira-Filho, E.C. Acute Toxicity of Commercial Wildfire Retardants to Two Daphniid Species (Ceriodaphnia dubia and Daphnia magna). Toxics 2024, 12, 548. [Google Scholar] [CrossRef]
- Giménez, A.; Pastor, E.; Zárate, L.; Planas, E.; Arnaldos, J. Long-term forest fire retardants: A review of quality, effectiveness, application, and environmental considerations. Int. J. Wildland Fire 2004, 13, 1–15. [Google Scholar] [CrossRef]
- Fiedler, N.C.; Larcerda, G.R.; Ramalho, A.H.C.; Berude, L.C.; Das Neves, F.P.; Rodrigues, C.K. Firefighting combat with fire retardants at different concentrations. Floresta 2020, 50, 1107–1112. [Google Scholar] [CrossRef]
- Ozeri, L.; Blaustein, L.; Polevikov, A.; Kneitel, J.; Rahav, E.; Horwitz, R. Effects of a Fire Retardant on the Near Eastern Fire Salamander Salamandra infraimmaculata and Aquatic Community Structure: An Experimental Approach. Hydrobiologia 2021, 848, 4713–4729. [Google Scholar] [CrossRef]
- Rehmann, C.R.; Jackson, P.R.; Puglis, H.J. Predicting the Spatiotemporal Exposure of Aquatic Species to Intrusions of Fire Retardant in Streams with Limited Data. Sci. Total Environ. 2021, 782, 146879. [Google Scholar] [CrossRef]
- Puglis, H.J.; Iacchetta, M.; Mackey, C.M. Toxicity of Wildland Firefighting Chemicals in Pulsed Exposures to Rainbow Trout and Fathead Minnows. Environ. Toxicol. Chem. 2022, 41, 1711–1720. [Google Scholar] [CrossRef]
- Braunbeck, T.; Kais, B.; Lammer, E.; Otte, J.; Schneider, K.; Stengel, D.; Strecker, R. The Fish Embryo Test (FET): Origin, Applications, and Future. Environ. Sci. Pollut. Res. Int. 2015, 22, 16247–16261. [Google Scholar] [CrossRef]
- OECD. Test No. 215: Fish, Juvenile Growth Test. OECD Guidel. Test. Chem. 2000, 301, 16. [Google Scholar]
- Carlsson, G.; Patring, J.; Kreuger, J.; Norrgren, L.; Oskarsson, A. Toxicity of 15 Veterinary Pharmaceuticals in Zebrafish (Danio rerio) Embryos. Aquat. Toxicol. 2013, 126, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, J.; Cuevas, E.; Ali, S.F.; Paule, M.G. Zebrafish model in drug safety assessment. Curr. Pharm. Des. 2014, 20, 5416–5429. [Google Scholar] [CrossRef]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T. Is the Fish Embryo Toxicity Test (FET) with the Zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef]
- OECD. Test guideline 236: Fish Embryo Acute Toxicity (FET) Test. Report 2013, 2, 1–22. [Google Scholar]
- Wilson, E.W.; Castro, V.; Chaves, R.; Espinosa, M.; Rodil, R.; Quintana, J.B.; Vieira, M.N.; Santos, M.M. Using Zebrafish Embryo Bioassays Combined with High-Resolution Mass Spectrometry Screening to Assess Ecotoxicological Water Bodies Quality Status: A Case Study in Panama Rivers. Chemosphere 2021, 272, 129823. [Google Scholar] [CrossRef] [PubMed]
- Euroforte. Firout. Euroforte. 2017. Available online: https://euroforte.com.br/firout/ (accessed on 10 February 2024).
- Perimeter Solutions. PHOS-CHEK LC95 Series. Perimeter Solutions. 2023. Available online: https://www.perimeter-solutions.com/en/fire-safety-fire-retardants/phos-cheklc95-series/ (accessed on 16 March 2024).
- MEFA, L. MEFA Ecológico. 2017. Available online: http://www.mefaecologic.com.br/#produto (accessed on 5 March 2025).
- EMBRAPA. Manual of Soil Analysis Methods; National Research Center of Soils: Rio de Janeiro, Brazil, 1997. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ulman, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Development. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological Effects of Pharmaceuticals in Aquatic Systems—Impacts through Behavioural Alterations. Philos. Trans. R. Soc. B. 2014, 369, 20130580. [Google Scholar] [CrossRef] [PubMed]
- Engeszer, R.E.; Patterson, L.B.; Rao, A.A.; Parichy, D.M. Zebrafish in the Wild: A Review of Natural History and New Notes from the Field. Zebrafish 2007, 4, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Loewengart, G. Toxicity of boron to rainbow trout: A weight-of-the-evidence assessment. Environ. Toxicol. Chem. 2001, 20, 796–803. [Google Scholar] [CrossRef]
- Rowe, R.I.; Bouzan, C.; Nabili, S.; Eckhert, C.D. The response of trout and zebrafish embryos to low and high boron concentrations is U-shaped. Biol. Trace Elem. Res. 1998, 66, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.I.; Eckhert, C.D. Boron is required in zebrafish. J. Exp. Biol. 1999, 202, 1649–1654. [Google Scholar] [CrossRef]
- Eckhert, C.D. Boron stimulates embryonic trout growth. J. Nutr. 1998, 128, 2488–2493. [Google Scholar] [CrossRef]
- Sutter, G.; Gonzalez Flores, B.; Laskey, G.; Jurak, S. Investigating the impacts of ammonium phosphate-based fire retardants on cyanobacteria (Anabaena) growth. Humboldt J. Microbiol. 2023, 23, 4. Available online: https://digitalcommons.humboldt.edu/hjm/vol23/iss1/4 (accessed on 20 January 2025).
- Sinha, A.K.; Diricx, M.; Chan, L.P.; Liew, H.J.; Kumar, V.; Blust, R.; De Boeck, G. Expression Pattern of Potential Biomarker Genes Related to Growth, Ion Regulation and Stress in Response to Ammonia Exposure, Food Deprivation and Exercise in Common Carp (Cyprinus carpio). Aquat. Toxicol. 2012, 122, 93–105. [Google Scholar] [CrossRef]
- Parvathy, A.J.; Das, B.C.; Jifiriya, M.J.; Varghese, T.; Pillai, D.; Rejish Kumar, V.J. Ammonia Induced Toxico-Physiological Responses in Fish and Management Interventions. Rev. Aquac. 2023, 15, 452–479. [Google Scholar] [CrossRef]
- Thurston, R.V.; Russo, R.C.; Vinogradov, G.A. Ammonia Toxicity to Fishes: Effect of pH on the Toxicity of the Unionized Ammonia Species. Environ. Sci. Technol. 1981, 15, 837–840. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Zheng, J.-A.; Huang, S.-C.; Hung, G.-Y.; Horng, J.-L. Ammonia exposure impairs lateral-line hair cells and mechanotransduction in zebrafish embryos. Chemosphere 2020, 257, 127170. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Frumin, G.T.; Gildeeva, I.M. Eutrophication of water bodies—A global environmental problem. Russ. J. Gen. Chem. 2014, 84, 2483–2488. [Google Scholar] [CrossRef]
- Li, M.; Yu, N.; Qin, J.G.; Li, E.; Du, Z.; Chen, L. Effects of ammonia stress, dietary linseed oil, and Edwardsiella ictaluri challenge on juvenile darkbarbel catfish. Fish Shellfish. Immunol. 2014, 38, 158–165. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A. Ecological and Toxicological Effects of Inorganic Nitrogen Pollution in Aquatic Ecosystems: A Global Assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, H.J. Nitrate toxicity in Siberian sturgeon (Acipenser baeri). Aquaculture 2006, 253, 688–693. [Google Scholar] [CrossRef]
- Barbieri, E.; Bondioli, A.C.V. Acute Toxicity of Ammonia in Pacu Fish (Piaractus mesopotamicus, Holberg, 1887) at Different Temperature Levels. Aquac. Res. 2015, 46, 565–571. [Google Scholar] [CrossRef]
- Wells, J.B.; Little, E.E.; Calfee, R.D. Behavioral Response of Young Rainbow Trout (Oncorhynchus mykiss) to Forest Fire–Retardant Chemicals in the Laboratory. Environ. Toxicol. Chem. 2004, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.; Crunkilton, R.L. Acute and Chronic Toxicity of Nitrate to Fathead Minnows (Pimephales promelas), Ceriodaphnia dubia and Daphnia magna. Environ. Toxicol. Chem. 2000, 19, 2918–2922. [Google Scholar] [CrossRef]
- Moore, A.P.; Bringolf, R.B. Comparative Toxicity of Nitrate to Common and Imperiled Freshwater Mussel Glochidia and Larval Fishes. Arch. Environ. Contam. Toxicol. 2020, 78, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.C.R.; Santos, J.L.D.; Porawski, M.; Schaefer, P.G.; Maurer, R.L.; Matte, U.D.S.; Silveira, T.R.D. Implementação de um Novo Modelo de Experimentação Animal: Zebrafish. Rev. HCPA 2009, 29, 100–103. [Google Scholar]
- Pereira, A.C.; Gonçalves, B.B.; Brito, R.d.S.; Vieira, L.G.; Lima, E.d.O.; Rocha, T.L. Comparative Developmental Toxicity of Iron Oxide Nanoparticles and Ferric Chloride to Zebrafish (Danio rerio) after Static and Semi-Static Exposure. Chemosphere 2020, 254, 126792. [Google Scholar] [CrossRef]
- Holman, W.F.; Macek, K.J. An aquatic safety assessment of linear alkylbenzene sulfonate (LAS): Chronic effects on fathead minnows. Trans. Am. Fish. Soc. 1980, 109, 122–131. [Google Scholar]
- Mariani, L.; De Pascale, D.; Faraponova, O.; Tornambè, A.; Sarni, A.; Giuliani, S.; Ruggiero, G.; Onorati, F.; Magalett, E. The use of a test battery in marine ecotoxicology: The acute toxicity of sodium dodecyl sulfate. Environ. Toxicol. 2006, 21, 297–443. [Google Scholar] [CrossRef]
- Freitas, E.C.; Rocha, O. Acute and chronic effects of atrazine and sodium dodecyl sulfate on the tropical freshwater cladoceran Pseudosida ramosa. Ecotoxicology 2012, 21, 1347–1357. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Saha, N.C. Consequences of sodium dodecyl sulfate exposure on the antioxidant status and steroidogenesis in fish gonad. Environ. Sci. Pollut. Res. 2021, 28, 19247–19259. [Google Scholar] [CrossRef]
- Tallarico, L.F.; Silva, F.N.V.D.; Miranda, M.S.; Nakano, E. Sensitivity assessment of Biomphalaria glabrata (SAY, 1818) using reference substance sodium dodecyl sulfate for ecotoxicological analyzes. Ecotoxicology 2024, 33, 1135–1144. [Google Scholar] [CrossRef]
- Domingues, I.; Oliveira, R.; Lourenço, J.; Koppe, C.; Mendo, S.; Soares, A.M.V.M. Biomarkers as a Tool to Assess Effects of Chromium (VI): Comparison of Responses in Zebrafish Early Life Stages and Adults. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Kristofco, L.A.; Haddad, S.P.; Chambliss, C.K.; Brooks, B.W. Differential uptake of and sensitivity to diphenhydramine in embryonic and larval zebrafish. Environ. Toxicol. Chem. 2018, 37, 1175–1181. [Google Scholar] [CrossRef]
- Oliveira, R.; Grisolia, C.K.; Monteiro, M.S.; Soares, A.M.; Domingues, I. Multilevel Assessment of Ivermectin Effects Using Different Zebrafish Life Stages. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 187, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Rawson, D.M.; Zhang, T.; Kalicharan, D.; Jogebloed, W.L. Field Emission Scanning Electron Microscopy and Transmission Electron Microscopy Studies of the Chorion, Plasma Membrane and Syncytial Layers of the Gastrula-Stage Embryo of the Zebrafish Brachy Danio rerio: A Consideration of the Structural and Functional Relationships with Respect to Cryoprotectant Penetration. Aquac. Res. 2001, 31, 325–336. [Google Scholar] [CrossRef]
- Wang, S.; Zhuang, C.; Du, J.; Wu, C.; You, H. The Presence of MWCNTs Reduces Developmental Toxicity of PFOS in Early Life Stage of Zebrafish. Environ. Pollut. 2017, 222, 201–209. [Google Scholar] [CrossRef]
- Hill, A.J.; Bello, S.M.; Prasch, A.L.; Peterson, R.E.; Heideman, W. Water permeability and TCDD-induced edema in zebrafish early-life stages. Toxicol. Sci. 2003, 78, 78–87. [Google Scholar] [CrossRef]
- Samson, J.C.; Shenker, J. The teratogenic effects of methylmercury on early development of the zebrafish Danio rerio. Aquat. Toxicol. 2000, 48, 343–354. [Google Scholar] [CrossRef]
- Hallare, A.V.; Schirling, M.; Luckenbach, T.; Köhler, H.R.; Triebskorn, R. Combined effects of temperature and cadmium on developmental parameters and biomarker responses in zebrafish (Danio rerio) embryos. J. Therm. Biol. 2005, 30, 7–17. [Google Scholar] [CrossRef]
- Fuiman, L.A. Special considerations of fish eggs and larval. In Fishery Science: The Unique Contributions of Early Life Stage; Fuiman, L.A., Werner, R.G., Eds.; Blackwell Science: Oxford, UK, 2002; pp. 1–32. [Google Scholar]
- Jezierska, B.; Lugowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish: A review. Fish Physiol. Biochem. 2009, 35, 625–640. [Google Scholar] [CrossRef]
- Oliveira, R.; Domingues, I.; Grisolia, C.K.; Soares, A.M. Effects of Triclosan on Zebrafish Early-Life Stages and Adults. Environ. Sci. Pollut. Res. Int. 2009, 16, 679–688. [Google Scholar] [CrossRef]
- Bai, W.; Tian, W.; Zhang, Z.; He, H.; Ma, Y.; Liu, N.; Chai, Z. Effects of Copper Nanoparticles on the Development of Zebrafish Embryos. J. Nanosci. Nanotechnol. 2010, 10, 8670–8676. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.B.; Lin, S.; Nisbet, R.M. Quantitative Adverse Outcome Pathway Analysis of Hatching in Zebrafish with CuO Nanoparticles. Environ. Sci. Technol. 2015, 49, 11817–11824. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Ansari, B.A. Effects of Heavy Metals on the Embryo and Larvae of Zebrafish, Danio rerio (Cyprinidae). Sch. Acad. J. Biosci. 2015, 3, 52–56. [Google Scholar]
- Cruz, A.; Serrano, M.; Navarro, E.; Luna, B.; Moreno, J.M. Effect of a Long-Term Fire Retardant (Fire Trol 934®) on the Germination of Nine Mediterranean Type Shrub Species. Environ. Toxicol. 2005, 20, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Angeler, D.G.; Sanchez, B.; Garcia, G.; Moreno, J.M. Community Ecotoxicology: Invertebrate Emergence from Fire Trol 934 Contaminated Vernal Pool and Salt Marsh Sediments under Contrasting Photoperiod and Temperature Regimes. Aquat. Toxicol. 2006, 78, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Mizuki, H.; Uezu, K.; Kawano, T.; Kadono, T.; Kobayashi, M.; Hatae, S.; Oba, Y.; Iwamoto, S.; Mitumune, S.; Nagatomo, Y.; et al. Novel Environmental Friendly Soap-Based Fire-Fighting Agent. J. Environ. Eng. Manag. 2007, 16, 403–408. [Google Scholar]
- Bixby, R.J.; Cooper, S.D.; Gresswell, R.E.; Brown, L.E.; Dahm, C.N.; Dwire, K.A. Fire Effects on Aquatic Ecosystems: An Assessment of the Current State of Science. Freshw. Sci. 2015, 34, 1340–1350. [Google Scholar] [CrossRef]
- Gaikowski, M.P.; Hamilton, S.J.; Buhl, K.J.; McDonald, S.F.; Summers, C.H. Acute toxicity of firefighting chemical formulations to four life stages of fathead minnow. Ecotoxicol. Environ. Saf. 1996, 34, 252–263. [Google Scholar] [CrossRef]
- Irwin, E.G.; Culligan, P.J.; Fischer-Kowalski, M.; Law, K.L.; Murtugudde, R.; Pfirman, S. Bridging Barriers to Advance Global Sustainability. Nat. Sustain. 2018, 1, 324–326. [Google Scholar] [CrossRef]

| Fire Retardant Type | N-Borate | N-Phosphate+ | N-Phosphate− | ||||
|---|---|---|---|---|---|---|---|
| Concentration mg/L (%) | Control | Lowest 13.5 (0.001) | Highest 432 (0.032) | Lowest 74 (0.005) | Highest 1181 (0.080) | Lowest 3.32 (0.0003) | Highest 111 (0.01) |
| F− | <DL | <DL | <DL | <DL | <DL | <DL | <DL |
| Cl− | 65.54 | 69.96 | 81.04 | 59.69 | 68.54 | 62.45 | 64.17 |
| NO2− | <DL | <DL | <DL | <DL | <DL | <DL | <DL |
| Br− | 0.25 | 0.26 | 0.32 | 0.17 | 0.30 | 0.24 | 0.30 |
| NO3− | 9.48 | 10.14 | 10.2 | 7.37 | 9.64 | 9.55 | 9.61 |
| PO43− | <DL | <DL | <DL | <DL | <DL | <DL | <DL |
| SO42− | 0.56 | 0.57 | 0.57 | 18.26 | 90.65 | 0.88 | 4.42 |
| Li | <DL | <DL | <DL | <DL | <DL | <DL | <DL |
| Na+ | 69.74 | 74.78 | 74.9 | 55.28 | 70 | 68.67 | 65 |
| NH4+ | 1.37 | 0.38 | 10.26 | 23.15 | 101 | <DL | 3.38 |
| K+ | 2.97 | 3.22 | 3.05 | 2.46 | 3.73 | 3.02 | 2.95 |
| Ca2+ | 5.58 | 6.05 | 6.45 | 4.83 | 5.94 | 5.93 | 5.80 |
| Mg2+ | 8.10 | 8.80 | 9.0 | 6.41 | 8.71 | 7.80 | 7.64 |
| pH | 7.1 | 6.98 | 7.2 * | 6.97 | 7 | 7.25 | 7.55 |
| Conductivity (μS.cm−1) | 442 | 442 | 562 | 583 | 1196 | 443 | 483 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, D.Q.; Piau, T.B.; Henke-Oliveira, C.; Oliveira-Filho, E.C.; Grisolia, C.K. Ecotoxicity of Fire Retardants to Zebrafish (Danio rerio) in Early Life Stages. J. Xenobiot. 2025, 15, 79. https://doi.org/10.3390/jox15030079
Brito DQ, Piau TB, Henke-Oliveira C, Oliveira-Filho EC, Grisolia CK. Ecotoxicity of Fire Retardants to Zebrafish (Danio rerio) in Early Life Stages. Journal of Xenobiotics. 2025; 15(3):79. https://doi.org/10.3390/jox15030079
Chicago/Turabian StyleBrito, Darlan Quinta, Tathyana Benetis Piau, Carlos Henke-Oliveira, Eduardo Cyrino Oliveira-Filho, and Cesar Koppe Grisolia. 2025. "Ecotoxicity of Fire Retardants to Zebrafish (Danio rerio) in Early Life Stages" Journal of Xenobiotics 15, no. 3: 79. https://doi.org/10.3390/jox15030079
APA StyleBrito, D. Q., Piau, T. B., Henke-Oliveira, C., Oliveira-Filho, E. C., & Grisolia, C. K. (2025). Ecotoxicity of Fire Retardants to Zebrafish (Danio rerio) in Early Life Stages. Journal of Xenobiotics, 15(3), 79. https://doi.org/10.3390/jox15030079








